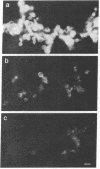
Abstract
The nucleocapsid of vesicular stomatitis virus (VSV) was introduced into the cytoplasm of Saccharomyces cerevisiae by low pH-dependent fusion of the viral envelope with the spheroplast plasma membrane. This led to de novo synthesis of the three major structural proteins of the virus--the G, N, and M proteins--as shown by immunoprecipitation of [35S]methionine-labeled spheroplast lysates. In NaDodSO4/polyacrylamide gel electrophoresis, M and N proteins comigrated with those of the virion, whereas the yeast-made G protein migrated as two bands with apparent molecular sizes of 60 and 70 kDa. Both polypeptides appeared to be N-glycosylated, since only one polypeptide with the apparent molecular mass of approximately equal to 55 kDa was produced in the presence of tunicamycin. Phase separation into Triton X-114 suggested that the unglycosylated G protein was membrane bound. According to immunofluorescent surface staining of live spheroplasts, at least part of the G protein was transported to the plasma membrane. Spheroplasts expressing the VSV genes could be fused together by low pH to form polykaryons, indicating that G protein synthetized by yeast was fusogenic--i.e., biologically active.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D., Huang A. S., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus, II. An RNA polymerase in the virion. Proc Natl Acad Sci U S A. 1970 Jun;66(2):572–576. doi: 10.1073/pnas.66.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann J. E., Tokuyasu K. T., Singer S. J. Passage of an integral membrane protein, the vesicular stomatitis virus glycoprotein, through the Golgi apparatus en route to the plasma membrane. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1746–1750. doi: 10.1073/pnas.78.3.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein M., Hoffmann W., Ammerer G., Schekman R. Characterization of a gene product (Sec53p) required for protein assembly in the yeast endoplasmic reticulum. J Cell Biol. 1985 Dec;101(6):2374–2382. doi: 10.1083/jcb.101.6.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Boy-Marcotte E., Jacquet M. A Dictyostelium discoideum DNA fragment complements a Saccharomyces cerevisiae ura3 mutant. Gene. 1982 Dec;20(3):433–439. doi: 10.1016/0378-1119(82)90212-8. [DOI] [PubMed] [Google Scholar]
- Cabezón T., De Wilde M., Herion P., Loriau R., Bollen A. Expression of human alpha 1-antitrypsin cDNA in the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6594–6598. doi: 10.1073/pnas.81.21.6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer J. H., Lea K., Slightom J. L. Expression of phaseolin cDNA genes in yeast under control of natural plant DNA sequences. Proc Natl Acad Sci U S A. 1985 Jan;82(2):334–338. doi: 10.1073/pnas.82.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois-Dalcq M. E., Doller E. W., Haspel M. V., Holmes K. V. Cell tropism and expression of mouse hepatitis viruses (MHV) in mouse spinal cord cultures. Virology. 1982 Jun;119(2):317–331. doi: 10.1016/0042-6822(82)90092-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmon B., Novick P., Schekman R. Compartmentalized assembly of oligosaccharides on exported glycoproteins in yeast. Cell. 1981 Aug;25(2):451–460. doi: 10.1016/0092-8674(81)90063-5. [DOI] [PubMed] [Google Scholar]
- Gahmberg N., Kuismanen E., Keränen S., Pettersson R. F. Uukuniemi virus glycoproteins accumulate in and cause morphological changes of the Golgi complex in the absence of virus maturation. J Virol. 1986 Mar;57(3):899–906. doi: 10.1128/jvi.57.3.899-906.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitzeman R. A., Hagie F. E., Levine H. L., Goeddel D. V., Ammerer G., Hall B. D. Expression of a human gene for interferon in yeast. Nature. 1981 Oct 29;293(5835):717–722. doi: 10.1038/293717a0. [DOI] [PubMed] [Google Scholar]
- Jabbar M. A., Sivasubramanian N., Nayak D. P. Influenza viral (A/WSN/33) hemagglutinin is expressed and glycosylated in the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2019–2023. doi: 10.1073/pnas.82.7.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuismanen E., Hedman K., Saraste J., Pettersson R. F. Uukuniemi virus maturation: accumulation of virus particles and viral antigens in the Golgi complex. Mol Cell Biol. 1982 Nov;2(11):1444–1458. doi: 10.1128/mcb.2.11.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehle L., Cohen R. E., Ballou C. E. Carbohydrate structure of yeast invertase. Demonstration of a form with only core oligosaccharides and a form with completed polysaccharide chains. J Biol Chem. 1979 Dec 10;254(23):12209–12218. [PubMed] [Google Scholar]
- Makarow M. Endocytosis in Saccharomyces cerevisiae: internalization of alpha-amylase and fluorescent dextran into cells. EMBO J. 1985 Jul;4(7):1861–1866. doi: 10.1002/j.1460-2075.1985.tb03861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarow M. Endocytosis in Saccharomyces cerevisiae: internalization of enveloped viruses into spheroplasts. EMBO J. 1985 Jul;4(7):1855–1860. doi: 10.1002/j.1460-2075.1985.tb03860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlin K., Bainton D. F., Pesonen M., Louvard D., Genty N., Simons K. Transepithelial transport of a viral membrane glycoprotein implanted into the apical plasma membrane of Madin-Darby canine kidney cells. I. Morphological evidence. J Cell Biol. 1983 Sep;97(3):627–637. doi: 10.1083/jcb.97.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor J., Dobson M. J., Roberts N. A., Tuite M. F., Emtage J. S., White S., Lowe P. A., Patel T., Kingsman A. J., Kingsman S. M. Efficient synthesis of enzymatically active calf chymosin in Saccharomyces cerevisiae. Gene. 1983 Sep;24(1):1–14. doi: 10.1016/0378-1119(83)90126-9. [DOI] [PubMed] [Google Scholar]
- Novick P., Ferro S., Schekman R. Order of events in the yeast secretory pathway. Cell. 1981 Aug;25(2):461–469. doi: 10.1016/0092-8674(81)90064-7. [DOI] [PubMed] [Google Scholar]
- Novick P., Field C., Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980 Aug;21(1):205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Pesonen M., Ansorge W., Simons K. Transcytosis of the G protein of vesicular stomatitis virus after implantation into the apical plasma membrane of Madin-Darby canine kidney cells. I. Involvement of endosomes and lysosomes. J Cell Biol. 1984 Sep;99(3):796–782. doi: 10.1083/jcb.99.3.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel H., Kondor-Koch C., Garoff H. Cell surface expression of fusogenic vesicular stomatitis virus G protein from cloned cDNA. EMBO J. 1984 Jul;3(7):1477–1483. doi: 10.1002/j.1460-2075.1984.tb01999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riezman H. Endocytosis in yeast: several of the yeast secretory mutants are defective in endocytosis. Cell. 1985 Apr;40(4):1001–1009. doi: 10.1016/0092-8674(85)90360-5. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Gallione C. J. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus G and M proteins determined from cDNA clones containing the complete coding regions. J Virol. 1981 Aug;39(2):519–528. doi: 10.1128/jvi.39.2.519-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. A., Duncan M. J., Moir D. T. Heterologous protein secretion from yeast. Science. 1985 Sep 20;229(4719):1219–1224. doi: 10.1126/science.3939723. [DOI] [PubMed] [Google Scholar]
- Svoboda A. Mating reaction in yeast protoplasts. Arch Microbiol. 1976 Nov 2;110(23):313–318. doi: 10.1007/BF00690244. [DOI] [PubMed] [Google Scholar]
- Tkacz J. S., Lampen O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975 Jul 8;65(1):248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Tooze J., Tooze S., Warren G. Replication of coronavirus MHV-A59 in sac- cells: determination of the first site of budding of progeny virions. Eur J Cell Biol. 1984 Mar;33(2):281–293. [PubMed] [Google Scholar]
- Wen D. Z., Schlesinger M. J. Regulated expression of Sindbis and vesicular stomatitis virus glycoproteins in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3639–3643. doi: 10.1073/pnas.83.11.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Matlin K., Helenius A. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J Cell Biol. 1981 Jun;89(3):674–679. doi: 10.1083/jcb.89.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]