Abstract
Heteropentameric neuronal nicotinic receptors assemble so that the canonical acetylcholine-binding sites are located at the interfaces between two pairs of subunits, while the fifth subunit does not participate in a canonical transmitter-binding site. Several subunits are considered to be unable to participate in forming a functional receptor when they occupy a position that would contribute to such a site, including the α5 subunit. The α5 subunit is of interest because of its apparent involvement in nicotine dependence and in the control of dopamine release. We have examined this question using α4 and β2 subunits in concatemeric constructs with the α5 subunit, expressed in Xenopus oocytes. Using dimeric constructs of α4 and β2 subunits expressed with free α5 and pentameric constructs incorporating a single copy of α5, we find that the α5 subunit can occupy the position of a nonbinding subunit, or replace a β2 subunit participating in a canonical binding site. The resulting receptors functionally resemble pentamers assembled with two copies of α4 and three copies of β2. Functional receptors apparently cannot be formed with α5 subunits in both canonical binding sites. These observations extend the present ideas on the possible positions in the pentamer that may be occupied by the α5 subunit, and suggest that additional physiologic or pharmacological subtypes of neuronal nicotinic receptors may be present in neurons.
Introduction
Pentameric ligand-gated ion channels are members of a gene family including the nicotinic, GABAA, glycine, and serotonin type A receptors in vertebrates and a number of related channels in invertebrates and prokaryotes. The neuronal nicotinic receptors can form as pentamers of a single subunit (the α7 subunit), but many are heteropentameric and contain both α (α2–α6) and β (β2–β4) subunits in a variety of stoichiometries (Gotti et al., 2007). Recently the α5 subunit has been of particular interest since it was found that a nonsynonymous coding variant of this subunit is significantly associated with an increased risk of developing nicotine dependence (Bierut et al., 2008). Further, the level of α5 in the medial habenula determines the aversive response to nicotine (Frahm et al., 2011), and receptors containing the α5 subunit along with α4 and β2 are critical in regulating dopamine release in the dorsal striatum (Exley et al., 2012). In heteropentameric receptors the canonical acetylcholine (ACh) binding sites are located at the interface between an α and a β subunit, in which the α subunit contributes the principal (or +) face and the β subunit the complementary (or −) face (Gotti et al., 2007; Mazzaferro et al., 2011; see Fig. 1). This means that there is one subunit in the heteropentamer that does not contribute to such a canonical site (the fifth subunit). It has been proposed that the α5 subunit, in particular, cannot incorporate into a functional receptor in a position at which it contributes to a canonical binding site (Brown et al., 2007; Kuryatov et al., 2008). However, it can incorporate efficiently into the fifth subunit position and thereby affect the properties of the pentameric receptor (Gerzanich et al., 1998; Groot-Kormelink et al., 2001; Kuryatov et al., 2008). It should be noted that recent studies have shown that the fifth subunit in α4β2 receptors can participate in a novel agonist-binding site; in particular, adjacent α4 subunits form an ACh-binding site in which the fifth subunit contributes the principal face (Harpsøe et al., 2011; Mazzaferro et al., 2011). This expands the possible physiologic importance of incorporating the α5 subunit.
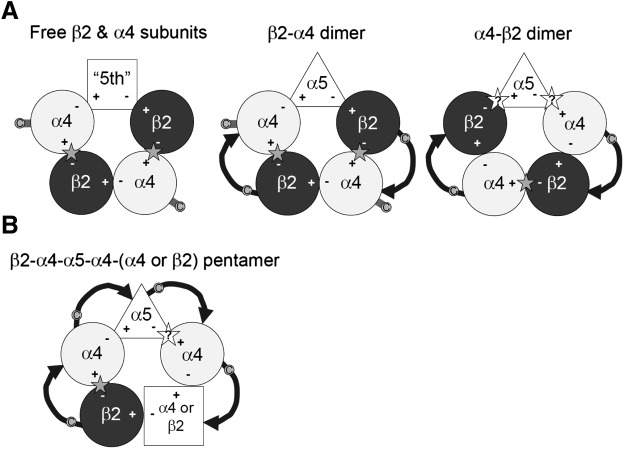
Fig. 1.
Structure of α4β2 receptor pentamers. (A) Receptors composed of free α4 and β2 subunits (left) and receptors expressed when a dimeric concatemer is expressed with a free α5 subunit (middle and right). The receptors are diagrammed as viewed from the extracellular space. The subunits are arranged in a rosette around the ion channel. Canonical ACh-binding sites (indicated by stars) are located at the interface between the α4 (contributing the principal or + face) and the β2 subunit. The fifth subunit (indicated by the square) does not participate in an interface contributing to a canonical site. The middle panel shows the pentamer formed when β2-α4 dimers are expressed with a free α5 subunit (indicated by a triangle). The free subunit occupies the position of the fifth subunit. The right panel shows the pentamer formed when βα4-β2 dimers are expressed with a free subunit. The free subunit occupies a position participating in a canonical binding interface, indicated by stars with question marks. The dimers are shown with the linking sequence as a curved line with the arrowhead indicating the N- to C-terminal direction. (B) The β2-α4-α5-α4-(α4 or β2) pentameric concatemer containing the α5 subunit in the middle position. In this construct one α4/β2 interface is formed (indicated by the stars with dashed outline) and an α4/α5 interface. The subunit order is as proposed in Mazzaferro et al. (2011), but because the α5 has an α4 subunit on either side, the same α4/α5 interface would be formed irrespective of a reversal of order.
We examined the ability of the α5 subunit to form functional receptors with the α4 and β2 subunits, using concatemeric constructs of subunits to define the number and position of subunits in the pentamer. The results indicate that the α5 subunit can assemble in the place of a β2 subunit at a position that would form a canonical ACh-binding site.
Materials and Methods
Constructs and Expression.
We used human α4 (NM000744), β2 (NM000748), and α5 (NM000745) subunits. The generation of the dimeric constructs α4-β2 and β2-α4 is described in Jin and Steinbach (2011). The pentameric constructs β2-α4-β2-α4-α4 and β2-α4-β2-α4-β2 are described in Carbone et al. (2009). The α5 subunit was inserted into a pentamer by mutational insertion of the appropriate linker sequences: the sequences for the signal peptide at the 5′ end and the stop codon at the 3′ end were removed and replaced by the linker. This construct was then restriction digested and inserted into the position of the middle β2 subunit in the pentamers. All constructs were fully sequenced through the inserted receptor sequence. In the pentamers, subunits were excised using the appropriate restriction enzymes and sequenced independently to verify that each copy was intact. RNA was synthesized using the mMessage mMachine T7 kit (Ambion, Austin, TX). The concentration of RNA was estimated from the optical density measured at 260 nm.
Xenopus oocytes were prepared in Dr. C. Zorumski's laboratory (Washington University, St. Louis, MO) using an approved protocol. Oocytes were injected with 12–20 ng of cRNA in a volume of 18–23 nl. Oocytes were maintained at 18°C for 2–7 days before physiologic study.
Electrophysiology.
Standard methods were used for two-electrode voltage clamp of Xenopus oocytes (Jin and Steinbach, 2011), using an OC-725C voltage clamp (Warner Instruments, Hamden, CT). Oocytes were clamped at −50 mV, and all recordings were made at room temperature (23–25°C). Currents were filtered at 20 Hz, then digitized at 50 Hz (Digidata 1200 interface; Molecular Devices, Sunnyvale, CA) and stored using pClamp 8.0 (Molecular Devices). Transients were analyzed with Clampfit (Molecular Devices). Oocyte recordings were performed in a small chamber that was continuously perfused with saline. Drug applications were made using a manually controlled perfusion system. The system was made with glass, stainless steel, or Teflon components to reduce steroid adsorption. The applications were relatively slow, with bath exchange times of ∼1 second. The external solution contained (in mM): 96 NaCl, 2 KCl, 1.8 BaCl2, 1 MgCl2, and 10 HEPES; pH 7.3. External Ca2+ was replaced with Ba2+ to avoid activation of Ca2+-activated channels. We did not use atropine to block muscarinic receptors, as it potentiates α4β2 receptors (Zwart and Vijverberg, 1997). Occasional oocytes showed delayed responses to ACh; these oocytes were discarded.
The concentration-response relationship for activation by ACh was characterized for data from each cell using nonlinear regression in SigmaPlot (Systat Software, Chicago, IL) by fitting the Hill equation:
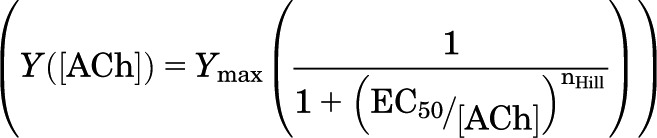 |
where Y is the response to a concentration of ACh, Ymax is the maximal response, EC50 is the concentration producing half-maximal activation, and nHill is the Hill coefficient. Concentration-response data were collected for an individual cell, and data were normalized to the response to 1 mM ACh. The fit was rejected if the estimated error in any fit parameter was >60% of the fit value, and all parameter estimates for that fit were discarded. The relationship was analyzed for each cell, and overall mean values were then calculated for oocytes injected with that set of constructs.
Potentiation by 17β-estradiol or physostigmine is strongest for low concentrations of ACh (Paradiso et al., 2001; Curtis et al., 2002; Smulders et al., 2005). Because the EC50 for activation by ACh depends on the subunit combinations expressed (see Results and Discussion), each oocyte was tested with 1 mM ACh to estimate the maximal response. A low concentration of ACh, chosen to be able to evoke <20% of the maximal current, was then applied. After the response to ACh had reached a stable level, the application was switched to ACh plus 10 μM drug. The application was switched to bathing solution, followed by repeat of the control low concentration. The relative response in the presence of drug to that in the absence of drug was then calculated. Drug was not preapplied. ACh or ACh plus drug was applied for 10–20 seconds, and applications were separated by 3–4 minutes to allow full washout.
Values are presented as arithmetic mean ± S.E. (number of observations).
Drugs.
17β-Estradiol and ACh were purchased from Sigma-Aldrich (St. Louis, MO). 5I A85380 (5-iodo-3-[2(S)-azetidinylmethoxy]pyridine) and physostigmine hemisulfate (physostigmine) were purchased from Tocris (Ellisville, MO). 17β-Estradiol was prepared as a 20 mM stock solution in dimethylsulfoxide and diluted into external solution on the day of an experiment. ACh was prepared as a 1 M stock solution in bath solution and stored frozen at −20°C. 5I A85380 was prepared as a 50 μM stock solution in bath solution and stored frozen at −20°C. Physostigmine was prepared as a 10 mM stock in deionized water and stored frozen at −20°C. Working solutions were prepared on the day of experiments.
Results and Discussion
We used concatemeric constructs to express receptors containing α5 subunits in a defined stoichiometry and position in nicotinic α4β2* receptors. Receptors were expressed in Xenopus oocytes and responses were determined using two-electrode voltage clamp.
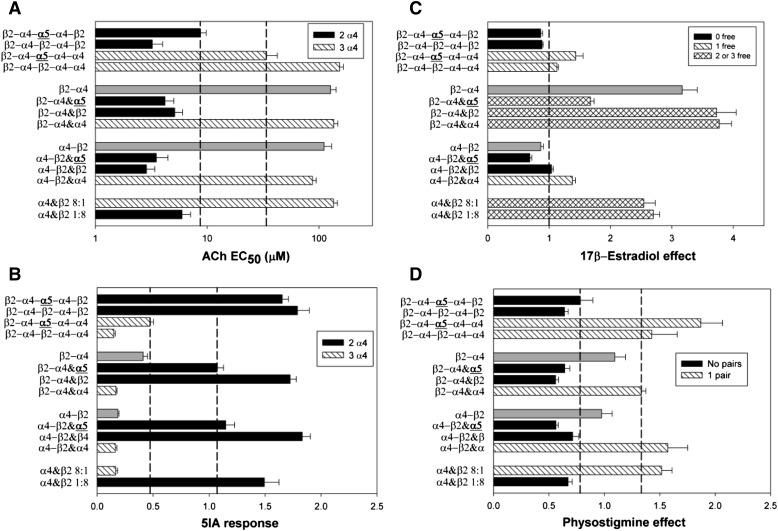
Initially we used dimeric concatemers containing a single α4 and a single β2 subunit, in either α4-β2 or β2-α4 orientation. Previous work (Zhou et al., 2003; Jin and Steinbach, 2011) has shown that these concatemers assemble in the pentamer in a counterclockwise fashion (Fig. 1), so that a free additional subunit takes the position either of the fifth (nonbinding) subunit when expressed with the β2-α4 concatemer or of a subunit contributing to the canonical ACh-binding site interface when expressed with the α4-β2 concatemer. To assess the number and position of subunits in the receptor pentamer, we used pharmacological tests. We determined the concentration of ACh that produced a half-maximal response (ACh EC50), the relative current elicited by a 1 μM concentration of 5I 85380 (compared with 1 mM ACh), and the ability of 17β-estradiol to potentiate responses to a low concentration of ACh. Previous work has shown that receptors containing three copies of α4 have an ACh EC50 near 100 μM while those with two copies have an ACh EC50 of <10 μM (Nelson et al., 2003; Moroni et al., 2006; Jin and Steinbach, 2011). 5I A85380 is a more efficacious agonist on receptors containing three copies of β2, with the response to 1 μM 5I A85380 larger than that to high ACh (∼1.4×), while for receptors with three copies of α4 the response is less (∼0.3×) (Zwart et al., 2006; Jin and Steinbach, 2011). Finally, for 17β-estradiol to potentiate responses there must be a free (untethered) C terminus for an α4 subunit (Paradiso et al., 2001; Zhou et al., 2003; Jin and Steinbach, 2011). In addition, we tested the ability of physostigmine to potentiate responses to a low concentration of ACh. As shown in Fig. 2 and Table 1, potentiation by physostigmine is seen when there are two adjacent α4 subunits. We also determined the response to a high concentration of ACh (1 mM) as an estimate of the level of expression of functional receptors. The results are presented in Fig. 2 and Table 1.
Fig. 2.
Graphical depiction of the pharmacological profiles. (A and B) Responses to agonists [(A) ACh EC50 (note logarithmic abscissa); (B) response to 1 μM 5I A85380 relative to 1 mM ACh], while C and D show the effects of two modulating drugs [(C) effect of 10 μM 17β-estradiol; (D) 10 μM physostigmine]. The panels are formatted in the same way: at the bottom are free subunits at two different cRNA ratios, then a group of four receptors containing the α4-β2 concatemer, four receptors containing the β2-α4 concatemer, and four pentamers. The position of the α5 subunit is emphasized in bold and underlined. The bars are filled to indicate predicted structural features. In each panel, the bars corresponding to dimers expressed without a free subunit are filled with gray. In A and B, the solid fill indicates receptor predicted to have two copies of α4 and diagonal hatching indicates three copies of α4. In C, solid fill indicates no free (untethered) α4 C termini, diagonal hatching one free C terminus, and cross-hatching two or three free C termini. In D, solid fill indicates no adjacent α4 subunits, while diagonal hatching indicates a pair of adjacent α4 subunits. The bars in the panels show mean values ± S.E. Data values are presented in Table 1.
TABLE 1.
Summary of results
The first column gives the subunits injected. The first set of four rows gives results for receptors predicted to contain three copies of α4 and two copies of β2, while the second set gives results for two copies of α4 and three of β2. The third set of four rows shows data for receptors containing the α5 subunit, again ordered based on the predicted number of copies of α4. The final set of two rows shows data for dimeric concatemers expressed in the absence of a free subunit; the pattern of properties is distinct from a dimer expressed with a free subunit (although closest to receptors formed with a free α4 subunit). The second column gives the number of α4 subunits predicted to be in the receptor, while the third and fourth columns give the ACh EC50 and the response to 1 μM 5I A85380 relative to the response of the same oocyte to 1 mM ACh. The fifth and sixth columns give whether the predicted receptor has adjacent α4 subunits and the observed effect of physostigmine. The seventh and eighth columns give the predicted number of untethered α4 C termini in the receptor and the observed effect of 17β-estradiol. The final column gives the response to 1 mM ACh. All data are mean ± S.E. (number of observations).
| Subunits | No. of α4 Subunits | ACh EC50 | 5I A85380 Relative Response | Adjacent α4? | Physostigmine Effect | No. of Free α4 C Termini | 17 β-Estradiol Effect | Maximal Response |
|---|---|---|---|---|---|---|---|---|
| μM | relative to ACh | fold | fold | −nA | ||||
| α4&β2 8:1 | 3 | 135 ± 11 (32) | 0.17 ± 0.02 (17) | Yes | 1.52 ± 0.09 (18) | 3 | 2.54 ± 0.18 (23) | 13,451 ± 1459 (45) |
| α4-β2&α4 | 3 | 87 ± 7 (46) | 0.16 ± 0.01 (41) | Yes | 1.57 ± 0.18 (21) | 1 | 1.38 ± 0.05 (35) | 7238 ± 1179 (68) |
| β2-α4&α4 | 3 | 135 ± 12 (52) | 0.17 ± 0.01 (56) | Yes | 1.33 ± 0.04 (34) | 3 | 3.77 ± 0.20 (11) | 14,454 ± 1301 (77) |
| β2-α4-β2-α4-α4 | 3 | 153 ± 12 (9) | 0.15 ± 0.01 (12) | Yes | 1.43 ± 0.23 (8) | 1 | 1.13 ± 0.02 (20) | 2020 ± 265 (25) |
| α4&β2 1:8 | 2 | 6 ± 1 (18) | 1.49 ± 0.13 (18) | No | 0.67 ± 0.04 (11) | 2 | 2.70 ± 0.11 (4) | 1103 ± 208 (43) |
| α4-β2&β2 | 2 | 3 ± 1 (14) | 1.83 ± 0.07 (19) | No | 0.71 ± 0.06 (6) | 0 | 1.04 ± 0.03 (5) | 341 ± 98 (32) |
| β2-α4&β2 | 2 | 5 ± 1 (40) | 1.72 ± 0.05 (60) | No | 0.56 ± 0.03 (15) | 2 | 3.73 ± 0.32 (7) | 819 ± 114 (73) |
| β2-α4-β2-α4-β2 | 2 | 3 ± 1 (16) | 1.79 ± 0.11 (7) | No | 0.64 ± 0.03 (14) | 0 | 0.88 ± 0.02 (8) | 110 ± 23 (20) |
| β2-α4-α5-α4-α4 | 3 | 34 ± 9 (2) | 0.47 ± 0.03 (8) | Yes | 1.87 ± 0.20 (6) | 1 | 1.43 ± 0.12 (12) | 24 ± 5 (30) |
| β2-α4&α5 | 2 | 4 ± 1 (20) | 1.07 ± 0.06 (31) | No | 0.64 ± 0.04 (20) | 2 | 1.68 ± 0.06 (7) | 399 ± 67 (45) |
| α4-β2&α5 | 2 | 4 ± 1 (9) | 1.15 ± 0.08 (15) | No | 0.56 ± 0.02 (19) | 0 | 0.69 ± 0.03 (5) | 436 ± 124 (20) |
| β2-α4-α5-α4-β2 | 2 | 9 ± 1 (13) | 1.65 ± 0.06 (17) | No | 0.78 ± 0.11 (9) | 0 | 0.86 ± 0.03 (10) | 27 ± 4 (36) |
| β2-α4 | ? | 127 ± 15 (52) | 0.41 ± 0.04 (72) | No | 1.09 ± 0.10 (13) | ≥2 | 3.17 ± 0.25 (6) | 5155 ± 781 (82) |
| α4-β2 | ? | 111 ± 18 (24) | 0.19 ± 0.01 (24) | No | 0.98 ± 0.09 (9) | 0 | 0.87 ± 0.05 (8) | 859 ± 160 (37) |
We will use abbreviations for the constructs studied. Concatemers are named with subunits in order from the N terminus, so α4-β2 indicates that the dimer begins with α4 subunit sequence. When more than one construct was used, the constructs are separated by “&,” so α4&β2 1:8 indicates that both α4 and β2 subunit cRNA were injected, at a 1:8 mass ratio.
The inclusion of three copies of α4 (α4&β2 8:1, α4-β2&α4, β2-α4&α4, or β2-α4-β2-α4-α4) resulted in receptors that had a large EC50 for ACh (∼100 μM), had a small relative response to 5I A85380 (<0.3), and showed potentiation by physostigmine (ratio > 1.2). In addition, the response to 17β-estradiol reflected whether there was a free (untethered) C terminus for at least one α4 subunit (e.g., comparing α4-β2&β2 to β2-α4&β2). In contrast, inclusion of three copies of β2 (α4&β2 1:8, α4-β2&β2, β2-α4&β2, or β2-α4-β2-α4-β2) resulted in receptors that had a small EC50 for ACh (<10 μM), had a large relative response to 5I A85380 (>1.0), and showed block by physostigmine (<0.7). We also tested responses to the dimeric concatemers injected alone. Overall, these properties were more similar to receptors containing three copies of α4 (Fig. 2; Table 1), but clearly distinct from either dimer expressed with free β2 subunits.
We then expressed dimeric constructs with free α5 subunits. We tested both wild-type α5 and α5(D398N), as the α5(D398N) variant is associated with an increased risk of developing nicotine dependence (Bierut et al., 2008). However, we saw no difference between these constructs in any of the parameters measured, so the results have been pooled. Injected oocytes expressed functional receptors, although the response to saturating concentrations of ACh was reduced compared with when α4 was used as the free subunit (Table 1). In this case the pharmacological properties of the receptors were very similar to those of receptors containing three copies of β2 (Fig. 2; Table 1). The properties of the receptors containing free α5 subunits are clearly distinct from those of receptors when the dimers are expressed in the absence of a free subunit, as shown in Fig. 2 and Table 1. Accordingly, even though the dimeric constructs can assemble to produce functional surface receptors, in the presence of free α5 subunits the majority of the functional receptors contain the free subunit in addition to the dimer. These results suggest that the α5 subunit can replace either the subunit that does not contribute to a canonical binding site (when expressed with the β2-α4 dimer) or a subunit contributing to a binding site (when expressed with the α4-β2 dimer). However, it is also possible that the presence of the α5 subunit caused the dimers to assemble in different orientations, so that in association with the β2-α4 dimer the α5 subunit actually occupied the position that does not contribute to a canonical binding site.
To address this concern we used pentameric concatemers. We first confirmed that the β2-α4-β2-α4-β2 and β2-α4-β2-α4-α4 constructs behaved as would be predicted from the expected structure (Carbone et al., 2009; Mazzaferro et al., 2011). With β2-α4-β2-α4-α4 the properties were consistent with there being three copies of α4 present: the ACh EC50 was high; the response to 1 μM 5I A85380 was low. Furthermore, 17β-estradiol potentiated the response, indicating that the C-terminal α4 sequence was present, and physostigmine potentiation indicated that there were adjacent α4 subunits. In contrast, with β2-α4-β2-α4-β2 the properties of the receptor were consistent with only two copies of α4 being present (Fig. 2; Table 1), without either a free α4 C terminus (indicated by the absence of potentiation by 17β-estradiol) or adjacent α4 subunits (indicated by the absence of potentiation by physostigmine). To examine the properties of receptors containing α5 we replaced the central β2 subunit in each pentamer so that α5 was flanked on both sides by an α4 subunit (Fig. 1). In these constructs α5 would occupy the position of β2 irrespective of whether the concatemer assembled in a clockwise direction (as found by Mazzaferro et al., 2011) or a counterclockwise direction in the pentameric receptor. As shown in Figs. 2 and 3 and Table 1, the inclusion of the α5 subunit resulted in receptors whose properties resemble those of the receptors formed by the original, β2-containing, pentameric concatemers. That is, the pharmacological properties of the receptors containing α5 in place of β2 support the idea that each receptor contains the subunits in a single pentameric construct. They are not the result of receptors assembled with contributions from multiple concatemers or concatemers with subunits derived from proteolysis of concatemers. These results strongly support the idea that the α5 subunit can assemble to produce functional receptors even when it occupies the position of a β2 subunit at a canonical ACh-binding site.
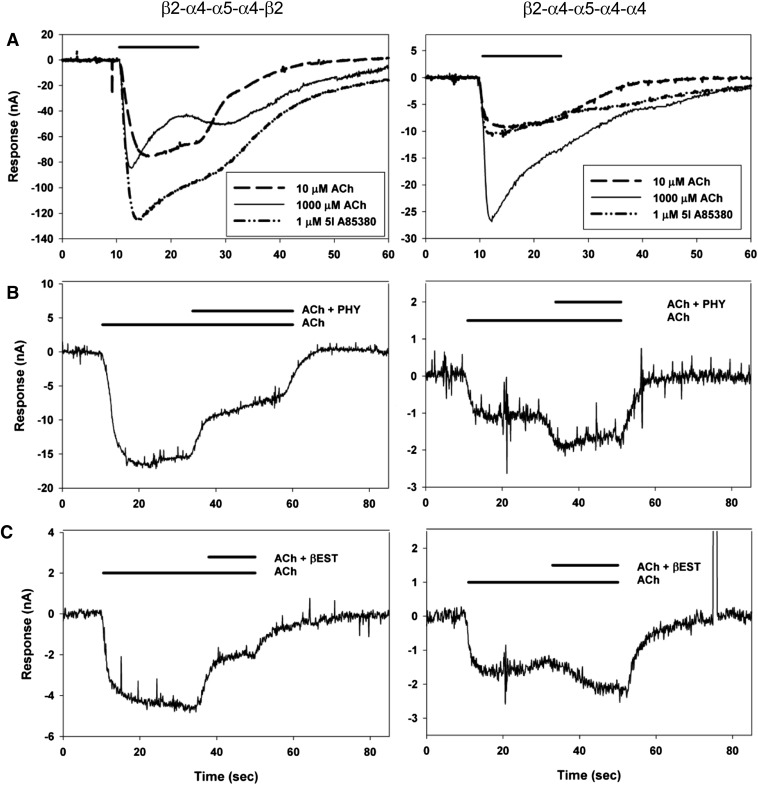
Fig. 3.
Responses of pentamers containing α5. The left column shows responses of receptors formed from β2-α4-α5-α4-β2 pentamers, and the right column from β2-α4-α5-α4-α4. (A) Responses to agonists; note that in the left column 5I A85380 produces a larger response than 1 mM ACh, and that 10 μM ACh is clearly a more than half-maximal concentration. In contrast, in the right column 5I A85380 produces a smaller response than 1 mM ACh, and 10 μM ACh is clearly a less than half-maximal concentration. (B and C) Responses to 0.3 μM ACh; then the application is switched to 0.3 μM ACh plus 15 μM physostigmine (PHY; B) or 10 μM 17β-estradiol (βEST; C). Both drugs inhibit responses of β2-α4-α5-α4-β2 pentamers but potentiate responses of β2-α4-α5-α4-α4. Each frame in A shows responses from one oocyte, while each frame in B and C shows responses from separate oocytes.
A concern in using subunit concatemers is that the linker sequences or the physical constraints imposed in linking subunits will alter receptor properties. In the case of these constructs, this does not seem to be the case. Examination of the data in Table 1 indicates that for receptors containing α4 and β2 subunits, the properties of receptors containing three copies of α4 are indistinguishable for receptors derived from free subunits, dimers, and pentamers except for the expected effects on potentiation by 17β-estradiol. Similarly, for receptors containing two copies of α4, the properties are indistinguishable (again, except for 17β-estradiol). Accordingly, there is no indication that the linkers per se affect properties for the present studies.
We also generated pentameric β2-α5-β2-α4-α4 concatemers in which the second position (occupied by α4) was replaced by α5. In this case the α5 subunit would be constrained to replace an α4 subunit that contributes to a canonical binding site. These constructs expressed so poorly that reliable data could not be obtained (data not shown). Previous work has shown that the α5 subunit does not assemble to produce functional receptors when expressed with the β2, β3, or β4 subunits (Boulter et al., 1990), so it seems unlikely that the α5 subunit can replace an α4 subunit at a position producing a canonical ACh-binding site. We found that no functional receptors were produced when we injected free α4 and free α5 subunits (data not shown), which indicates that there must be at least one α4/β2 interface in an α4β2α5 receptor.
The efficiency of expression of functional surface receptors containing the α5 subunit appears to be low. A previous study (Kuryatov et al., 2008) found that surface expression of α4β2* was reduced by α5, although total (surface plus intracellular) expression was actually increased. They proposed that the α5 subunit might enhance formation of unproductive oligomers that could not associate to form pentamers and successfully traffic to the cell surface (Kuryatov et al., 2008).
In sum, the α5 subunit is not restricted to occupying only the position in a receptor of the subunit that does not contribute to a canonical binding site. In the pentameric constructs we used, the α5 subunit contributes to both α4/α5 and α5/α4 interfaces, in the first case replacing a β2 subunit at an interface containing a canonical site and in the second case possibly contributing to a noncanonical (α4/α4) site (Harpsøe et al., 2011; Mazzaferro et al., 2011). When expressed with the α4-β2 dimeric construct, the α5 subunit would contribute to interfaces containing a canonical site, most likely replacing β2 to generate an α4/α5 site. However, it is not clear that the α4/α5 interface actually forms a functional ACh-binding site. Previous work has shown that it is possible to mutate residues in a canonical binding site to render that site ineffective, and still form functional (albeit impaired) receptors (Mazzaferro et al., 2011). Still, functional receptors can form with α5 replacing a subunit contributing to a canonical site. The physiologic consequences of an α4β2* receptor containing the α5 subunit in place of a β2 subunit at a canonical binding site are not yet known, since in the assays that we have used the α4β2* receptor containing the α5 subunit in place of a β2 subunit at a canonical binding site is indistinguishable from one with β2 or α5 in the fifth subunit position. Additional experiments will be required to define the consequences, including a possible effect on the ability of nicotine to upregulate the surface expression of the receptor (Kishi and Steinbach, 2006; Kuryatov et al., 2008; Mao et al., 2008).
Abbreviations
- 5I A85380
5-iodo-3-[2(S)-azetidinylmethoxy]pyridine
- ACh
acetylcholine
Authorship Contributions
Participated in research design: Jin, Steinbach.
Conducted experiments: Jin.
Contributed new reagents or analytic tools: Bermudez, Jin.
Performed data analysis: Jin, Steinbach.
Wrote or contributed to the writing of the manuscript: Bermudez, Jin, Steinbach.
Footnotes
This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant R01-NS22356]. J.H.S. is the Russell and Mary Shelden Professor of Anesthesiology.
References
- Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, Saccone NL, Saccone SF, Bertelsen S, Fox L, et al. (2008) Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry 165:1163–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter J, O’Shea-Greenfield A, Duvoisin RM, Connolly JG, Wada E, Jensen A, Gardner PD, Ballivet M, Deneris ES, McKinnon D, et al. (1990) α 3, α 5, and β 4: three members of the rat neuronal nicotinic acetylcholine receptor-related gene family form a gene cluster. J Biol Chem 265:4472–4482 [PubMed] [Google Scholar]
- Brown RW, Collins AC, Lindstrom JM, Whiteaker P. (2007) Nicotinic α5 subunit deletion locally reduces high-affinity agonist activation without altering nicotinic receptor numbers. J Neurochem 103:204–215 [DOI] [PubMed] [Google Scholar]
- Carbone AL, Moroni M, Groot-Kormelink PJ, Bermudez I. (2009) Pentameric concatenated (α4)(2)(β2)(3) and (α4)(3)(β2)(2) nicotinic acetylcholine receptors: subunit arrangement determines functional expression. Br J Pharmacol 156:970–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis L, Buisson B, Bertrand S, Bertrand D. (2002) Potentiation of human α4β2 neuronal nicotinic acetylcholine receptor by estradiol. Mol Pharmacol 61:127–135 [DOI] [PubMed] [Google Scholar]
- Exley R, McIntosh JM, Marks MJ, Maskos U, Cragg SJ. (2012) Striatal α5 nicotinic receptor subunit regulates dopamine transmission in dorsal striatum. J Neurosci 32:2352–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frahm S, Slimak MA, Ferrarese L, Santos-Torres J, Antolin-Fontes B, Auer S, Filkin S, Pons S, Fontaine JF, Tsetlin V, et al. (2011) Aversion to nicotine is regulated by the balanced activity of β4 and α5 nicotinic receptor subunits in the medial habenula. Neuron 70:522–535 [DOI] [PubMed] [Google Scholar]
- Gerzanich V, Wang F, Kuryatov A, Lindstrom J. (1998) α 5 Subunit alters desensitization, pharmacology, Ca++ permeability and Ca++ modulation of human neuronal α 3 nicotinic receptors. J Pharmacol Exp Ther 286:311–320 [PubMed] [Google Scholar]
- Gotti C, Moretti M, Gaimarri A, Zanardi A, Clementi F, Zoli M. (2007) Heterogeneity and complexity of native brain nicotinic receptors. Biochem Pharmacol 74:1102–1111 [DOI] [PubMed] [Google Scholar]
- Groot-Kormelink PJ, Boorman JP, Sivilotti LG. (2001) Formation of functional α3β4α5 human neuronal nicotinic receptors in Xenopus oocytes: a reporter mutation approach. Br J Pharmacol 134:789–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpsøe K, Ahring PK, Christensen JK, Jensen ML, Peters D, Balle T. (2011) Unraveling the high- and low-sensitivity agonist responses of nicotinic acetylcholine receptors. J Neurosci 31:10759–10766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Steinbach JH. (2011) A portable site: a binding element for 17β-estradiol can be placed on any subunit of a nicotinic α4β2 receptor. J Neurosci 31:5045–5054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi M, Steinbach JH. (2006) Role of the agonist binding site in up-regulation of neuronal nicotinic α4β2 receptors. Mol Pharmacol 70:2037–2044 [DOI] [PubMed] [Google Scholar]
- Kuryatov A, Onksen J, Lindstrom J. (2008) Roles of accessory subunits in α4β2(*) nicotinic receptors. Mol Pharmacol 74:132–143 [DOI] [PubMed] [Google Scholar]
- Mao D, Perry DC, Yasuda RP, Wolfe BB, Kellar KJ. (2008) The α4β2α5 nicotinic cholinergic receptor in rat brain is resistant to up-regulation by nicotine in vivo. J Neurochem 104:446–456 [DOI] [PubMed] [Google Scholar]
- Mazzaferro S, Benallegue N, Carbone A, Gasparri F, Vijayan R, Biggin PC, Moroni M, Bermudez I. (2011) Additional acetylcholine (ACh) binding site at α4/α4 interface of (α4β2)2α4 nicotinic receptor influences agonist sensitivity. J Biol Chem 286:31043–31054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni M, Zwart R, Sher E, Cassels BK, Bermudez I. (2006) α4β2 nicotinic receptors with high and low acetylcholine sensitivity: pharmacology, stoichiometry, and sensitivity to long-term exposure to nicotine. Mol Pharmacol 70:755–768 [DOI] [PubMed] [Google Scholar]
- Nelson ME, Kuryatov A, Choi CH, Zhou Y, Lindstrom J. (2003) Alternate stoichiometries of α4β2 nicotinic acetylcholine receptors. Mol Pharmacol 63:332–341 [DOI] [PubMed] [Google Scholar]
- Paradiso K, Zhang J, Steinbach JH. (2001) The C terminus of the human nicotinic α4β2 receptor forms a binding site required for potentiation by an estrogenic steroid. J Neurosci 21:6561–6568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smulders CJ, Zwart R, Bermudez I, van Kleef RG, Groot-Kormelink PJ, Vijverberg HP. (2005) Cholinergic drugs potentiate human nicotinic α4β2 acetylcholine receptors by a competitive mechanism. Eur J Pharmacol 509:97–108 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Nelson ME, Kuryatov A, Choi C, Cooper J, Lindstrom J. (2003) Human α4β2 acetylcholine receptors formed from linked subunits. J Neurosci 23:9004–9015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart R, Broad LM, Xi Q, Lee M, Moroni M, Bermudez I, Sher E. (2006) 5-I A-85380 and TC-2559 differentially activate heterologously expressed α4β2 nicotinic receptors. Eur J Pharmacol 539:10–17 [DOI] [PubMed] [Google Scholar]
- Zwart R, Vijverberg HP. (1997) Potentiation and inhibition of neuronal nicotinic receptors by atropine: competitive and noncompetitive effects. Mol Pharmacol 52:886–895 [DOI] [PubMed] [Google Scholar]