Abstract
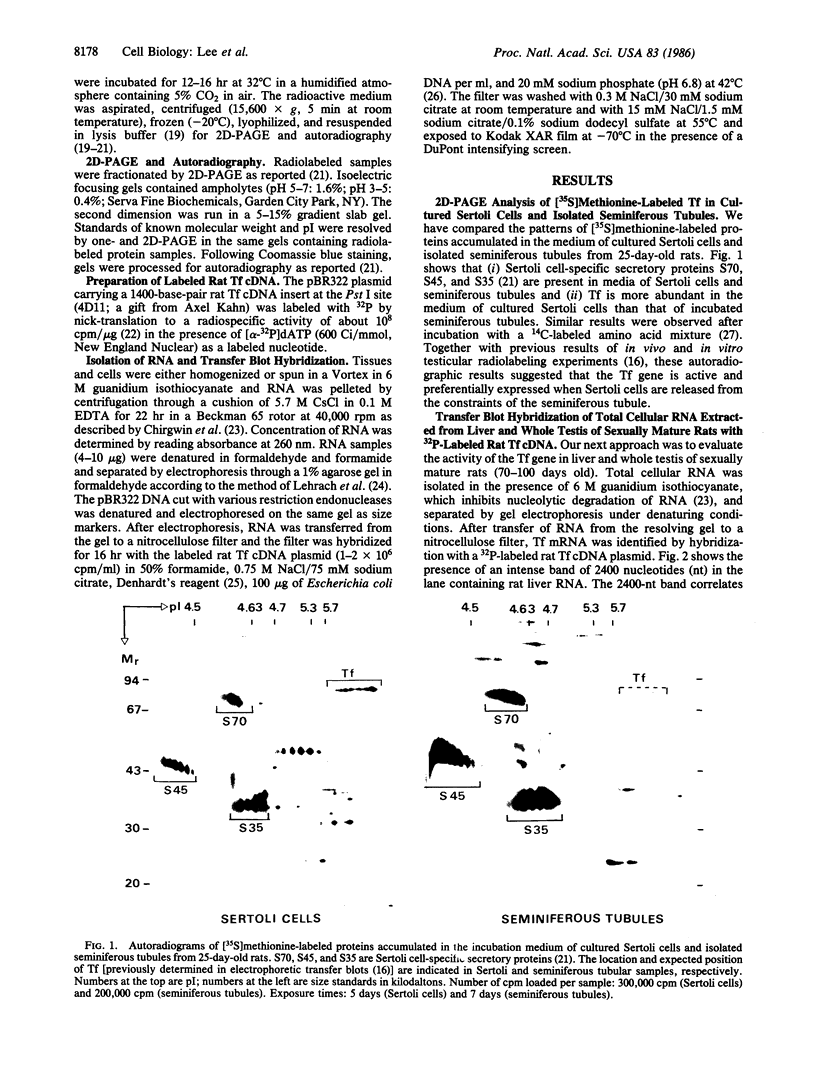
Two-dimensional polyacrylamide gel electrophoresis of proteins and transfer blot hybridization of RNA have been used to study the activity and expression of the rat transferrin gene in cultured Sertoli cells and in whole testis and isolated seminiferous tubules of sexually immature and mature rats. Although the transferrin gene in cultured Sertoli cells is actively engaged in the transcription of mRNA and the mRNA is translated into a secretory product, little transferrin mRNA and transferrin protein are present in whole testes and isolated seminiferous tubules. Sertoli cells upon culturing show a time-dependent transferrin gene activation, and abundant transferrin mRNA can be detected 6 hr after plating. A similar study using intact seminiferous tubular segments from the same rats failed to show a comparable temporal activation of the transferrin gene. Results of this study, together with previous experimental data, suggest that Sertoli cells in vivo are most likely not actively engaged in the synthesis of a testicular transferrin but, instead, rely mainly on plasma transferrin contributed by the liver. In vitro, Sertoli cells, released from the physiological constraints that operate in vivo, rapidly activate the transferrin gene, resulting in abundant newly synthesized Sertoli cell transferrin product.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdullah M., Crowell J. A., Tres L. L., Kierszenbaum A. L. Fetuin: a serum component associated with rat Sertoli and spermatogenic cells in coculture. J Cell Physiol. 1986 Jun;127(3):463–472. doi: 10.1002/jcp.1041270317. [DOI] [PubMed] [Google Scholar]
- Aisen P., Leibman A., Zweier J. Stoichiometric and site characteristics of the binding of iron to human transferrin. J Biol Chem. 1978 Mar 25;253(6):1930–1937. [PubMed] [Google Scholar]
- Aisen P., Listowsky I. Iron transport and storage proteins. Annu Rev Biochem. 1980;49:357–393. doi: 10.1146/annurev.bi.49.070180.002041. [DOI] [PubMed] [Google Scholar]
- Barnes D., Sato G. Serum-free cell culture: a unifying approach. Cell. 1980 Dec;22(3):649–655. doi: 10.1016/0092-8674(80)90540-1. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- DePhilip R. M., Kierszenbaum A. L. Hormonal regulation of protein synthesis, secretion, and phosphorylation in cultured rat Sertoli cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6551–6555. doi: 10.1073/pnas.79.21.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Dym M., Fawcett D. W. The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod. 1970 Dec;3(3):308–326. doi: 10.1093/biolreprod/3.3.308. [DOI] [PubMed] [Google Scholar]
- Ekblom P., Thesleff I., Saxén L., Miettinen A., Timpl R. Transferrin as a fetal growth factor: acquisition of responsiveness related to embryonic induction. Proc Natl Acad Sci U S A. 1983 May;80(9):2651–2655. doi: 10.1073/pnas.80.9.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes S. D., Bucci L. R., Lipshultz L. I., Smith R. G. Transferrin binds specifically to pachytene spermatocytes. Endocrinology. 1983 Nov;113(5):1916–1918. doi: 10.1210/endo-113-5-1916. [DOI] [PubMed] [Google Scholar]
- Idzerda R. L., Huebers H., Finch C. A., McKnight G. S. Rat transferrin gene expression: tissue-specific regulation by iron deficiency. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3723–3727. doi: 10.1073/pnas.83.11.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Mintz B. Receptor-mediated endocytosis of transferrin in developmentally totipotent mouse teratocarcinoma stem cells. J Biol Chem. 1981 Apr 10;256(7):3245–3252. [PubMed] [Google Scholar]
- Kierszenbaum A. L., Crowell J. A., Shabanowitz R. B., DePhilip R. M., Tres L. L. Protein secretory patterns of rat Sertoli and peritubular cells are influenced by culture conditions. Biol Reprod. 1986 Aug;35(1):239–251. doi: 10.1095/biolreprod35.1.239. [DOI] [PubMed] [Google Scholar]
- Kierszenbaum A. L., Feldman M., Lea O., Spruill W. A., Tres L. L., Petrusz P., French F. S. Localization of androgen-binding protein in proliferating Sertoli cells in culture. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5322–5326. doi: 10.1073/pnas.77.9.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierszenbaum A. L., Spruill W. A., White M. G., Tres L. L., Perkins J. P. Rat Sertoli cells acquire a beta-adrenergic response during primary culture. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2049–2053. doi: 10.1073/pnas.82.7.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Ashwell G., van Renswoude J., Harford J. B., Bridges K. R. Binding of apotransferrin to K562 cells: explanation of the transferrin cycle. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2263–2266. doi: 10.1073/pnas.80.8.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Levin M. J., Tuil D., Uzan G., Dreyfus J. C., Kahn A. Expression of the transferrin gene during development of non-hepatic tissues: high level of transferrin mRNA in fetal muscle and adult brain. Biochem Biophys Res Commun. 1984 Jul 18;122(1):212–217. doi: 10.1016/0006-291x(84)90461-3. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Schreiber G., Dryburgh H., Millership A., Matsuda Y., Inglis A., Phillips J., Edwards K., Maggs J. The synthesis and secretion of rat transferrin. J Biol Chem. 1979 Dec 10;254(23):12013–12019. [PubMed] [Google Scholar]
- Shabanowitz R. B., Kierszenbaum A. L. Newly synthesized proteins in seminiferous intertubular and intratubular compartments of the rat testis. Biol Reprod. 1986 Aug;35(1):179–190. doi: 10.1095/biolreprod35.1.179. [DOI] [PubMed] [Google Scholar]
- Skinner M. K., Cosand W. L., Griswold M. D. Purification and characterization of testicular transferrin secreted by rat Sertoli cells. Biochem J. 1984 Mar 1;218(2):313–320. doi: 10.1042/bj2180313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland R., Delia D., Schneider C., Newman R., Kemshead J., Greaves M. Ubiquitous cell-surface glycoprotein on tumor cells is proliferation-associated receptor for transferrin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4515–4519. doi: 10.1073/pnas.78.7.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tres L. L., Kierszenbaum A. L. Viability of rat spermatogenic cells in vitro is facilitated by their coculture with Sertoli cells in serum-free hormone-supplemented medium. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3377–3381. doi: 10.1073/pnas.80.11.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tres L. L., Smith E. P., Van Wyk J. J., Kierszenbaum A. L. Immunoreactive sites and accumulation of somatomedin-C in rat Sertoli-spermatogenic cell co-cultures. Exp Cell Res. 1986 Jan;162(1):33–50. doi: 10.1016/0014-4827(86)90424-6. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Omary M. B. Human cell surface glycoprotein related to cell proliferation is the receptor for transferrin. Proc Natl Acad Sci U S A. 1981 May;78(5):3039–3043. doi: 10.1073/pnas.78.5.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzan G., Frain M., Park I., Besmond C., Maessen G., Trépat J. S., Zakin M. M., Kahn A. Molecular cloning and sequence analysis of cDNA for human transferrin. Biochem Biophys Res Commun. 1984 Feb 29;119(1):273–281. doi: 10.1016/0006-291x(84)91648-6. [DOI] [PubMed] [Google Scholar]
- Wada H. G., Hass P. E., Sussman H. H. Transferrin receptor in human placental brush border membranes. Studies on the binding of transferrin to placental membrane vesicles and the identification of a placental brush border glycoprotein with high affinity for transferrin. J Biol Chem. 1979 Dec 25;254(24):12629–12635. [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright W. W., Parvinen M., Musto N. A., Gunsalus G. L., Phillips D. M., Mather J. P., Bardin C. W. Identification of stage-specific proteins synthesized by rat seminiferous tubules. Biol Reprod. 1983 Aug;29(1):257–270. doi: 10.1095/biolreprod29.1.257. [DOI] [PubMed] [Google Scholar]
- Yang F., Lum J. B., McGill J. R., Moore C. M., Naylor S. L., van Bragt P. H., Baldwin W. D., Bowman B. H. Human transferrin: cDNA characterization and chromosomal localization. Proc Natl Acad Sci U S A. 1984 May;81(9):2752–2756. doi: 10.1073/pnas.81.9.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]