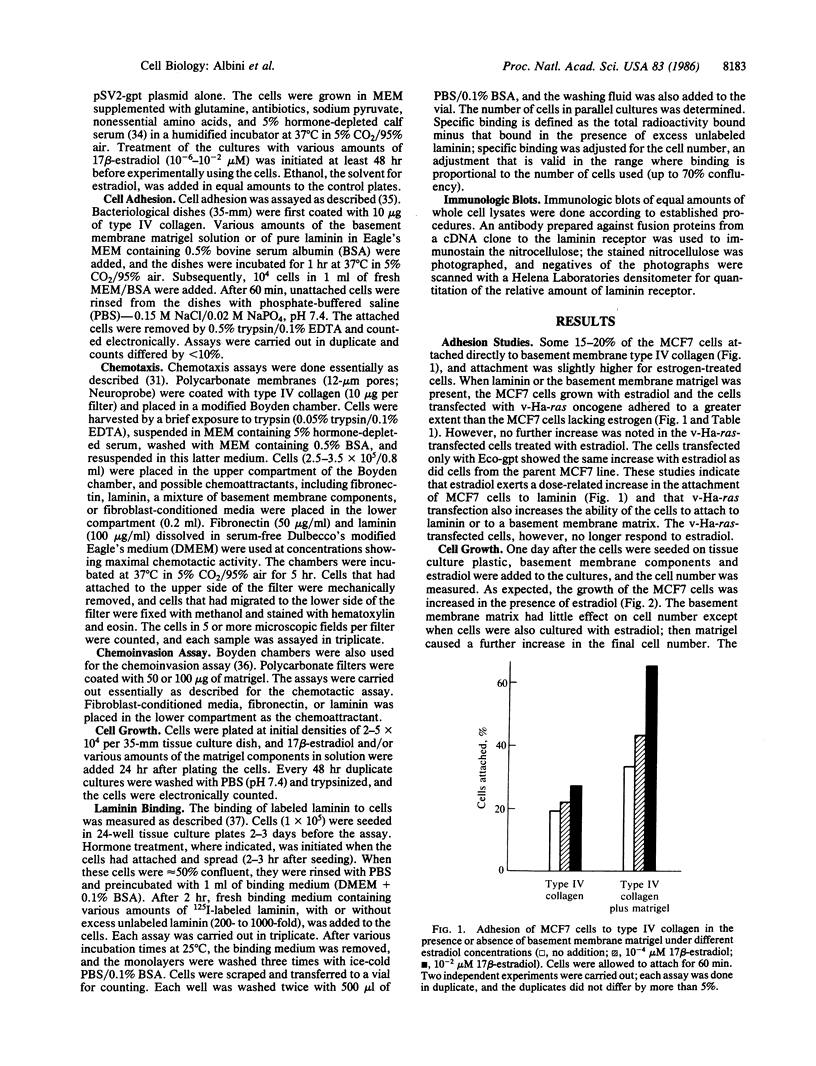
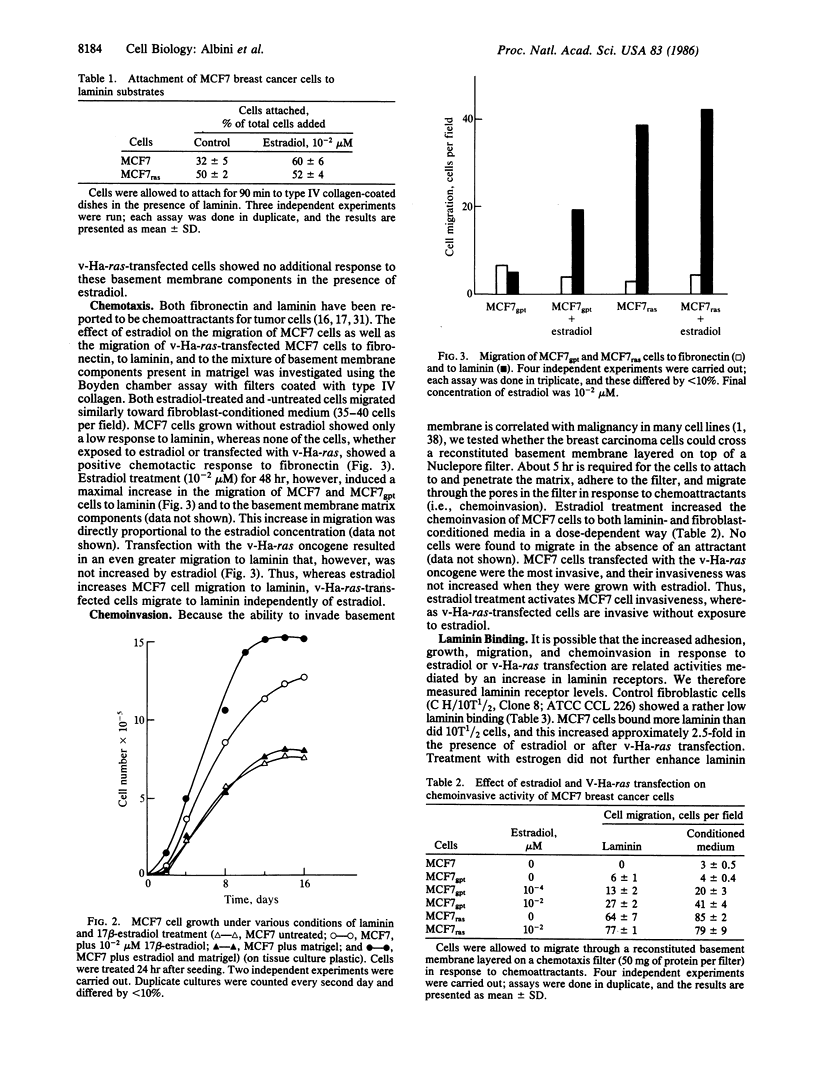
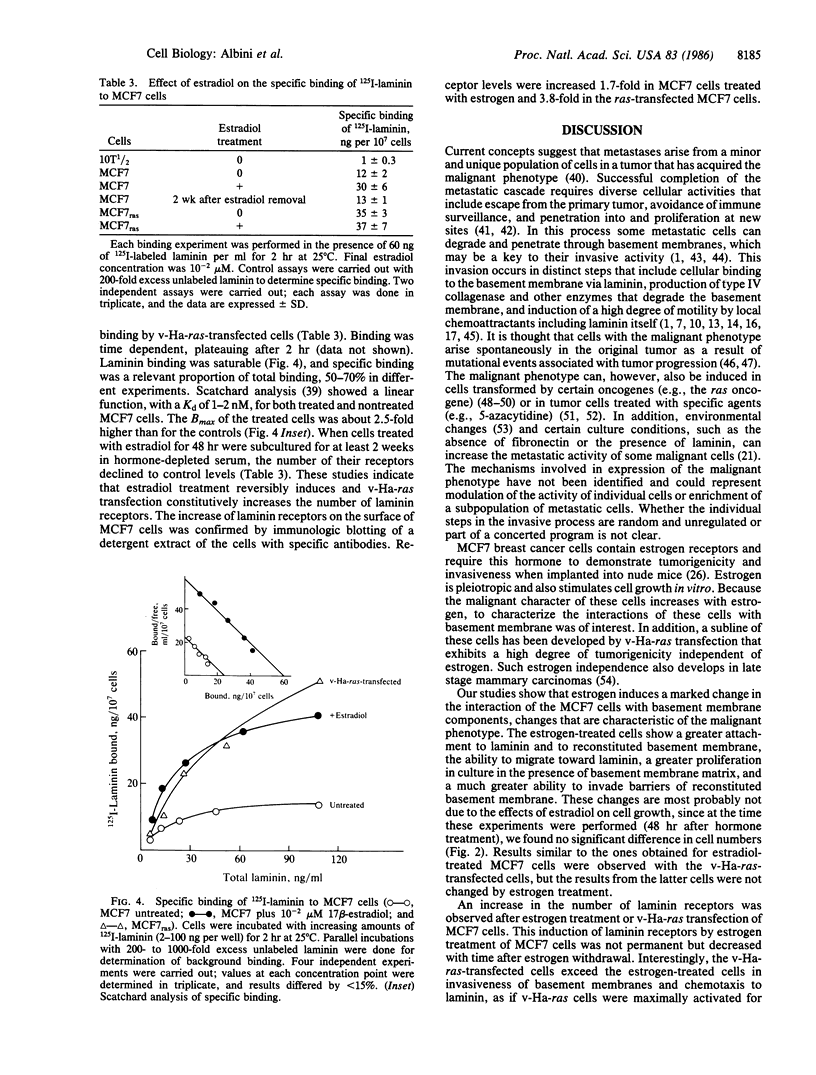
Abstract
The interaction of a line of human breast carcinoma cells (MCF7) with basement membrane components, particularly laminin, was altered by exposure of the cells to estrogen as well as by transfection of the cells with the v-Ha-ras oncogene. In both cases, the cells show a greater ability to attach to a laminin substrate, to migrate to laminin, to grow in the presence of a basement membrane matrix, and to cross barriers of reconstituted basement membrane. These responses were associated with an increase in the expression of laminin receptors. It is postulated that the increase in the invasive behavior of the cells treated with estrogen or transfected with v-Ha-ras is related to the increased number of laminin receptors and their interaction with laminin. Estrogen had no discernible effect on the v-Ha-ras transfected cells. It appears that in the MCF7 cells, the malignant phenotype is under hormonal control and that this control is bypassed after v-Ha-ras transfection.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albini A., Allavena G., Parodi S., Santi L. Comparison of the chemotactic response to conditioned medium of BALB/c3T3 fibroblasts and their SV 40 transformants. Tumori. 1985 Apr 30;71(2):97–100. doi: 10.1177/030089168507100202. [DOI] [PubMed] [Google Scholar]
- Berthois Y., Katzenellenbogen J. A., Katzenellenbogen B. S. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2496–2500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondy G. P., Wilson S., Chambers A. F. Experimental metastatic ability of H-ras-transformed NIH3T3 cells. Cancer Res. 1985 Dec;45(12 Pt 1):6005–6009. [PubMed] [Google Scholar]
- Dickson R. B., Bates S. E., McManaway M. E., Lippman M. E. Characterization of estrogen responsive transforming activity in human breast cancer cell lines. Cancer Res. 1986 Apr;46(4 Pt 1):1707–1713. [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Fidler I. J., Kripke M. L. Metastasis results from preexisting variant cells within a malignant tumor. Science. 1977 Aug 26;197(4306):893–895. doi: 10.1126/science.887927. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. Tumor heterogeneity and the biology of cancer invasion and metastasis. Cancer Res. 1978 Sep;38(9):2651–2660. [PubMed] [Google Scholar]
- Greig R. G., Koestler T. P., Trainer D. L., Corwin S. P., Miles L., Kline T., Sweet R., Yokoyama S., Poste G. Tumorigenic and metastatic properties of "normal" and ras-transfected NIH/3T3 cells. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3698–3701. doi: 10.1073/pnas.82.11.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata K., Usui T., Koshiba H., Maruyama Y., Oikawa I., Freeman A. E., Shiramatsu K., Hayasaka H. Effects of basement membrane matrix on the culture of fetal mouse hepatocytes. Gan. 1983 Oct;74(5):687–692. [PubMed] [Google Scholar]
- Hopper K. E., Adelmann B. C., Gentner G., Gay S. Recongnition by guinea-pig peritoneal exudate cells of conformationally different states of the collagen molecule. Immunology. 1976 Feb;30(2):249–259. [PMC free article] [PubMed] [Google Scholar]
- Horwitz K. B., McGuire W. L. Estrogen control of progesterone receptor in human breast cancer. Correlation with nuclear processing of estrogen receptor. J Biol Chem. 1978 Apr 10;253(7):2223–2228. [PubMed] [Google Scholar]
- Hujanen E. S., Terranova V. P. Migration of tumor cells to organ-derived chemoattractants. Cancer Res. 1985 Aug;45(8):3517–3521. [PubMed] [Google Scholar]
- Jones P. A., DeClerck Y. A. Destruction of extracellular matrices containing glycoproteins, elastin, and collagen by metastatic human tumor cells. Cancer Res. 1980 Sep;40(9):3222–3227. [PubMed] [Google Scholar]
- Kasid A., Lippman M. E., Papageorge A. G., Lowy D. R., Gelmann E. P. Transfection of v-rasH DNA into MCF-7 human breast cancer cells bypasses dependence on estrogen for tumorigenicity. Science. 1985 May 10;228(4700):725–728. doi: 10.1126/science.4039465. [DOI] [PubMed] [Google Scholar]
- Klebe R. J. Isolation of a collagen-dependent cell attachment factor. Nature. 1974 Jul 19;250(463):248–251. doi: 10.1038/250248a0. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., McGarvey M. L., Hassell J. R., Star V. L., Cannon F. B., Laurie G. W., Martin G. R. Basement membrane complexes with biological activity. Biochemistry. 1986 Jan 28;25(2):312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., McGarvey M. L., Liotta L. A., Robey P. G., Tryggvason K., Martin G. R. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982 Nov 23;21(24):6188–6193. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- Kramer R. H., Bensch K. G., Wong J. Invasion of reconstituted basement membrane matrix by metastatic human tumor cells. Cancer Res. 1986 Apr;46(4 Pt 2):1980–1989. [PubMed] [Google Scholar]
- Laurie G. W., Leblond C. P., Martin G. R. Localization of type IV collagen, laminin, heparan sulfate proteoglycan, and fibronectin to the basal lamina of basement membranes. J Cell Biol. 1982 Oct;95(1):340–344. doi: 10.1083/jcb.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. T. Patterns of metastasis and natural courses of breast carcinoma. Cancer Metastasis Rev. 1985;4(2):153–172. doi: 10.1007/BF00050693. [DOI] [PubMed] [Google Scholar]
- Lesot H., Kühl U., Mark K. Isolation of a laminin-binding protein from muscle cell membranes. EMBO J. 1983;2(6):861–865. doi: 10.1002/j.1460-2075.1983.tb01514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine E. L., Birk D. E., Raska K., Jr Attachment to and degradation of collagen substrata by adenovirus-transformed cells of varying tumorigenicity. Coll Relat Res. 1984 Jan;4(1):49–61. doi: 10.1016/s0174-173x(84)80028-x. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Kleinerman J., Catanzaro P., Rynbrandt D. Degradation of basement membrane by murine tumor cells. J Natl Cancer Inst. 1977 May;58(5):1427–1431. doi: 10.1093/jnci/58.5.1427. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Thorgeirsson U. P., Garbisa S. Role of collagenases in tumor cell invasion. Cancer Metastasis Rev. 1982;1(4):277–288. doi: 10.1007/BF00124213. [DOI] [PubMed] [Google Scholar]
- Liotta L. A. Tumor invasion and metastases: role of the basement membrane. Warner-Lambert Parke-Davis Award lecture. Am J Pathol. 1984 Dec;117(3):339–348. [PMC free article] [PubMed] [Google Scholar]
- Lippman M., Bolan G., Huff K. The effects of estrogens and antiestrogens on hormone-responsive human breast cancer in long-term tissue culture. Cancer Res. 1976 Dec;36(12):4595–4601. [PubMed] [Google Scholar]
- Malinoff H. L., McCoy J. P., Jr, Varani J., Wicha M. S. Metastatic potential of murine fibrosarcoma cells is influenced by cell surface laminin. Int J Cancer. 1984 May 15;33(5):651–655. doi: 10.1002/ijc.2910330516. [DOI] [PubMed] [Google Scholar]
- Malinoff H. L., Wicha M. S. Isolation of a cell surface receptor protein for laminin from murine fibrosarcoma cells. J Cell Biol. 1983 May;96(5):1475–1479. doi: 10.1083/jcb.96.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy J. B., Basara M. L., Palm S. L., Sas D. F., Furcht L. T. The role of cell adhesion proteins--laminin and fibronectin--in the movement of malignant and metastatic cells. Cancer Metastasis Rev. 1985;4(2):125–152. doi: 10.1007/BF00050692. [DOI] [PubMed] [Google Scholar]
- McCarthy J. B., Palm S. L., Furcht L. T. Migration by haptotaxis of a Schwann cell tumor line to the basement membrane glycoprotein laminin. J Cell Biol. 1983 Sep;97(3):772–777. doi: 10.1083/jcb.97.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensing H., Albini A., Krieg T., Pontz B. F., Müller P. K. Enhanced chemotaxis of tumor-derived and virus-transformed cells to fibronectin and fibroblast-conditioned medium. Int J Cancer. 1984 Jan 15;33(1):43–48. doi: 10.1002/ijc.2910330109. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Cancer metastasis. Organ colonization and the cell-surface properties of malignant cells. Biochim Biophys Acta. 1982 Dec 21;695(2):113–176. doi: 10.1016/0304-419x(82)90020-8. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Poste G. Tumor cell diversity and host responses in cancer metastasis--part I--properties of metastatic cells. Curr Probl Cancer. 1982 Dec;7(6):1–83. doi: 10.1016/s0147-0272(82)80017-2. [DOI] [PubMed] [Google Scholar]
- Orkin R. W., Gehron P., McGoodwin E. B., Martin G. R., Valentine T., Swarm R. A murine tumor producing a matrix of basement membrane. J Exp Med. 1977 Jan 1;145(1):204–220. doi: 10.1084/jem.145.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormerod E. J., Everett C. A., Hart I. R. Enhanced experimental metastatic capacity of a human tumor line following treatment with 5-azacytidine. Cancer Res. 1986 Feb;46(2):884–890. [PubMed] [Google Scholar]
- Rao N. C., Barsky S. H., Terranova V. P., Liotta L. A. Isolation of a tumor cell laminin receptor. Biochem Biophys Res Commun. 1983 Mar 29;111(3):804–808. doi: 10.1016/0006-291x(83)91370-0. [DOI] [PubMed] [Google Scholar]
- Rizzino A., Terranova V., Rohrbach D., Crowley C., Rizzino H. The effects of laminin on the growth and differentiation of embryonal carcinoma cells in defined media. J Supramol Struct. 1980;13(2):243–253. doi: 10.1002/jss.400130212. [DOI] [PubMed] [Google Scholar]
- Schirrmacher V. Shifts in tumor cell phenotypes induced by signals from the microenvironment. Relevance for the immunobiology of cancer metastasis. Immunobiology. 1980 Jul;157(2):89–98. doi: 10.1016/S0171-2985(80)80091-X. [DOI] [PubMed] [Google Scholar]
- Shafie S. M. Estrogen and the growth of breast cancer: new evidence suggests indirect action. Science. 1980 Aug 8;209(4457):701–702. doi: 10.1126/science.6994231. [DOI] [PubMed] [Google Scholar]
- Shearman P. J., Longenecker B. M. Clonal variation and functional correlation of organ-specific metastasis and an organ-specific metastasis-associated antigen. Int J Cancer. 1981 Mar 15;27(3):387–395. doi: 10.1002/ijc.2910270319. [DOI] [PubMed] [Google Scholar]
- Smith H. S., Liotta L. A., Hancock M. C., Wolman S. R., Hackett A. J. Invasiveness and ploidy of human mammary carcinomas in short-term culture. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1805–1809. doi: 10.1073/pnas.82.6.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soule H. D., Vazguez J., Long A., Albert S., Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst. 1973 Nov;51(5):1409–1416. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- Talmadge J. E., Fidler I. J. Cancer metastasis is selective or random depending on the parent tumour population. Nature. 1982 Jun 17;297(5867):593–594. doi: 10.1038/297593a0. [DOI] [PubMed] [Google Scholar]
- Terranova V. P., Hujanen E. S., Loeb D. M., Martin G. R., Thornburg L., Glushko V. Use of a reconstituted basement membrane to measure cell invasiveness and select for highly invasive tumor cells. Proc Natl Acad Sci U S A. 1986 Jan;83(2):465–469. doi: 10.1073/pnas.83.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova V. P., Hujanen E. S., Martin G. R. Basement membrane and the invasive activity of metastatic tumor cells. J Natl Cancer Inst. 1986 Aug;77(2):311–316. [PubMed] [Google Scholar]
- Terranova V. P., Liotta L. A., Russo R. G., Martin G. R. Role of laminin in the attachment and metastasis of murine tumor cells. Cancer Res. 1982 Jun;42(6):2265–2269. [PubMed] [Google Scholar]
- Terranova V. P., Rao C. N., Kalebic T., Margulies I. M., Liotta L. A. Laminin receptor on human breast carcinoma cells. Proc Natl Acad Sci U S A. 1983 Jan;80(2):444–448. doi: 10.1073/pnas.80.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova V. P., Williams J. E., Liotta L. A., Martin G. R. Modulation of the metastatic activity of melanoma cells by laminin and fibronectin. Science. 1984 Nov 23;226(4677):982–985. doi: 10.1126/science.6505678. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson U. P., Turpeenniemi-Hujanen T., Williams J. E., Westin E. H., Heilman C. A., Talmadge J. E., Liotta L. A. NIH/3T3 cells transfected with human tumor DNA containing activated ras oncogenes express the metastatic phenotype in nude mice. Mol Cell Biol. 1985 Jan;5(1):259–262. doi: 10.1128/mcb.5.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]
- Trainer D. L., Kline T., Mallon F., Greig R., Poste G. Effect of 5-azacytidine on DNA methylation and the malignant properties of B16 melanoma cells. Cancer Res. 1985 Dec;45(12 Pt 1):6124–6130. [PubMed] [Google Scholar]
- Turpeenniemi-Hujanen T., Thorgeirsson U. P., Rao C. N., Liotta L. A. Laminin increases the release of type IV collagenase from malignant cells. J Biol Chem. 1986 Feb 5;261(4):1883–1889. [PubMed] [Google Scholar]
- Welch D. R., Tomasovic S. P. Implications of tumor progression on clinical oncology. Clin Exp Metastasis. 1985 Jul-Sep;3(3):151–188. doi: 10.1007/BF01786761. [DOI] [PubMed] [Google Scholar]


