Abstract
Fibroblasts are the major mesenchymal cell type in connective tissue and deposit the collagen and elastic fibers of the extracellular matrix (ECM)1. Even within a single tissue fibroblasts exhibit remarkable functional diversity, but it is not known whether this reflects the existence of a differentiation hierarchy or is a response to different environmental factors. Here we show, using transplantation assays and lineage tracing, that the fibroblasts of skin connective tissue arise from two distinct lineages. One forms the upper dermis, including the dermal papilla that regulates hair growth and the arrector pili muscle (APM), which controls piloerection. The other forms the lower dermis, including the reticular fibroblasts that synthesise the bulk of the fibrillar ECM, and the pre-adipocytes and adipocytes of the hypodermis. The upper lineage is required for hair follicle formation. In wounded adult skin, the initial wave of dermal repair is mediated by the lower lineage and upper dermal fibroblasts are recruited only during re-epithelialisation. Epidermal beta-catenin activation stimulates expansion of the upper dermal lineage, rendering wounds permissive for hair follicle formation. Our findings explain why wounding is linked to formation of ECM-rich scar tissue that lacks hair follicles2-4. They also form a platform for discovering fibroblast lineages in other tissues and for examining fibroblast changes in ageing and disease.
At E12.5 mouse epidermis comprises one or two cell layers and the dermis appears homogeneous in composition (Fig. 1a)5. By E18.5 the epidermis is fully stratified, hair follicles, with associated DP, are forming and the upper (papillary) dermis is distinguishable from the lower (reticular) dermis because of its higher cellular density (Fig. 1a). By P2 the hypodermis has formed, comprising differentiated adipocytes and pre-adipocytes (Fig. 1a). We identified markers of different fibroblast subpopulations at each developmental stage, based on prior studies6,7 and the availability of antibodies for live cell sorting.
Figure 1. Morphological and molecular markers of embryonic and postnatal fibroblasts.
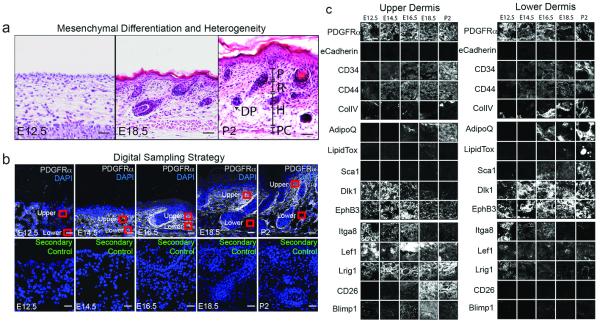
(a-c) Sections of mouse back skin. (a) H&E staining. P: papillary dermis; R: reticular dermis; H: hypodermis; P: panniculus carnosus; DP: dermal papilla. (b) 10μm sections immunostained with PDGFRa antibodies and secondary antibody control with DAPI nuclear counterstain. Red squares show areas digitally sampled for the tissue screen. (c) Tissue screen of upper and lower dermis. 3 biological replicates for each stain were performed. Images are representative samples of sections shown Fig. E1, E2. Scale bars: 50 μm.
The pan-fibroblast marker platelet derived growth factor receptor alpha (PDGFRa) is expressed in upper and lower dermis at all stages of development (Fig. 1b)8. In contrast there were temporal and spatial changes in expression of 18 other fibroblast markers (Fig. 1b-c; Fig. E1-3). From E16.5 CD26 and B lymphocyte induced maturation protein (Blimp1/Prdm1) were selectively expressed in the upper dermis, while Sca1 was selectively expressed in the lower dermis. Delta-like homologue 1 (Dlk1) and Lrig1 were expressed throughout the dermis at E12.5, with Dlk1 expression persisting in the lower dermis from E18.5 and Lrig1 expression persisting in the upper dermis. The changes in abundance of CD26+, Sca1+ and Dlk1+ cells were confirmed by flow cytometry of GFP+ dermal cells from PDGFRaH2BeGFP mice8 (Fig. E3). Several of the markers were also differentially expressed in neonatal human skin (Fig. E4).
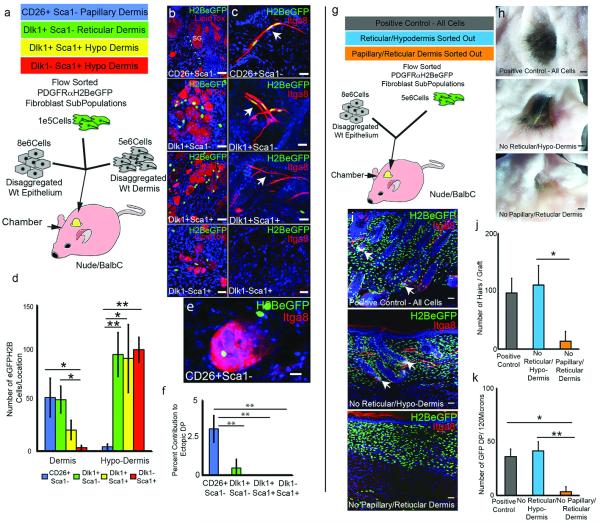
To evaluate the differentiation potential of different dermal fibroblasts (Fig. 2a), cells were flow sorted from P2 PDGFRaH2BeGFP dermis8 (Fig. E5c-e), combined with unlabelled epidermal and dermal cells and injected into chambers implanted into nude/BalbC mice9. Papillary dermal cells (CD26+Sca1-) contributed exclusively to the upper dermis (Fig. 2b-d; Fig. E6a), including the dermal papilla and APM (Fig. 2, e, f). Hypodermal fibroblasts (Dlk1+Sca1+ and Dlk1-Sca1+) differentiated into adipocytes but not into APM or dermal papilla (Fig. 2b-d, Fig. E6c, d). Dlk1+Sca1− cells, located primarily at the boundary between reticular dermis and hypodermis (Fig. E5a, b), contributed to all dermal mesenchymal compartments (Fig. 2b-d, Fig. E6b). However, while we cannot rule out the existence of multipotent fibroblasts in adult skin, Dlk1+Sca1− cells did not persist after P10, when Dlk1 was no longer expressed in the dermis (Fig. E4d and data not shown).
Figure 2. Skin reconstitution assays.
(a) Experimental set up for (b-f). (b-c) Grafts immunostained with DAPI counterstain (blue). SG: sebaceous gland. Arrows: APM. (d-f) Contribution of PDGFRaH2BeGFP cells to dermal compartments. (g) Experimental set up for (h-k). (h) Macroscopic views of grafts. (i) Contribution of GFP+ cells to grafts. Arrows: GFP+ DP. (j) Hair follicles per graft. (k) GFP+ DP per 120 microns graft. N=3 biological replicates per experiment. *P <0.05; **P <0.005. Horizontal whole mounts shown. Scale bars: (b-c) 40 μm (e) 30 μm (h) 2.5mm (i) 50 μm.
The number of hair follicles was higher in grafts containing upper dermal (CD26+Sca1-) cells than Dlk1-Sca1+ hypodermal cells (Fig. E6e-i). Dlk1-Sca1+ cells are a subpopulation of Lin-CD34+CD29+Sca1+ adipocyte precursors, which also express Pdgfra (Fig. E5f-i)10. To establish whether hypodermal cells were less effective at supporting hair follicle formation we compared control grafts containing unfractionated P2 PDGFRaH2BeGFP+ cells with grafts in which we excluded papillary/reticular cells (CD26+/Dlk1+/Sca1− cells depleted) or reticular/hypodermal cells (Dlk1+/-Sca1+ cells depleted) ((Fig. 2g-k; Fig. E6j-l). Since CD26, Dlk1 and Sca1 are not expressed in the DP (data not shown) all grafts contained DP cells. The contribution of GFP+ cells to the grafts was extensive in all cases (Fig. 2i). Hair follicle formation was similar in the grafts of unfractionated dermal cells and those depleted of reticular and hypodermal cells. In contrast, when the papillary and reticular dermal cells were excluded very few hair follicles formed (Fig. 2h-k). Thus P2 skin contains cells that are restricted to forming either the upper or lower dermal lineages on skin reconstitution, the upper dermal cells being required for hair follicle formation.
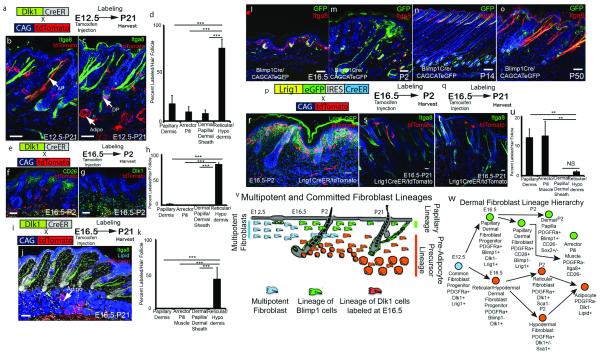
We next performed lineage tracing with specific promoters. We crossed Dlk1CreER mice with Rosa-CAG fl/stop/fl tdTomato mice, then labelled with Tamoxifen at E12.5 (when all fibroblasts are Dlk1+; Fig. 1c) or E16.5 (when only reticular fibroblasts express Dlk1; Fig. 1c) and harvested at P2 or P21 (Fig. 3a-k). Flow cytometry established that the labelling efficiency was over 95% (Fig. E7a-c). When Dlk1 expressing cells were labelled at E12.5 all of the dermal mesenchymal compartments (Pdgfra+) were labelled (Fig. 3a-d). In contrast, when cells were labelled at E16.5 the only labelled fibroblasts were reticular and hypodermal cells, including mature adipocytes (Fig. 3e-k). Therefore after E16.5 dermal fate restriction occurs, such that cells expressing Dlk1 only give rise to the lower dermal lineages. We confirmed this by examining adipogenesis of dermal cells in culture (Fig. E3d-g; see Supplementary Information)6,11.
Figure 3. Fibroblast lineage commitment during skin development.
(a, e, i, p, q) Labelling strategies. (b, c, f, g, j, l-o, r-t) Contribution of labelled cells to different dermal compartments. AP: arrector pili muscle; DP: dermal papilla; Adipo: adipocyte. (d, h, k, u) Average number labeled cells per unit area of dermis (defined by hair follicle spacing). (v, w) Schematic summary of lineage restriction. 3 biological replicates analyzed per experiment. Sections counterstained with DAPI. NS – Not statistically significant different; **P <0.005, ***P <0.0005. Scale bars: 50 μm.
We next performed lineage tracing with Blimp1Cre. Between E16.5 and E18.5 Blimp1 was expressed in the DP, dermal sheath and papillary dermis (Fig. 1c; Fig. E7d-e)12,13, but from P2 through adulthood dermal Blimp1 expression was confined to the DP and dermal sheath (Fig. E7f-g). Blimp1 expressing epidermal and endothelial cells (Fig. E7h-i)13-15 were excluded from analysis based on co-expression of relevant markers. The fidelity of the Blimp1 promoter was established by flow sorting (Fig. E7j-k).
At all time points DP and dermal sheath cells were labelled in Blimp1Cre x CAGCATeGFP mice (Fig. 3l-o). At E16.5 the papillary dermis was unlabeled (Fig. 3l), but at E18.5 the papillary dermis contained GFP positive cells, consistent with the pattern of endogenous Blimp1 expression (Fig. E7e, h). From P14 onwards all APM were GFP positive and only a few GFP+ cells remained in the papillary dermis (Fig. 3n, o). Thus P2 papillary dermal fibroblasts are the progenitors of the APM. Labeled papillary fibroblasts never contributed to the reticular dermis or hypodermis.
We used Lrig1 as an additional marker for lineage tracing16 (Fig. 1c; Fig. 3r-u; Fig. E7l-q). The specificity of the Lrig1-GFP-IRES-CreER reporter line17 was confirmed by flow cytometry (Fig. E7n). P2 dermal cells from Lrig1-GFP-IRES-CreER x Rosa-CAG fl/stop/fl tdTomato crosses treated with Tamoxifen at E16.5 expressed PDGFRa and CD26 but not Sca1 (Fig. E7n-q). At P2 labelling was restricted to the papillary dermis (Fig. 3r, Fig. E7m). At P21 the APM and papillary dermis were labelled, while the reticular dermis and fat were unlabelled (Fig. 3s-u). Our data suggest that loss of papillary dermis after the first hair follicle cycle8,18 is due to differentiation of papillary fibroblasts into the APM, which was further confirmed by clonal analysis in PDGFRaCreER x CAGCATeGFP mice (Fig. E8). It is interesting that the papillary dermis is more pronounced in human skin, which has fewer hair follicles and therefore fewer APM, than mouse skin5. We conclude that from E16.5 the developing dermis undergoes fate restriction such that cells in the upper dermis that express Lrig1 or Blimp1 give rise to the DP, APM and papillary fibroblasts, while Dlk1-positive cells in the lower dermis give rise to the reticular dermis, hypodermis and adipocyte layer (Fig. 3v, w).
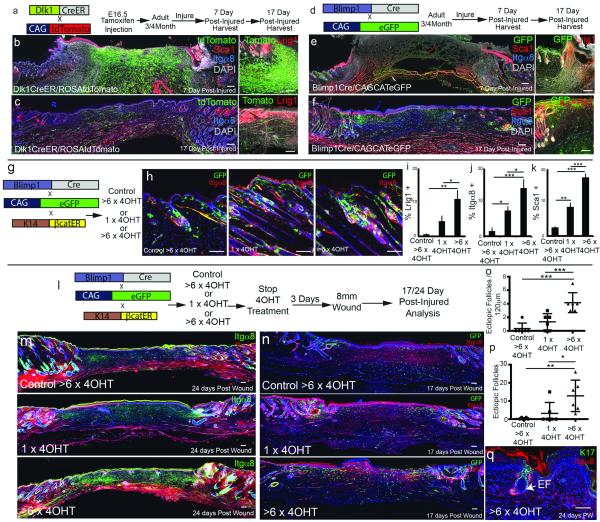
To investigate how fibroblasts of different lineages contribute to skin repair, we created full thickness wounds in adult back skin. We ruled out any contribution of bone marrow derived cells to the Pdgfra+ dermal compartment (Figure E9a-k; Supplementary Information). When PDGFRaCreER x lox/stop/lox tdTomato mice that had been injected with Tamoxifen at E17.5 were wounded in adulthood, we found tdTomato+ cells throughout the repairing dermis (Fig. E9l-n). When we examined wound healing in Dlk1CreER x lox/stop/lox tdTomato mice that had been injected with Tamoxifen at E16.5 to selectively label the lower dermis (Fig. 4a-c; Fig. E10a, b), a large proportion of wound fibroblasts, including cells that expressed the myofibroblast marker alpha-smooth muscle actin19 (Fig. E10b), were tdTomato-positive (Fig. 4b-c). In adult skin of Blimp1Cre x lox/stop/loxGFP mice only upper dermal fibroblasts and CD31-positive endothelial cells were GFP positive (Fig. 4e). 7 days after wounding no GFP-positive fibroblasts were present in the wound bed (Fig. 4e; Fig. E10c-d) and at day 17 GFP-positive fibroblasts were exclusively detected immediately underneath the reformed epidermis (Fig. 4f; Fig. E10d).
Figure 4. Contribution of fibroblast lineages in adult skin.
(a, d, g, l) Labelling strategies. (b, c, e, f, h, m, n, q) Histological analysis of horizontal whole mounts. (i-k) Flow cytometric quantification of fibroblast markers. (o, p) Quantitation of ectopic hair follicles in (o) 17 day wound bed whole mount sections and (p) entire 24 day wound bed. (i-k) n = 3, (o, p) n = 6 biological replicates. *P <0.05, **P <0.005, ***P <0.0005. Scale bars: 200μm (b, c, e, f), 100μm (m, n, q) or 50μm (higher magnification views).
We conclude that the first wave of dermal regeneration depends on cells of the reticular dermis and hypodermis, which are not capable of inducing hair follicles (Fig. 2i-k) and which elaborate the collagenous ECM characteristic of fibrosis6. The upper dermal lineage is not repopulated until re-epithelialisation and contributes exclusively to the papillary dermis. This explains both the rapid reconstitution of the skin adipocyte layer after wounding19 and the absence of hair follicles in newly closed wounds20.
Sustained activation of the canonical Wnt pathway in adult epidermis induces growth of existing hair follicles (anagen), formation of new hair follicles21,22, fibroblast proliferation and dermal ECM remodelling8. To examine the impact on the different dermal lineages we used K14ΔNβ-cateninER transgenic mice. One application of Tamoxifen induces anagen, while repeated applications stimulate ectopic hair follicle formation21. Epidermal β-catenin activation led to expansion of the upper and lower dermal compartments, the effects being more pronounced in mice that received multiple applications of Tamoxifen (Fig. 4h-k; Fig. E10e-g). The relative locations of the different dermal cells were unchanged (Fig. E9o-p). Expansion of the upper dermal lineage was evident in K14ΔNβ-cateninER x Blimp1Cre/CAGCATeGFP mice (Fig. 4g, h). Since epidermal Wnt signalling promotes hair follicle growth, the expansion of the lower dermis could explain why the skin adipocyte layer increases in thickness during anagen22.
To test whether epidermal β-catenin activation prior to wounding would promote hair follicle formation, K14ΔNβ-cateninER/Blimp1Cre/CAGCATeGFP mice were given repeated applications of Tamoxifen and subsequently wounded (Fig. 4l). This led to increased numbers of Itga8+ and Lrig1+ fibroblasts in the wound bed (Fig. 4m; Fig. E10h), an increased number of cells marked by Blimp1Cre (Fig. 4n) and a pronounced stimulation of hair follicle formation (Fig. 4o-q). We conclude that dermal remodelling in response to epidermal β-catenin activation leads to expansion of the upper and lower dermis, the increase in the upper papillary dermis being permissive for new hair follicle formation. It will be interesting to identify the downstream signalling pathways involved, one candidate being Hedgehog21. Wnt signalling regulates cells of the immune system23 and a role for infiltrating γδ T cells in promoting hair follicle formation has recently been described24. The Wnt effector transcription factors Lef1 and Tcf3 were differentially expressed by upper and lower dermal fibroblasts (Fig. E5d-e), raising the intriguing possibility that different fibroblast lineages differ in their Wnt responsiveness and interactions with inflammatory cells24.
In conclusion, the lineage hierarchies we have described reveal the cellular origins of the heterogeneous architecture of the dermis and provide a mechanistic basis for understanding the changes that take place during repair of adult skin wounds and in response to epidermal Wnt activation. We now have the opportunity to uncover events that result in dermal lineage specification, and discover how the different lineages contribute to the changes that occur during aging25 and tumour formation1.
Methods
Transgenic mice
Mice were maintained on a C57/Blk6 and CBA F1 background. PDGFRaCreER28 or Dlk1CreERt (ICS) mice were bred with CAGCATeGFP29 or ROSA26tdTomato (Jax Labs - 007905). PDGFRaeH2BeGFP mice30 were obtained from Jackson Laboratories. Blimp1GFP14 and Blimp1Cre (Jax Labs - 008827) 13 mice have been described previously. K14ΔNβ-cateninER mice have previously been described. Tamoxifen (Sigma Aldrich) was injected intraperitoneally into adult mice. 2mg of Tamoxifen in 100ul of acetone was administered topically to the back skin of pups. Bone marrow reconstitution experiments and wounding (8 mm diameter full thickness wounds) were performed as described previously26. All experiments were performed according to the guidelines of the UK Government Animals (Scientific Procedures) Act 1986 and underwent ethical review by Cambridge University and King’s College London. Experiments were performed on female mice; however, the markers evaluated showed the same distribution in males. All experiments were performed on 3 or more female mice.
Flow cytometry
P2 disaggregated dermal preparations were prepared as previously described 11 and labelled with the antibodies listed below.
RT-PCR
This was performed as previously described27. Analysis was performed by the delta-Ct method. Primer probe sets were purchased from Applied Biosystems and used according to manufacturer’s recommendations: Ly6a/Sca1 - Mm00726565_s1; Dlk1/Pref1 - Mm00438422_m1; CD26/DPP4 - Mm00494538_m1; pparg -Mm01184322_m1; Zfp423 - Mm00473699_m1; Acta2 - Mm01546133_m1 Axin 2 – Mm01266783_m1; Dkk1 – Mm00438422_m1; Lef1 – MM01310389_m1; Tcf3 – Mm01188714_m1; Tcf7l2 – Mm00501505_m1; Wnt5a – Mm00437347_m1; Blimp1 – Mm01187285_m1.
Chamber grafting assay
This procedure was performed as previously described9. 8 × 106 wild type adult dorsal epidermal cells were combined with 5 × 106 wild type P2 dermal cells and 105 flow sorted PDGFRaH2BeGFP P2 dermal cells.
Histology and microscopy
5 μm frozen sections and horizontal whole mounts were stained with the antibodies listed. The tissue array (Fig. 1c) was constructed from digitally excised images of areas of upper and lower dermis (Fig. 1b). All microscopy was performed on a Leica SP5 or Nikon A1 confocal microscope and images were analyzed in Image J and Adobe Photoshop CS6.
Horizontal whole mounts were prepared as previously described11. Skin was collected and fixed in 4%PFA for 15-30 minutes then embedded in cryomolds. Sections were cut on a cryostat at a thickness of between 50-150 μm. Instead of attaching sections with OCT onto slides, forceps were used to place sections into 1xPBS at room temperature. The 1XPBS dissolved away the OCT and tissue was then ready for staining in 300-500μl of PB buffer (1XPBS containing 0.5% skim milk, 0.25% Cold Water Fish Skin Gelatin, 0.5%Triton X100) in Eppendorf tubes. Sections could be stored for 3-6 months in 1xPBS at 4°C. Staining is subsequently performed by incubation with primary antibody overnight at 4°C on a rocker. Secondary antibodies were incubated for at least 2 hours at room temperature after 1 wash in 1xPBS for 1 hour. The tissue was then mounted on coverslips with a small volume of 100% glycerol and analysed by confocal microscopy.
Antibodies
The following antibodies were used: Itga8 (R&D Systems AF4076), Dlk1 (R&D Systems AF1144), CD26 (R&D Systems AF954), Sca1(R&D Systems AF1226), PDGFRa (R&D Systems AF1062), Fabp4 (R&D Systems AF1443), AdipoQ (R&D Systems AF1119), Ephb3 (R&D Systems AF432), Lrig1 (R&D Systems AF3688), Podplanin (R&D Systems AF3244), Transglutaminase2 (R&D Systems AF4376), Blimp1 (eBioscience 14-5963), CD26 (eBioscience 45-0261), Sca1 (eBioscience 56-5981-82), PDGFRa (eBioscience 14-140182), eCadherin (eBioscience 17-1449), CD34 (eBioscience 17-0349-42), CD44 (eBioscience 130441), CD45 (eBioscience 12-0451), CD31 (eBioscience 25-0311-82), GFP (Invitrogen A11122), Lipidtox (Rockland H34476), tdTomato (MBL 600-401-379), Dlk1 (Millipore D187-5), ColIV (Cell Signaling AB8201), Lef1 (Cell Signaling 2330S), Vimentin (Cell Signaling 5741S). The alpha-smooth muscle actin antibody was a gift from Frank Nestle, King’s College London.
Clonal growth of fibroblast subpopulations in hydrogels
Flow sorted PDGFRa populations were seeded into Extracel hydrogels (Glycosan Biosystems, Salt Lake City, UT), which contain cross-linked gelatin and hyaluronic acid14, in individual wells of 24 well plates at a density of 5 × 104 cells per well. Cells were grown in Adipogenic Medium (R&D Systems) or standard Growth Medium (DMEM supplemented with 10% bovine serum) for 1 week. Cultures were fixed with 4% PFA, washed in PBS and stained with Lipidtox (Invitrogen 1:500) and DAPI. Spheres were scored positive for adipogenesis if they contained at least one lipid positive cell.
Graphing and Statistical Analysis
All graphs were generated using Excel, GraphPad Prism 6 and Adobe Illustrator CS4 software. Data are means ± standard error of the mean (SEM). A One way ANOVA parametric test was performed for experiments with P < 0.05 considered significant.
Sample size for animal experiments was determined on the basis of pilot experiments. In the case of the skin reconstitution assays, animals were excluded from analysis only if grafts were unsuccessful (i.e. chamber falling out 1-2 days after grafting).
Supplementary Material
Acknowledgements
This work was funded by the Wellcome Trust (FMW, ACFS), the Medical Research Council (FMW, ACFS) and the European Union FP7 programme: TUMIC (FMW), HEALING (FMW) and EpigeneSys (ACFS). BML is the recipient of a FEBS long-term fellowship. KK is the recipient of a MRC PhD Studentship. The authors acknowledge financial support from the Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy’s & St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust. Input from Maria Mastrogiannaki, Andreas Reimer and Britta Trappmann is gratefully acknowledged. The authors also thank John Connelly and George Theocharidis for providing tissue and the KCL Hodgkins BSU staff, in particular Joan Castle, Claire Pearce and Dionne Cooper, for technical support.
Footnotes
Supplementary information line Supplementary information can be found in the accompanying PDF file.
Author Information Reprints and permissions information is available at ww.nature.com/reprints.
The authors have no competing financial interests.
References
- 1.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat. Rev. Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 2.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 3.Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 4.Shah M, Foreman DM, Ferguson MW. Neutralising antibody to TGF-beta 1,2 reduces cutaneous scarring in adult rodents. J. Cell Sci. 1994;107:1137–1157. doi: 10.1242/jcs.107.5.1137. [DOI] [PubMed] [Google Scholar]
- 5.Van Exan RJ, Hardy MH. The differentiation of the dermis in the laboratory mouse. Am. J. Anat. 1984;169:149–164. doi: 10.1002/aja.1001690204. [DOI] [PubMed] [Google Scholar]
- 6.Dulauroy S, Di Carlo SE, Langa F, Eberl G, Peduto L. Lineage tracing and genetic ablation of ADAM12(+) perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat. Med. 2012;18:1262–1270. doi: 10.1038/nm.2848. [DOI] [PubMed] [Google Scholar]
- 7.Janson DG, Saintigny G, van Adrichem A, Mahe C, El Ghalbzouri A. Different gene expression patterns in human papillary and reticular fibroblasts. J. Invest. Dermatol. 2012;132:2565–2572. doi: 10.1038/jid.2012.192. [DOI] [PubMed] [Google Scholar]
- 8.Collins CA, Kretzschmar K, Watt FM. Reprogramming adult dermis to a neonatal state through epidermal activation of beta-catenin. Development. 2011;138:5189–5199. doi: 10.1242/dev.064592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen KB, Driskell RR, Watt FM. Assaying proliferation and differentiation capacity of stem cells using disaggregated adult mouse epidermis. Nature Protocols. 2010;5:898–911. doi: 10.1038/nprot.2010.39. [DOI] [PubMed] [Google Scholar]
- 10.Festa E, et al. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146:761–771. doi: 10.1016/j.cell.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Driskell RR, et al. Clonal growth of dermal papilla cells in hydrogels reveals intrinsic differences between Sox2-positive and -negative cells in vitro and in vivo. J. Invest. Dermatol. 2012;132:1084–1093. doi: 10.1038/jid.2011.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lesko MH, Driskell RR, Kretzschmar K, Goldie SJ, Watt FM. Sox2 modulates the function of two distinct cell lineages in mouse skin. Dev. Biol. 2013;382:15–26. doi: 10.1016/j.ydbio.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robertson EJ, et al. Blimp1 regulates development of the posterior forelimb, caudal pharyngeal arches, heart and sensory vibrissae in mice. Development. 2007;134:4335–4345. doi: 10.1242/dev.012047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horsley V, et al. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell. 2006;126:597–609. doi: 10.1016/j.cell.2006.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magnusdottir E, et al. Epidermal terminal differentiation depends on B lymphocyte-induced maturation protein-1. Proc. Natl Acad Sci USA. 2007;104:14988–14993. doi: 10.1073/pnas.0707323104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomez C, Chua W, Miremadi A, Quist S, Headon DJ, Watt FM. The interfollicular epidermis of adult mouse tail comprises two distinct cell lineages that are differentially regulated by Wnt, Edaradd, and Lrig1. Stem Cell Reports. 2013;1:19–27. doi: 10.1016/j.stemcr.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Page ME, Lombard P, Ng F, Gottgens B, Jensen KB. The epidermis comprises autonomous compartments maintained by distinct stem cell populations. Cell Stem Cell. 2013 doi: 10.1016/j.stem.2013.07.010. doi:10.1016/j.stem.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egawa G, Osawa M, Uemura A, Miyachi Y, Nishikawa S. Transient expression of ephrin b2 in perinatal skin is required for maintenance of keratinocyte homeostasis. J. Invest. Dermatol. 2009;129:2386–2395. doi: 10.1038/jid.2009.105. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt BA, Horsley V. Intradermal adipocytes mediate fibroblast recruitment during skin wound healing. Development. 2013;140:1517–1527. doi: 10.1242/dev.087593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito M, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- 21.Silva-Vargas V, et al. Beta-catenin and Hedgehog signal strength can specify number and location of hair follicles in adult epidermis without recruitment of bulge stem cells. Dev. Cell. 2005;9:121–131. doi: 10.1016/j.devcel.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 22.Plikus MV, et al. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451:340–344. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Staal FJ, Luis TC, Tiemessen MM. WNT signalling in the immune system: WNT is spreading its wings. Nat. Rev. Immunol. 2008;8:581–593. doi: 10.1038/nri2360. [DOI] [PubMed] [Google Scholar]
- 24.Gay D, et al. Fgf9 from dermal gammadelta T cells induces hair follicle neogenesis after wounding. Nature Med. 2013;19:916–923. doi: 10.1038/nm.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giangreco A, Qin M, Pintar JE, Watt FM. Epidermal stem cells are retained in vivo throughout skin aging. Aging Cell. 2008;7:250–259. doi: 10.1111/j.1474-9726.2008.00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arwert EN, et al. Tumor formation initiated by nondividing epidermal cells via an inflammatory infiltrate. Proc. Natl Acad. Sci. USA. 2010;107:19903–19908. doi: 10.1073/pnas.1007404107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Driskell RR, Giangreco A, Jensen KB, Mulder KW, Watt FM. Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis. Development. 2009;136:2815–2823. doi: 10.1242/dev.038620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rivers LE, et al. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nature Neurosci. 2008;11:1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawamoto S, et al. A novel reporter mouse strain that expresses enhanced green fluorescent protein upon Cre-mediated recombination. FEBS Letts. 2000;470:263–268. doi: 10.1016/s0014-5793(00)01338-7. [DOI] [PubMed] [Google Scholar]
- 30.Hamilton TG, Klinghoffer RA, Corrin PD, Soriano P. Evolutionary divergence of platelet-derived growth factor alpha receptor signaling mechanisms. Mol. Cell Biol. 2003;23:4013–4025. doi: 10.1128/MCB.23.11.4013-4025.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.