Abstract
Numerous new and innovative approaches for repairing damaged myocardium are currently under investigation, with several encouraging results. In addition to the progression of stem cell-based approaches and gene therapy/silencing methods, evidence continues to emerge that protein therapeutics may be used to directly promote cardiac repair and even regeneration. However, proteins are often limited in their therapeutic potential by short local half-lives and insufficient bioavailability and/or bioactivity, and many academic laboratories studying cardiovascular diseases are more comfortable with molecular and cellular biology compared with protein biochemistry. Protein engineering has been employed broadly to overcome weaknesses traditionally associated with protein therapeutics and has the potential to specifically enhance the efficacy of molecules for cardiac repair. Yet protein engineering as a strategy has not yet been employed in the development of cardiovascular therapeutics to the degree that it has in other fields. In this review, we discuss the role of engineered proteins in cardiovascular therapies to date. Further, we address the promise of applying emerging protein engineering technologies to cardiovascular medicine and the barriers that must be overcome to enable the ultimate success of this approach.
Keywords: proteins, heart failure, peptides, heart diseases, receptors
An explosion of interest in new therapies for heart repair has occurred recently. Most notably, clinical trials of cell-based cardiovascular therapy have garnered much attention and preliminary results seem encouraging1, 2. However, the mechanism of action of cell-based therapies remains unclear, and many details with regard to characterization, quality control and delivery of cells remain to be worked out prior to widespread therapeutic application. Nonetheless, the reported efficacy of cardiac cell-based therapies in human trials has generated interest in additional therapeutic development pathways. Indeed, the identification of the release of paracrine-acting proteins as one mechanism by which cell-based therapies in the heart may improve cardiac function3 has spurred renewed interest in protein-based therapeutic approaches for cardiac repair.
Although protein therapeutics are increasingly becoming mainstream in numerous fields including cancer and inflammatory diseases – 9 of the 20 top selling drugs in 2012 were proteins4 – proteins have a relatively low penetration in the cardiovascular market. This is not to suggest a lack of progress in the cardiovascular therapeutic development, as small molecule drugs such as statins5, 6 and anticoagulants7 have been huge successes. Rather, as overall new drug approvals shift predominantly to proteins, protein therapeutic development represents an area of opportunity for cardiovascular medicine. Protein therapies allow for more targeted interventions – with well-defined regulatory pathways, improved scalability, and potentially reduced overall cost – compared to cell-based approaches. Furthermore, proteins have much larger surface areas than the small molecules of typical orally bioavailable drugs, and thus many of the molecular targets that are probably not “druggable” with small molecules can be targeted with proteins. A classic example of this concept is that no clinically successful small molecule activates the insulin receptor, and therefore Type I diabetes patients must inject insulin.
With regard to proteins for cardiovascular therapy, much effort has been put into enhancing cardiac microvasculature formation. More recently, the potential of proteins to induce cardiomyocyte proliferation has been identified8, increasing optimism that protein-based approaches can be effective for cardiac regeneration. However, protein therapeutics often have limitations, including poor bioavailability, undesirable pharmacokinetics and biodistribution profiles, and off-target effects9–12. One approach to overcome these deficiencies is protein engineering, a strategy that may not only render a therapeutic concept feasible, but may generate the intellectual property necessary for development in patients.
The term “protein engineering” is a general one that covers many techniques for modifying proteins, including the use of molecular display technologies to enable targeted delivery13, 14, structural modification of proteins to impart enhanced properties such as resistance to enzymatic degradation via rational design15 or directed evolution16, fusion of proteins to polymers or other protein domains to promote immune evasion17, 18, incorporation of desired properties or functional groups via non-canonical amino acids19, 20, and numerous others. Despite this promise and widespread application in fields such as oncology, engineered protein therapeutics have so far failed to become a major part of the cardiologist’s toolkit. Here we review the use of protein therapeutic approaches in the heart to date, with emphasis on therapies that have reached the clinical trial stage. We then highlight emerging and promising techniques in protein engineering and discuss how, when combined with new discoveries in molecular cardiology, these approaches might lead to a new generation of therapeutics for repairing the heart.
Protein Engineering for Cardiovascular Therapeutics to Date
Though none have yet achieved widespread clinical use, numerous engineered proteins have been applied to cardiovascular diseases in clinical trials. Many other native protein therapeutic approaches have also been applied toward cardiac repair, and several of these may benefit from protein engineering. Some of the earliest and most high profile examples of protein engineering technology for cardiovascular therapy have been clinical failures. However, other examples have proven more successful, and ongoing clinical trials offer exciting possibilities.
Tumor Necrosis Factor (TNF) Antagonists
One of the most prominent, and ultimately unsuccessful, examples of translation of protein engineering technology for cardiovascular therapy involved the TNF antagonist etanercept (Enbrel®). Developed based on research by Beutler and colleagues21, etanercept is a dimeric fusion protein comprising the Fc region of human Immunoglobulin G1 and the soluble human TNF Receptor 2 (TNFR2) domain that is approved for treatment of various forms of arthritis. Following the description of overexpression of TNF in heart failure22, it was hypothesized that TNF inhibition could be an effective treatment modality. However, despite initial promising results in carefully performed studies, both pre-clinically23, 24 and in patients25, 26, two large scale clinical trials revealed that not only did etanercept not show clinical benefit in chronic heart failure (CHF) patients, but it may in fact increase the risk of CHF-associated morbidity and mortality27. Moreover, the chimeric monoclonal antibody infliximab (Remicade®), a TNF inhibitor that works through a different mechanism than etanercept28, 29, also did not improve the clinical outcome from heart failure patients, with high doses showing profound adverse effects30. It should be noted that both etanercept and infliximab are efficacious against various forms of arthritis, and infliximab is highly effective against Crohn’s disease. Thus protein engineering-based antagonism of TNF has proven to be a case of a successful product development pathway that has so far been unsuccessfully applied to cardiovascular therapy despite compelling pre-clinical investigation.
Natriuretic Peptides
The natriuretic peptides offer an example of a promising application of protein engineering to cardiovascular therapy. Atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP), also known as brain natriuretic peptide and basic natriuretic peptide, have similar activities and are well established as useful biomarkers in the guidance of cardiovascular therapy31, 32. In addition, exogenous administration of BNP has been shown to improve multiple parameters in patients with systolic heart failure33. However, BNP as a therapeutic (nesritide) may be limited due to its hypotensive effects 34. C-type natriuretic protein (CNP) causes a lower hypotensive response than ANP and BNP, owing to its lack of activity on arteries due to differential receptor expression35. Additionally, a related peptide isolated from snake venom, Dendroaspis Natriuretic Peptide (DNP), acts similarly to ANP and BNP but is highly potent and resistant to enzymatic degradation36. In order to leverage the advantageous properties of CNP and DNP, Burnett and colleagues synthesized a chimeric natriuretic peptide that comprises domains of both (CD-NP)37. Initial results from clinical trials for this engineered protein as a heart failure therapy were promising38 and a follow-up trial may shed more light on the potential of CD-NP for clinical use39.
Insulin-like Growth Factor-1 (IGF-1)
IGF-1 is a protein with a similar molecular structure to insulin that has been shown to provide protection from the progression of heart failure in mice40. In humans, low serum levels of IGF-1 are associated with an increased risk of ischemic heart disease41. However the undesirable side effects of IGF-1 systemic delivery are well noted and include increased risk of diabetic retinopathy and cancer42–44. Thus, as of early 2013, there were only two active clinical trials examining IGF-1 (Mecasermin®) as a cardiovascular therapy45, 46. As an attempt to overcome these effects by promoting local delivery, Tokunou et. al. engineered an IGF-1 fusion with the heparin-binding (HB) domain of heparin-binding epidermal growth factor to make HB-IGF, which proved effective in stimulating chondrocyte biosynthesis47. Additionally, Hubbell and colleagues engineered a variant of IGF-1 with increased immobilization capacity within fibrin that improved smooth muscle cell proliferation48, introducing the possibility of a co-factorial local delivery approach. Notably, an IGF-1 modified to enable interaction with self-assembling peptides for cardiac delivery has demonstrated efficacy in improved cardiac function following MI49, 50. Thus, though not yet applied in clinical trials, engineered variants of IGF-1 may yield therapeutics for cardiovascular therapy.
Stromal cell-derived Factor-1α (SDF-1)
One protein under active clinical investigation for cardiac regeneration – although not currently as a protein therapy – is SDF-1. SDF-1 is a chemokine that plays important roles in angiogenesis and leukocyte trafficking51. The discovery that SDF-1 induces stem cell homing to the heart following injury52, 53 spurred interest in its therapeutic application. However, SDF-1 is proteolytically cleaved by both matrix metalloproteinase-2 (MMP-2)54, 55 and dipeptidyl peptidase IV56, and thus the likelihood of retained bioactivity in the myocardium following injury – a highly inflammatory environment – is low. For this and other reasons, the only active clinical trial of SDF-1 for cardiac therapy employs plasmid delivery57, 58, which offers the potential for prolonged SDF-1 expression but is also limited by issues of safety and unpredictability common to gene therapy approaches. Protein engineering applied to SDF-1 offers an alternative; we developed a protease-resistant form of SDF-1 by mutating a single amino acid within the MMP-2 cleavage site15. This protease-resistant SDF-1 successfully induced endothelial progenitor cell recruitment to the heart following MI that resulted in improved cardiac function15 and led to increased angiogenesis and improved ventricular function following onset of myocardial ischemia59. Protein engineering efforts to improve delivery and tissue retention of SDF-1 have also been reported60, as has the creation of a polypeptide analog of SDF-1 that induced improved recovery after MI compared to the native protein61. Future synergy of these and other protein engineering strategies may allow for a therapeutic approach that overcomes the limitations of the native SDF-1 protein.
Granulocyte Colony-Stimulating Factor (G-CSF)
G-CSF is a glycoprotein that selectively induces a reduction of SDF-1 and an increase in the SDF-1 receptor CXCR4 in the bone marrow62. A majority of clinical trials involving G-CSF in the heart have focused on its use as an adjunct to cell therapy63–65, but others have assessed its potential as a primary therapy66–69 as G-CSF has been shown to prevent deleterious remodeling after MI70. A potential next step in these active lines of therapeutic investigation could involve the use of fusion proteins incorporating G-CSF moieties, as improved functionality relating to delivery71 and stability72 of G-CSF have already been reported.
Interleukin (IL) Receptor Antagonists
Interleukins are proteins that induce pleiotropic effects in a wide variety of cell types73. In the heart, IL-1 is a potent mediator of inflammation during ischemia/reperfusion injury74 and elevated plasma levels of IL-6 have been linked with increased risk of future MI75, thus their inhibition is of therapeutic interest. IL-1 has a naturally occurring competitive inhibitor for its receptor (IL-1R), the IL-1 related protein IL-1 receptor antagonist (IL-1Ra), also known as anakinra76. Both forced overexpression and injections of anakinra have been shown to reduce apoptosis following heart injury in rodents74, 76, and this drug has been applied in clinical trials with promising results77. In addition, IL-6 inhibition has been pursued via antagonism of its receptor (IL-6R or CD126) by an engineered, humanized monoclonal antibody, tocilizumab. Encouragingly, a human case report of tocilizumab administration resulting in successful improvement of cardiac dysfunction associated with multicentric Castleman disease by has been published78, and a clinical trial investigating the efficacy of tocilizumab in MI is ongoing79. Thus interleukin receptor antagonists may represent a promising path forward for protein engineering applied to cardiovascular therapy.
Erythropoietin (EPO)
A glycoprotein that regulates red blood cell production, EPO has well-documented cardioprotective properties and works via multiple mechanisms80, 81. Pre-clinical studies in both rat82 and rabbit83 acute MI models showed that EPO administration improves cardiac contractility and hemodynamic parameters, prompting human clinical trials. Initial findings indicated that EPO, while failing to improve left ventricular ejection fraction, may reduce heart failure in acute MI patients84. However, a more recent trial has cast doubt on the potential for EPO as a therapy for heart failure, indicating that it may lead to increased infarct size85. Owing to these results, focus has shifted towards examining the efficacy of low doses of EPO86, an approach that might be aided by applying protein engineering techniques to improve potency and stability of EPO, as has already been demonstrated87.
Neuregulin-1β (NRG)
NRG is an essential regulator of cardiovascular development and plays an important role in cardiovascular disease88. The cardioprotective potential of NRG has been well established in pre-clinical models89, 90, and enthusiasm for future therapeutic application of NRG in heart failure increased upon the report of its potential ability to induce cardiomyocyte proliferation8. Clinical trials with the epidermal growth factor (EGF)-like domain of NRG in heart failure have demonstrated safety and efficacy91, 92, and another NRG molecule – glial growth factor-2 (GGF-2) – is currently in clinical trials for heart failure93 and has shown efficacy in a pre-clinical model94. The nature of the interactions between the cognate receptor of NRG in the heart, ErbB4, and its preferred signal induction partner, ErbB2, may provide an opportunity for exploitation via protein engineering. Our group hypothesized that ErbB receptor interactions, which are prerequisite to signaling, could be biased away from the most commonly induced partnerships by receptor-ligand affinity interactions imposed by a bivalent ErbB ligand95. Bivalent NRG (NN) was shown to induce differential signaling compared to NRG95 and has superior cardioprotective efficacy compared to the EGF-like domain of NRG in a mouse model of doxorubicin-induced cardiomyopathy96. Further exploration of this strategy may reveal a benefit of protein engineering for NRG in cardiac repair and regeneration.
Vascular Endothelial Growth Factor (VEGF) and Fibroblast Growth Factor (FGF)
Due to their well-characterized mitogenic effects on endothelial cells in many contexts, VEGF97 and FGF98 – as well as their splice isoforms and variants – have been examined extensively for therapeutic vascularization in the heart over the last ~25 years99–101. However, negative outcomes of early clinical trials with both FGF102 and VEGF103 have stalled progress toward clinical translation of therapeutic vascularization approaches, with common limitations such as short half-life and systemic side effects cited as reasons for the failures. Pre-clinical protein engineering studies of improved stability of angiogenic growth factors through a variety of techniques are plentiful104–110, and the potential for local delivery of VEGF via fusion with a collagen-binding domain has been reported111. Yet, there are still no protein drugs for therapeutic vascularization of the heart used in clinical practice. Strategies that combine protein engineering with nanotechnology109 or other methods to improve delivery49, 112–115 may hold promise to enable protein-based therapies for therapeutic vascularization in the heart in the future.
Additional Therapeutic Avenues
Beyond what is mentioned above, additional ongoing cardiovascular therapeutic efforts may benefit from protein engineering. Like NRG, periostin has also been reported to induce cardiomyocyte proliferation116 and thus could be of further therapeutic interest. Growth differentiating factor 11 (GDF11) has recently been identified as a mediator of the reversal of age-related cardiac hypertrophy117 and could open a new area of exploration for protein therapeutics for the heart and other organs. Glucagon-like peptide-1-based therapies have been effective pre-clinically118, and the cyclic peptide approach currently employed119 may lend itself to refinement through protein engineering. Biased G protein-coupled receptor ligands hold intriguing promise120 and represent a template for future protein engineering strategies designed to promote selective receptor activation, such as has already been done for the ErbB receptor system95. Adipokines are peptides or proteins secreted by adipocytes that can have beneficial or detrimental effects on the cardiovascular system depending on when and for how long they are exposed to it (reviewed in 121, 122). Protein engineering techniques that impart enhanced control over tissue localization and delivery of this class of peptides/proteins may prove critical in enabling their eventual therapeutic application. Further, intravenous immunoglobulin administration may be effective against certain forms of heart failure123. Engineering of proteins has perhaps been most extensively applied to immunoglobulin molecules, and so any effects observed in ongoing clinical trials124 could potentially be augmented through robust protein engineering approaches. Antibody-based therapeutic approaches as well as other protein therapies for immunomodulation continue to emerge as potential treatments for atherosclerosis125. More recently, mimetic peptides and monoclonal antibodies have been developed to inhibit PCSK9 to lower cholesterol levels126. All of the aforementioned approaches have promise, and the further application of protein engineering techniques to augment these therapeutic strategies could potentially enhance their efficacy.
Therapeutic Frontiers in Protein Engineering
Despite the implementation of some protein therapeutics for cardiovascular therapy, many limitations still remain. The ability to target delivery of a protein, or any drug, directly to the heart would minimize the required dose as well as undesirable off-target effects; but this ability remains mostly beyond our current reach. In general, proteins have often been considered poor drug candidates drugs owing to low oral bioavailability, a lack of long-term stability and other characteristics, and this applies to proteins for cardiovascular therapy as well. Indeed, a friend of the authors’ – an organic chemist currently employed in drug discovery by a major pharmaceutical company – revealed that many drug discovery professionals with chemistry training are taught a common mantra: “proteins are not drugs”. The biotechnology revolution has helped to change this perception, and protein engineering is a significant reason why, with fields such as oncology brimming with new engineered protein therapeutics. However, this revolution has, for the most part, occurred outside of the realm of cardiovascular medicine. The existing cardiovascular therapeutics created via protein engineering noted above demonstrate only a fraction of the potential of the field. Innovative and exciting protein engineering approaches are currently being developed, with many already having been applied to non-cardiovascular therapeutics. Application of these burgeoning technologies to cardiovascular medicine – for example, to enable heart-specific targeted drug delivery, to enhance protein stability and circulation times, or to promote direct stimulation of specific pathways to promote cardiomyogenesis via a therapeutic protein – may enhance the utility of protein-based cardiac therapy.
Molecular Display
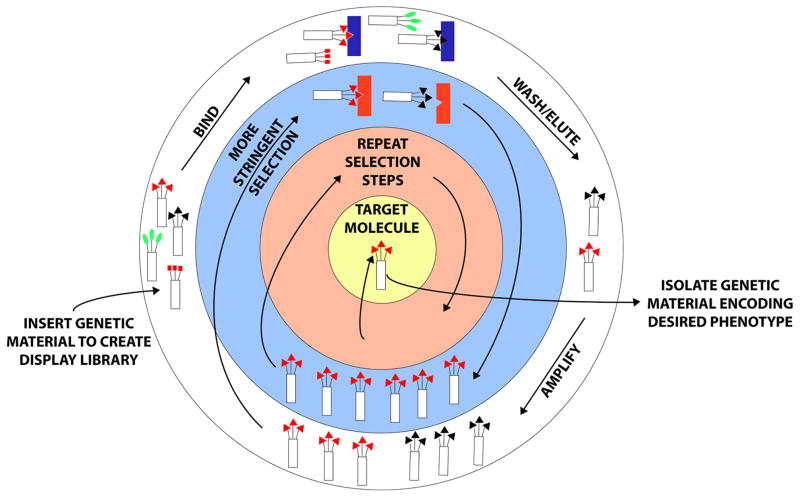
Molecular display encompasses techniques that present molecules – typically peptides or proteins – on the surface of a cell, virus, or other host entity. This approach enables a linkage of genotype and phenotype of the displayed molecule, as its coding information is hybridized with that of the carrier. Thus, selective enrichment is possible through successive propagation steps under specified conditions – a process known as panning – and large polypeptide libraries can be screened for desired properties or interactions (Figure 1).
Figure 1.
Molecular Display. A protein or peptide with specific properties can be derived via molecular display following multiple rounds of selection. Polypeptide libraries can be made or purchased and either inserted into molecular hosts such as phage or yeast or appended to carrier molecules such as mRNA or ribosomes. The schematic above is representative of phage or cell-surface display, where proteins are displayed on the surfaces of organisms that are subsequently exposed to various substrates, which serve as selection criteria. In the example above, protein-mediated binding of the organism with a specific substrate followed by washing away of non-specific binders and elution enables amplification of those organisms displaying proteins with the desired properties. Repetitive screening with additional or more stringent selection criteria enables ultimate isolation of an optimized protein sequence based on phenotype, which can then be linked with genotype following extraction of genetic material from the organism. Thus, molecular display facilitates discovery of information needed to design a protein with specific properties evolved to match nearly any chosen selection criteria.
The concept of molecular display arose following George Smith’s seminal report of phage display, the display of a foreign protein on the surface of filamentous phage13. Since then, numerous platform approaches for molecular display have been developed to enable high throughput screening of protein interactions. Technologies such as cell-surface display, ribosomal display, mRNA display and others have allowed for directed evolution of proteins to refine activity and stability. Phage display is the most commonly employed and is popular in the biotechnology industry, having been used in the development of numerous protein drugs127, especially antibodies128. Beyond directed evolution, phage display has been employed for panning of biological and/or tissue samples – known as biopanning – to select for peptide ligands that enable tissue-specific homing129 and/or enhanced tissue retention130 of drugs or drug carriers.
In addition to phage display, other display technologies have emerged and may have relevance for the development of cardiovascular therapies in the near future. Invented by Wittrup and colleagues14, yeast surface display enables, amongst other things, high throughput quantitative library screening via fluorescent-activated cell sorting (FACS) and has been used to evolve extremely high affinity antibodies131 and peptides132 that have facilitated molecular targeting, for example, to promote high-resolution vascular imaging133. Cell-free protein evolution methods such as ribosome display134 or mRNA display135 are not encumbered by transformation or expression limitations and also allow rapid evolution of high-affinity binding proteins. Overall, molecular display technologies are generally mature methods for selection and refinement of protein characteristics that have significant potential to promote cardiovascular protein therapies.
Engineered Protein Scaffolds
Antibodies have been the most successful protein therapeutics to date. However, antibodies have weaknesses as therapeutics, including limited tissue penetration due to their large size. To address this and other issues, numerous ‘engineered protein scaffolds’ (EPS) have been developed. For the purposes of protein engineering, EPS comprise a minimal polypeptide framework that can be based on either an immunoglobulin or a non-immunoglobulin molecule136. The framework is typically monomeric without disulfide bonds or glycosylation sites and is usually highly stable and readily soluble137. EPS are also typically able to be easily expressed in a host organism and have surface-accessible residues that allow incorporation of sequence diversity and subsequent selection via molecular display.
Numerous EPS have moved beyond the initial development stage towards clinical use137. Amongst the most well-developed are: Adnectins138, which are derived from type III fibronectin domains; Anticalins139, derived from lipocalin; Kunitz domains140, derived from Kunitz-type protease inhibitors; DARPins141, derived from ankyrin repeat proteins; Avimers142, derived from the A-domain of low density lipoprotein receptor; and many more. Ecallantide (Kalbitor®), a rationally designed Kunitz domain, was approved in 2009 for clinical use to treat hereditary angioedema143 and could potentially be used in cardiothoracic surgery as a replacement for aprotinin. Many other EPS are being tested for myriad applications137, and as molecular targets for cardiac repair and regeneration continue to be defined, EPS have the potential to play an important role in future development of cardiovascular therapeutics.
Non-Canonical Amino Acids (ncAAs)
The concept of incorporating non-canonical amino acids (also referred to as unnatural amino acids, non-natural amino acids, and artificial amino acids) site-specifically into proteins offers stunning potential: an avenue to attach any chemical functional group of interest to a protein, allowing for coupling with other proteins or molecules such as fluorophores or small molecule drugs using straightforward, efficient, and tunable chemical reactions. Although several variations exist, the general approach involves aminoacylation of a tRNA that has an incorporated suppressor anticodon with a ncAA. This chemical modification facilitates site-specific ncAA incorporation during mRNA translation at a nonsense codon site, which can be inserted (site-specifically) in a gene of interest using standard gene synthesis or molecular biology techniques144 (Figure 2). This development of ncAA technology was pioneered by Schultz and colleagues19, and this group and many others have contributed to important advances that have made it robust for imparting desired functionality to proteins for therapeutic applications. For example, ncAA incorporation enables site-specific PEGylation of therapeutic proteins145 to enhance evasion of the immune system and promote longer circulation times. This approach also allows for insertion of molecular staples – hydrocarbon linkers that can stabilize protein secondary structure in part by physically preventing entropic structural relaxation – into proteins to improve stability146. This strategy could be extended via expressed protein ligation techniques147 to fuse other engineered proteins for similar purposes148. Using a related process developed by Tirrell and colleagues, residue-specific ncAA incorporation, Interferonβ-1b was PEGylated for improved pharmacological properties149, 150. ncAA site-specific incorporation has further been utilized to generate bispecific antibodies151 and thus could be adapted to the creation of bivalent NRG molecules95 that might stimulate cardiomyogenesis. As ncAA technology continues to evolve, its potential for application towards cardiovascular therapy, which is already promising, should only be enhanced.
Figure 2.
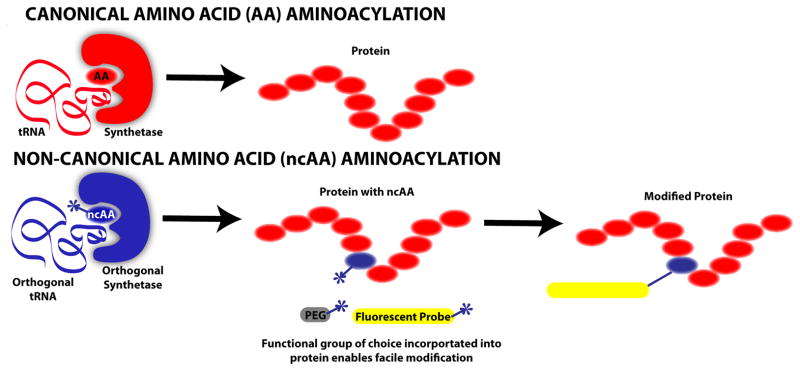
Incorporation of non-canonical amino acids (ncAA) into proteins. ncAA incorporation into proteins has been shown via multiple methods. The schematic above contrasts canonical amino acid (AA) incorporation into proteins with site-specific ncAA incorporation. Canonical AA incorporation proceeds following aminoacylation catalyzed by an endogenous aminoacyl-tRNA synthetase, which charges tRNA with a cognate amino acid. ncAA aminoacylation is possible via incorporation of a heterologous, orthogonal (i.e. not cross-reactive with host cell machinery) tRNA:synthetase pair into the cell responsible for protein production. Due to this incorporation, an orthogonal ncAA can be inserted site-specifically in response to a specific codon (typically a stop codon). A given ncAA typically has similar structure to a canonical AA except that a desired, atypical chemical functional group is included such that it is accessible to participate in a reaction. Thus, once translated, a protein incorporating a ncAA is readily modifiable with molecules such as polyethylene glycol (PEG), which improves circulation and limits immediate renal clearance, or with a molecule such as a fluorescent probe that facilitates imaging, as well as many other potential possibilities.
Enabling Methods for Drug Delivery
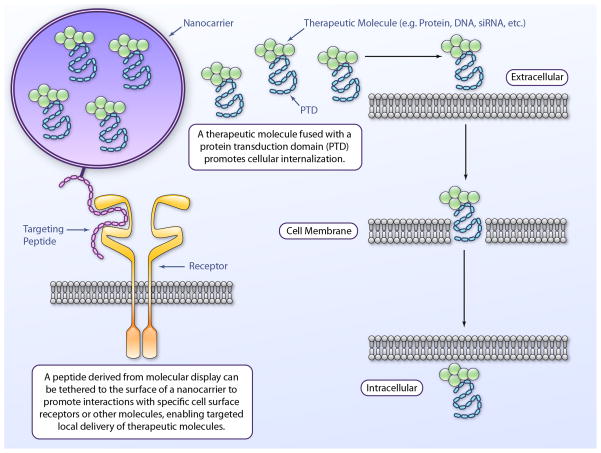
Protein engineering techniques have begun to be used for the development of proteins and peptides that enable more effective and efficient small molecule, protein and nucleic acid delivery across biological barriers and/or targeted to specific tissues. This is a critical consideration, as effective cardiovascular protein therapies will almost certainly require targeted or localized delivery to the heart. Some of these strategies have already been mentioned or implied, such as the functionalization of a therapeutic protein or a micro- or nanocarrier with a targeting sequence derived by molecular display or from an engineered protein scaffold. This general concept has also been applied to enable gene delivery with enhanced tissue specificity152. In addition, Dowdy and coworkers developed the concept of protein transduction domain (PTD)-containing fusion proteins for protein delivery153. In this method, the protein of interest is fused with a PTD – sometimes also referred to as a cell-penetrating peptide – such as that derived from the human immunodeficiency virus TAT protein153. This allows, amongst other things, proteins to cross biological barriers that would normally restrict them due to size and charge considerations154 (Figure 3). PTDs have also been used in gene delivery155. A conceptually similar fusion protein approach whereby the protein of interest is fused with an antibody or peptide that is transported across a biological barrier, such as the blood-brain barrier, by receptor-mediated transport has also been explored156 and is sometimes referred to as the “molecular Trojan horse” approach157. Some of these techniques have already been applied to facilitate targeted delivery to the myocardium158, and continued expansion of these and other efforts159 will likely prove critical to the ultimate success of protein-based cardiovascular therapies.
Figure 3.
Protein engineering methods to enable drug delivery. Numerous techniques within the realm of protein engineering could be employed to enhance delivery of small molecules, proteins and nucleic acids to the heart. Examples shown above include the targeting of a nanocarrier to a cell type of interest (e.g. cardiomyocytes, endothelial cells, etc.) by a peptide molecule derived via molecular display and the enhancement of cellular internalization of a therapeutic entity via fusion with a protein transduction domain (PTD).
Enabling Technologies for Cell-Based Therapy
In addition to providing an alternative for cell-based cardiac therapy, protein engineering can also be used to augment this therapeutic approach. Cardiac cell transplantation has been limited by poor localization of injected cells160, an ~90% death rate for cells within one week of the injection or implantation161, and, in the case of stem cells, uncontrolled cell proliferation or differentiation following transplantation162. Some of these limitations have been overcome in the most recent clinical trials1, 2, however the efficiency and efficacy of cell-based cardiac therapy can still be improved. Our group has employed a therapeutic approach utilizing molecularly designed self-assembling peptide scaffolds combined with engineered proteins to create a microenvironment for improved engraftment and regenerative potential of transplanted cells for myocardial regeneration15, 49, 113. In this case, self-assembly occurs through electrostatic interactions between heterospecific, complementary amino acid sequences; a peptide scaffold is engineered to express one sequence and the therapeutic protein entity is engineered to express the complement. This approach allows for nearly any chosen growth factor(s) to be controllably displayed or delivered along with cells, which may bind or otherwise interact with the peptide scaffold. In this way, differentiation and/or engraftment of transplanted cells can be improved. Moreover, the starting materials are easy to synthesize and purify on a large scale. This and other methods for enabling cell-based therapies for the heart provide yet another therapeutic frontier for cardiovascular protein engineering.
CONCLUSION
Protein engineering is a powerful approach with untapped potential for cardiovascular therapeutic development. As previously stated, about half of new drugs are proteins4, and many of these therapeutics were created via protein engineering. The application of this technology to therapeutic development should only increase in the future, and yet no engineered protein drugs are currently being routinely applied for treatment of cardiovascular diseases, perhaps owing to deficiencies in our current understanding of the molecular mechanisms of cardiac repair and regeneration, which is still evolving (the authors direct the reader to several extensive recent reviews on the topic163–166). From the standpoint of therapeutic development, two phenomena that contribute to myocardial repair and regeneration have been the focus of much of the effort: vascularization and cardiomyogenesis. While the fundamental processes that constitute vascularization – angiogenesis, arteriogenesis, and vasculogenesis – are relatively well defined, the mechanistic underpinnings of cardiomyogenesis remain the subject of controversy. Hopefully, advances in the molecular cardiology field and resolution of fundamental controversies within it will facilitate a leap forward for cardiovascular medicine through increased utilization of engineered protein therapeutics.
Acknowledgments
The authors apologize to those whose work could not be cited due to space limitations.
FUNDING SOURCES
Funding was provided by NIH grants K99 HL112905 (S.M.J.), AG040019 (R.T.L) and AG032977 (R.T.L.) and by the Harvard Stem Cell Institute (R.T.L).
Non-standard Abbreviations and Acronyms
- CHF
chronic heart failure
- HB
heparin-binding
- MI
myocardial infarction
- mRNA
messenger ribonucleic acid
- PEG
poly(ethylene glycol)
- TAT
trans-activator of transcription
- tRNA
transfer ribonucleic acid
Footnotes
DISCLOSURES
Brigham and Women’s Hospital has filed for patents pertaining to the described bivalent ligand technology, listing S.M.J. and R.T.L. as inventors. R.T.L. is a co-founder and co-owner of Provasculon, Inc., is a paid consultant to the company and serves on the company’s Board of Directors. Provasculon has interests in SDF-1 therapy, an area related to the topics discussed in this review. R.T.L.’s interests were reviewed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their institutional policies.
References
- 1.Bolli R, Chugh AR, D’Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (scipio): Initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Chugh AR, Beache GM, Loughran JH, Mewton N, Elmore JB, Kajstura J, Pappas P, Tatooles A, Stoddard MF, Lima JA, Slaughter MS, Anversa P, Bolli R. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: The scipio trial: Surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126:S54–64. doi: 10.1161/CIRCULATIONAHA.112.092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mirotsou M, Jayawardena TM, Schmeckpeper J, Gnecchi M, Dzau VJ. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J Mol Cell Cardiol. 2011;50:280–289. doi: 10.1016/j.yjmcc.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.List of top selling drugs for 2012. http://www.drugs.com/stats/top100/sales.
- 5.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated c-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 7.Sabatine MS, Cannon CP, Gibson CM, Lopez-Sendon JL, Montalescot G, Theroux P, Claeys MJ, Cools F, Hill KA, Skene AM, McCabe CH, Braunwald E. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with st-segment elevation. N Engl J Med. 2005;352:1179–1189. doi: 10.1056/NEJMoa050522. [DOI] [PubMed] [Google Scholar]
- 8.Bersell K, Arab S, Haring B, Kuhn B. Neuregulin1/erbb4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 9.Mahmood I, Green MD. Pharmacokinetic and pharmacodynamic considerations in the development of therapeutic proteins. Clin Pharmacokinet. 2005;44:331–347. doi: 10.2165/00003088-200544040-00001. [DOI] [PubMed] [Google Scholar]
- 10.Arakawa T, Prestrelski SJ, Kenney WC, Carpenter JF. Factors affecting short-term and long-term stabilities of proteins. Adv Drug Deliv Rev. 2001;46:307–326. doi: 10.1016/s0169-409x(00)00144-7. [DOI] [PubMed] [Google Scholar]
- 11.Tang L, Persky AM, Hochhaus G, Meibohm B. Pharmacokinetic aspects of biotechnology products. J Pharm Sci. 2004;93:2184–2204. doi: 10.1002/jps.20125. [DOI] [PubMed] [Google Scholar]
- 12.Sola RJ, Griebenow K. Glycosylation of therapeutic proteins: An effective strategy to optimize efficacy. BioDrugs. 2011;24:9–21. doi: 10.2165/11530550-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith GP. Filamentous fusion phage: Novel expression vectors that display cloned antigens on the virion surface. Science. 1985;228:1315–1317. doi: 10.1126/science.4001944. [DOI] [PubMed] [Google Scholar]
- 14.Boder ET, Wittrup KD. Yeast surface display for screening combinatorial polypeptide libraries. Nat Biotechnol. 1997;15:553–557. doi: 10.1038/nbt0697-553. [DOI] [PubMed] [Google Scholar]
- 15.Segers VF, Tokunou T, Higgins LJ, MacGillivray C, Gannon J, Lee RT. Local delivery of protease-resistant stromal cell derived factor-1 for stem cell recruitment after myocardial infarction. Circulation. 2007;116:1683–1692. doi: 10.1161/CIRCULATIONAHA.107.718718. [DOI] [PubMed] [Google Scholar]
- 16.Roberts BL, Markland W, Ley AC, Kent RB, White DW, Guterman SK, Ladner RC. Directed evolution of a protein: Selection of potent neutrophil elastase inhibitors displayed on m13 fusion phage. Proc Natl Acad Sci U S A. 1992;89:2429–2433. doi: 10.1073/pnas.89.6.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abuchowski A, van Es T, Palczuk NC, Davis FF. Alteration of immunological properties of bovine serum albumin by covalent attachment of polyethylene glycol. J Biol Chem. 1977;252:3578–3581. [PubMed] [Google Scholar]
- 18.Abuchowski A, McCoy JR, Palczuk NC, van Es T, Davis FF. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J Biol Chem. 1977;252:3582–3586. [PubMed] [Google Scholar]
- 19.Noren CJ, Anthony-Cahill SJ, Griffith MC, Schultz PG. A general method for site-specific incorporation of unnatural amino acids into proteins. Science. 1989;244:182–188. doi: 10.1126/science.2649980. [DOI] [PubMed] [Google Scholar]
- 20.Kiick KL, Saxon E, Tirrell DA, Bertozzi CR. Incorporation of azides into recombinant proteins for chemoselective modification by the staudinger ligation. Proc Natl Acad Sci U S A. 2002;99:19–24. doi: 10.1073/pnas.012583299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peppel K, Crawford D, Beutler B. A tumor necrosis factor (tnf) receptor-igg heavy chain chimeric protein as a bivalent antagonist of tnf activity. J Exp Med. 1991;174:1483–1489. doi: 10.1084/jem.174.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torre-Amione G, Kapadia S, Lee J, Durand JB, Bies RD, Young JB, Mann DL. Tumor necrosis factor-alpha and tumor necrosis factor receptors in the failing human heart. Circulation. 1996;93:704–711. doi: 10.1161/01.cir.93.4.704. [DOI] [PubMed] [Google Scholar]
- 23.Nakano M, Knowlton AA, Dibbs Z, Mann DL. Tumor necrosis factor-alpha confers resistance to hypoxic injury in the adult mammalian cardiac myocyte. Circulation. 1998;97:1392–1400. doi: 10.1161/01.cir.97.14.1392. [DOI] [PubMed] [Google Scholar]
- 24.Kurrelmeyer KM, Michael LH, Baumgarten G, Taffet GE, Peschon JJ, Sivasubramanian N, Entman ML, Mann DL. Endogenous tumor necrosis factor protects the adult cardiac myocyte against ischemic-induced apoptosis in a murine model of acute myocardial infarction. Proc Natl Acad Sci U S A. 2000;97:5456–5461. doi: 10.1073/pnas.070036297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deswal A, Bozkurt B, Seta Y, Parilti-Eiswirth S, Hayes FA, Blosch C, Mann DL. Safety and efficacy of a soluble p75 tumor necrosis factor receptor (enbrel, etanercept) in patients with advanced heart failure. Circulation. 1999;99:3224–3226. doi: 10.1161/01.cir.99.25.3224. [DOI] [PubMed] [Google Scholar]
- 26.Fichtlscherer S, Rossig L, Breuer S, Vasa M, Dimmeler S, Zeiher AM. Tumor necrosis factor antagonism with etanercept improves systemic endothelial vasoreactivity in patients with advanced heart failure. Circulation. 2001;104:3023–3025. doi: 10.1161/hc5001.101749. [DOI] [PubMed] [Google Scholar]
- 27.Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, Djian J, Drexler H, Feldman A, Kober L, Krum H, Liu P, Nieminen M, Tavazzi L, van Veldhuisen DJ, Waldenstrom A, Warren M, Westheim A, Zannad F, Fleming T. Targeted anticytokine therapy in patients with chronic heart failure: Results of the randomized etanercept worldwide evaluation (renewal) Circulation. 2004;109:1594–1602. doi: 10.1161/01.CIR.0000124490.27666.B2. [DOI] [PubMed] [Google Scholar]
- 28.Knight DM, Trinh H, Le J, Siegel S, Shealy D, McDonough M, Scallon B, Moore MA, Vilcek J, Daddona P, et al. Construction and initial characterization of a mouse-human chimeric anti-tnf antibody. Mol Immunol. 1993;30:1443–1453. doi: 10.1016/0161-5890(93)90106-l. [DOI] [PubMed] [Google Scholar]
- 29.Scallon BJ, Moore MA, Trinh H, Knight DM, Ghrayeb J. Chimeric anti-tnf-alpha monoclonal antibody ca2 binds recombinant transmembrane tnf-alpha and activates immune effector functions. Cytokine. 1995;7:251–259. doi: 10.1006/cyto.1995.0029. [DOI] [PubMed] [Google Scholar]
- 30.Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: Results of the anti-tnf therapy against congestive heart failure (attach) trial. Circulation. 2003;107:3133–3140. doi: 10.1161/01.CIR.0000077913.60364.D2. [DOI] [PubMed] [Google Scholar]
- 31.Troughton RW, Frampton CM, Yandle TG, Espiner EA, Nicholls MG, Richards AM. Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (n-bnp) concentrations. Lancet. 2000;355:1126–1130. doi: 10.1016/s0140-6736(00)02060-2. [DOI] [PubMed] [Google Scholar]
- 32.Burnett JC, Jr, Kao PC, Hu DC, Heser DW, Heublein D, Granger JP, Opgenorth TJ, Reeder GS. Atrial natriuretic peptide elevation in congestive heart failure in the human. Science. 1986;231:1145–1147. doi: 10.1126/science.2935937. [DOI] [PubMed] [Google Scholar]
- 33.Chen HH, Glockner JF, Schirger JA, Cataliotti A, Redfield MM, Burnett JC., Jr Novel protein therapeutics for systolic heart failure: Chronic subcutaneous b-type natriuretic peptide. J Am Coll Cardiol. 2012;60:2305–2312. doi: 10.1016/j.jacc.2012.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sackner-Bernstein JD, Skopicki HA, Aaronson KD. Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation. 2005;111:1487–1491. doi: 10.1161/01.CIR.0000159340.93220.E4. [DOI] [PubMed] [Google Scholar]
- 35.Wei CM, Aarhus LL, Miller VM, Burnett JC., Jr Action of c-type natriuretic peptide in isolated canine arteries and veins. Am J Physiol. 1993;264:H71–73. doi: 10.1152/ajpheart.1993.264.1.H71. [DOI] [PubMed] [Google Scholar]
- 36.Chen HH, Lainchbury JG, Burnett JC., Jr Natriuretic peptide receptors and neutral endopeptidase in mediating the renal actions of a new therapeutic synthetic natriuretic peptide dendroaspis natriuretic peptide. J Am Coll Cardiol. 2002;40:1186–1191. doi: 10.1016/s0735-1097(02)02127-7. [DOI] [PubMed] [Google Scholar]
- 37.Lisy O, Huntley BK, McCormick DJ, Kurlansky PA, Burnett JC., Jr Design, synthesis, and actions of a novel chimeric natriuretic peptide: Cd-np. J Am Coll Cardiol. 2008;52:60–68. doi: 10.1016/j.jacc.2008.02.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rose RA. Cd-np, a chimeric natriuretic peptide for the treatment of heart failure. Curr Opin Investig Drugs. 2010;11:349–356. [PubMed] [Google Scholar]
- 39. [02/26/13];Effects of chimeric natriuretic peptide versus bnp versus placebo in stable heart failure and moderate renal dysfunction. http://www.clinicaltrials.gov/ct2/show/NCT01407900.
- 40.Li Q, Li B, Wang X, Leri A, Jana KP, Liu Y, Kajstura J, Baserga R, Anversa P. Overexpression of insulin-like growth factor-1 in mice protects from myocyte death after infarction, attenuating ventricular dilation, wall stress, and cardiac hypertrophy. J Clin Invest. 1997;100:1991–1999. doi: 10.1172/JCI119730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Juul A, Scheike T, Davidsen M, Gyllenborg J, Jorgensen T. Low serum insulin-like growth factor i is associated with increased risk of ischemic heart disease: A population-based case-control study. Circulation. 2002;106:939–944. doi: 10.1161/01.cir.0000027563.44593.cc. [DOI] [PubMed] [Google Scholar]
- 42.Jabri N, Schalch DS, Schwartz SL, Fischer JS, Kipnes MS, Radnik BJ, Turman NJ, Marcsisin VS, Guler HP. Adverse effects of recombinant human insulin-like growth factor i in obese insulin-resistant type ii diabetic patients. Diabetes. 1994;43:369–374. doi: 10.2337/diab.43.3.369. [DOI] [PubMed] [Google Scholar]
- 43.Pollak M, Beamer W, Zhang JC. Insulin-like growth factors and prostate cancer. Cancer Metastasis Rev. 1998;17:383–390. doi: 10.1023/a:1006154108619. [DOI] [PubMed] [Google Scholar]
- 44.Wilkinson-Berka JL, Wraight C, Werther G. The role of growth hormone, insulin-like growth factor and somatostatin in diabetic retinopathy. Curr Med Chem. 2006;13:3307–3317. doi: 10.2174/092986706778773086. [DOI] [PubMed] [Google Scholar]
- 45. [02/24/13];Evaluation of the safety and efficacy of using insulin-like growth factor-1 in patients with a heart attack (resus-ami) http://www.clinicaltrials.gov/ct2/show/NCT01438086.
- 46. [02/24/13];Treatment of the low igf-1 syndrome associated with chronic heart failure: A randomized, placebo-controlled, double-blind study. (tosca2) http://www.clinicaltrials.gov/ct2/show/NCT01235273.
- 47.Tokunou T, Miller R, Patwari P, Davis ME, Segers VF, Grodzinsky AJ, Lee RT. Engineering insulin-like growth factor-1 for local delivery. FASEB J. 2008;22:1886–1893. doi: 10.1096/fj.07-100925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lorentz KM, Yang L, Frey P, Hubbell JA. Engineered insulin-like growth factor-1 for improved smooth muscle regeneration. Biomaterials. 2012;33:494–503. doi: 10.1016/j.biomaterials.2011.09.088. [DOI] [PubMed] [Google Scholar]
- 49.Davis ME, Hsieh PC, Takahashi T, Song Q, Zhang S, Kamm RD, Grodzinsky AJ, Anversa P, Lee RT. Local myocardial insulin-like growth factor 1 (igf-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc Natl Acad Sci U S A. 2006;103:8155–8160. doi: 10.1073/pnas.0602877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Padin-Iruegas ME, Misao Y, Davis ME, Segers VF, Esposito G, Tokunou T, Urbanek K, Hosoda T, Rota M, Anversa P, Leri A, Lee RT, Kajstura J. Cardiac progenitor cells and biotinylated insulin-like growth factor-1 nanofibers improve endogenous and exogenous myocardial regeneration after infarction. Circulation. 2009;120:876–887. doi: 10.1161/CIRCULATIONAHA.109.852285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kucia M, Jankowski K, Reca R, Wysoczynski M, Bandura L, Allendorf DJ, Zhang J, Ratajczak J, Ratajczak MZ. Cxcr4-sdf-1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol. 2004;35:233–245. doi: 10.1023/b:hijo.0000032355.66152.b8. [DOI] [PubMed] [Google Scholar]
- 52.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, Penn MS. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 53.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 54.McQuibban GA, Gong JH, Tam EM, McCulloch CA, Clark-Lewis I, Overall CM. Inflammation dampened by gelatinase a cleavage of monocyte chemoattractant protein-3. Science. 2000;289:1202–1206. doi: 10.1126/science.289.5482.1202. [DOI] [PubMed] [Google Scholar]
- 55.McQuibban GA, Butler GS, Gong JH, Bendall L, Power C, Clark-Lewis I, Overall CM. Matrix metalloproteinase activity inactivates the cxc chemokine stromal cell-derived factor-1. J Biol Chem. 2001;276:43503–43508. doi: 10.1074/jbc.M107736200. [DOI] [PubMed] [Google Scholar]
- 56.De La Luz Sierra M, Yang F, Narazaki M, Salvucci O, Davis D, Yarchoan R, Zhang HH, Fales H, Tosato G. Differential processing of stromal-derived factor-1alpha and stromal-derived factor-1beta explains functional diversity. Blood. 2004;103:2452–2459. doi: 10.1182/blood-2003-08-2857. [DOI] [PubMed] [Google Scholar]
- 57. [02/24/13];Study to evaluate the safety and efficacy of jvs-100 administered to adults with critical limb ischemia. http://www.clinicaltrials.gov/ct2/show/NCT01410331.
- 58.Penn MS, Mendelsohn FO, Schaer GL, Sherman W, Farr M, Pastore J, Rouy D, Clemens R, Aras R, Losordo DW. An open label dose escalation study to evaluate the safety of administration of non-viral sdf-1 plasmid to treat symptomatic ischemic heart failure. Circ Res. 2013 doi: 10.1161/CIRCRESAHA.111.300440. [DOI] [PubMed] [Google Scholar]
- 59.Kanki S, Segers VF, Wu W, Kakkar R, Gannon J, Sys SU, Sandrasagra A, Lee RT. Stromal cell-derived factor-1 retention and cardioprotection for ischemic myocardium. Circ Heart Fail. 2011;4:509–518. doi: 10.1161/CIRCHEARTFAILURE.110.960302. [DOI] [PubMed] [Google Scholar]
- 60.Ziegler M, Elvers M, Baumer Y, Leder C, Ochmann C, Schonberger T, Jurgens T, Geisler T, Schlosshauer B, Lunov O, Engelhardt S, Simmet T, Gawaz M. The bispecific sdf1-gpvi fusion protein preserves myocardial function after transient ischemia in mice. Circulation. 2012;125:685–696. doi: 10.1161/CIRCULATIONAHA.111.070508. [DOI] [PubMed] [Google Scholar]
- 61.Hiesinger W, Perez-Aguilar JM, Atluri P, Marotta NA, Frederick JR, Fitzpatrick JR, 3rd, McCormick RC, Muenzer JR, Yang EC, Levit RD, Yuan LJ, Macarthur JW, Saven JG, Woo YJ. Computational protein design to reengineer stromal cell-derived factor-1alpha generates an effective and translatable angiogenic polypeptide analog. Circulation. 2011;124:S18–26. doi: 10.1161/CIRCULATIONAHA.110.009431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, Ponomaryov T, Taichman RS, Arenzana-Seisdedos F, Fujii N, Sandbank J, Zipori D, Lapidot T. G-csf induces stem cell mobilization by decreasing bone marrow sdf-1 and up-regulating cxcr4. Nat Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 63.Kang HJ, Kim HS, Zhang SY, Park KW, Cho HJ, Koo BK, Kim YJ, Soo Lee D, Sohn DW, Han KS, Oh BH, Lee MM, Park YB. Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: The magic cell randomised clinical trial. Lancet. 2004;363:751–756. doi: 10.1016/S0140-6736(04)15689-4. [DOI] [PubMed] [Google Scholar]
- 64.Zohlnhofer D, Ott I, Mehilli J, Schomig K, Michalk F, Ibrahim T, Meisetschlager G, von Wedel J, Bollwein H, Seyfarth M, Dirschinger J, Schmitt C, Schwaiger M, Kastrati A, Schomig A. Stem cell mobilization by granulocyte colony-stimulating factor in patients with acute myocardial infarction: A randomized controlled trial. JAMA. 2006;295:1003–1010. doi: 10.1001/jama.295.9.1003. [DOI] [PubMed] [Google Scholar]
- 65.Ripa RS, Jorgensen E, Wang Y, Thune JJ, Nilsson JC, Sondergaard L, Johnsen HE, Kober L, Grande P, Kastrup J. Stem cell mobilization induced by subcutaneous granulocyte-colony stimulating factor to improve cardiac regeneration after acute st-elevation myocardial infarction: Result of the double-blind, randomized, placebo-controlled stem cells in myocardial infarction (stemmi) trial. Circulation. 2006;113:1983–1992. doi: 10.1161/CIRCULATIONAHA.105.610469. [DOI] [PubMed] [Google Scholar]
- 66. [02/25/13];Filgrastim for the promotion of collateral growth in patients with cad. http://www.clinicaltrials.gov/ct2/show/NCT00596479.
- 67. [02/25/13];Peg-granulocyte-colony stimulating factor (gcsf) for coronary collateral growth in coronary artery disease patients. http://www.clinicaltrials.gov/ct2/show/NCT00886509.
- 68. [02/25/13];Sitagliptin plus granulocyte-colony stimulating factor in acute myocardial infarction (sitagrami) http://www.clinicaltrials.gov/ct2/show/NCT00650143.
- 69. [02/25/13];The effect of granulocyte colony stimulating factor (g-csf) on myocardial function after acute anterior myocardial infarction, a prospective double blind randomized placebo controlled study. http://www.clinicaltrials.gov/ct2/show/NCT00756756.
- 70.Harada M, Qin Y, Takano H, Minamino T, Zou Y, Toko H, Ohtsuka M, Matsuura K, Sano M, Nishi J, Iwanaga K, Akazawa H, Kunieda T, Zhu W, Hasegawa H, Kunisada K, Nagai T, Nakaya H, Yamauchi-Takihara K, Komuro I. G-csf prevents cardiac remodeling after myocardial infarction by activating the jak-stat pathway in cardiomyocytes. Nat Med. 2005;11:305–311. doi: 10.1038/nm1199. [DOI] [PubMed] [Google Scholar]
- 71.Bai Y, Ann DK, Shen WC. Recombinant granulocyte colony-stimulating factor-transferrin fusion protein as an oral myelopoietic agent. Proc Natl Acad Sci U S A. 2005;102:7292–7296. doi: 10.1073/pnas.0500062102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang YS, Wen XF, Wu YL, Wang YF, Fan M, Yang ZY, Liu W, Zhou LF. Engineering a pharmacologically superior form of granulocyte-colony-stimulating factor by fusion with gelatin-like-protein polymer. Eur J Pharm Biopharm. 2010;74:435–441. doi: 10.1016/j.ejpb.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 73.Bocci V. Interleukins. Clinical pharmacokinetics and practical implications. Clin Pharmacokinet. 1991;21:274–284. doi: 10.2165/00003088-199121040-00004. [DOI] [PubMed] [Google Scholar]
- 74.Suzuki K, Murtuza B, Smolenski RT, Sammut IA, Suzuki N, Kaneda Y, Yacoub MH. Overexpression of interleukin-1 receptor antagonist provides cardioprotection against ischemia-reperfusion injury associated with reduction in apoptosis. Circulation. 2001;104:I308–I303. doi: 10.1161/hc37t1.094871. [DOI] [PubMed] [Google Scholar]
- 75.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 76.Abbate A, Salloum FN, Vecile E, Das A, Hoke NN, Straino S, Biondi-Zoccai GG, Houser JE, Qureshi IZ, Ownby ED, Gustini E, Biasucci LM, Severino A, Capogrossi MC, Vetrovec GW, Crea F, Baldi A, Kukreja RC, Dobrina A. Anakinra, a recombinant human interleukin-1 receptor antagonist, inhibits apoptosis in experimental acute myocardial infarction. Circulation. 2008;117:2670–2683. doi: 10.1161/CIRCULATIONAHA.107.740233. [DOI] [PubMed] [Google Scholar]
- 77.Abbate A, Kontos MC, Grizzard JD, Biondi-Zoccai GG, Van Tassell BW, Robati R, Roach LM, Arena RA, Roberts CS, Varma A, Gelwix CC, Salloum FN, Hastillo A, Dinarello CA, Vetrovec GW. Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (virginia commonwealth university anakinra remodeling trial [vcu-art] pilot study) Am J Cardiol. 2010;105:1371–1377. e1371. doi: 10.1016/j.amjcard.2009.12.059. [DOI] [PubMed] [Google Scholar]
- 78.Kanda J, Kawabata H, Yamaji Y, Ichinohe T, Ishikawa T, Tamura T, Furukawa Y, Kimura T, Kita T, Uchiyama T. Reversible cardiomyopathy associated with multicentric castleman disease: Successful treatment with tocilizumab, an anti-interleukin 6 receptor antibody. Int J Hematol. 2007;85:207–211. doi: 10.1532/IJH97.06186. [DOI] [PubMed] [Google Scholar]
- 79. [02/26/13];Effect of the interleukin-6 receptor antagonist tocilizumab in non-st elevation myocardial infarction. http://www.clinicaltrials.gov/ct2/show/NCT01491074.
- 80.Heeschen C, Aicher A, Lehmann R, Fichtlscherer S, Vasa M, Urbich C, Mildner-Rihm C, Martin H, Zeiher AM, Dimmeler S. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood. 2003;102:1340–1346. doi: 10.1182/blood-2003-01-0223. [DOI] [PubMed] [Google Scholar]
- 81.Lipsic E, Schoemaker RG, van der Meer P, Voors AA, van Veldhuisen DJ, van Gilst WH. Protective effects of erythropoietin in cardiac ischemia: From bench to bedside. J Am Coll Cardiol. 2006;48:2161–2167. doi: 10.1016/j.jacc.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 82.Calvillo L, Latini R, Kajstura J, Leri A, Anversa P, Ghezzi P, Salio M, Cerami A, Brines M. Recombinant human erythropoietin protects the myocardium from ischemia-reperfusion injury and promotes beneficial remodeling. Proc Natl Acad Sci U S A. 2003;100:4802–4806. doi: 10.1073/pnas.0630444100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Parsa CJ, Matsumoto A, Kim J, Riel RU, Pascal LS, Walton GB, Thompson RB, Petrofski JA, Annex BH, Stamler JS, Koch WJ. A novel protective effect of erythropoietin in the infarcted heart. J Clin Invest. 2003;112:999–1007. doi: 10.1172/JCI18200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Voors AA, Belonje AM, Zijlstra F, Hillege HL, Anker SD, Slart RH, Tio RA, van ‘t Hof A, Jukema JW, Peels HO, Henriques JP, Ten Berg JM, Vos J, van Gilst WH, van Veldhuisen DJ. A single dose of erythropoietin in st-elevation myocardial infarction. Eur Heart J. 2010;31:2593–2600. doi: 10.1093/eurheartj/ehq304. [DOI] [PubMed] [Google Scholar]
- 85.Najjar SS, Rao SV, Melloni C, Raman SV, Povsic TJ, Melton L, Barsness GW, Prather K, Heitner JF, Kilaru R, Gruberg L, Hasselblad V, Greenbaum AB, Patel M, Kim RJ, Talan M, Ferrucci L, Longo DL, Lakatta EG, Harrington RA. Intravenous erythropoietin in patients with st-segment elevation myocardial infarction: Reveal: A randomized controlled trial. JAMA. 2011;305:1863–1872. doi: 10.1001/jama.2011.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Minamino T, Toba K, Higo S, Nakatani D, Hikoso S, Umegaki M, Yamamoto K, Sawa Y, Aizawa Y, Komuro I. Design and rationale of low-dose erythropoietin in patients with st-segment elevation myocardial infarction (epo-ami-ii study): A randomized controlled clinical trial. Cardiovasc Drugs Ther. 2012;26:409–416. doi: 10.1007/s10557-012-6410-4. [DOI] [PubMed] [Google Scholar]
- 87.Egrie JC, Dwyer E, Browne JK, Hitz A, Lykos MA. Darbepoetin alfa has a longer circulating half-life and greater in vivo potency than recombinant human erythropoietin. Exp Hematol. 2003;31:290–299. doi: 10.1016/s0301-472x(03)00006-7. [DOI] [PubMed] [Google Scholar]
- 88.Odiete O, Hill MF, Sawyer DB. Neuregulin in cardiovascular development and disease. Circ Res. 2012;111:1376–1385. doi: 10.1161/CIRCRESAHA.112.267286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao YY, Sawyer DR, Baliga RR, Opel DJ, Han X, Marchionni MA, Kelly RA. Neuregulins promote survival and growth of cardiac myocytes. Persistence of erbb2 and erbb4 expression in neonatal and adult ventricular myocytes. J Biol Chem. 1998;273:10261–10269. doi: 10.1074/jbc.273.17.10261. [DOI] [PubMed] [Google Scholar]
- 90.Liu X, Gu X, Li Z, Li X, Li H, Chang J, Chen P, Jin J, Xi B, Chen D, Lai D, Graham RM, Zhou M. Neuregulin-1/erbb-activation improves cardiac function and survival in models of ischemic, dilated, and viral cardiomyopathy. J Am Coll Cardiol. 2006;48:1438–1447. doi: 10.1016/j.jacc.2006.05.057. [DOI] [PubMed] [Google Scholar]
- 91.Gao R, Zhang J, Cheng L, Wu X, Dong W, Yang X, Li T, Liu X, Xu Y, Li X, Zhou M. A phase ii, randomized, double-blind, multicenter, based on standard therapy, placebo-controlled study of the efficacy and safety of recombinant human neuregulin-1 in patients with chronic heart failure. J Am Coll Cardiol. 2010;55:1907–1914. doi: 10.1016/j.jacc.2009.12.044. [DOI] [PubMed] [Google Scholar]
- 92.Jabbour A, Hayward CS, Keogh AM, Kotlyar E, McCrohon JA, England JF, Amor R, Liu X, Li XY, Zhou MD, Graham RM, Macdonald PS. Parenteral administration of recombinant human neuregulin-1 to patients with stable chronic heart failure produces favourable acute and chronic haemodynamic responses. Eur J Heart Fail. 2011;13:83–92. doi: 10.1093/eurjhf/hfq152. [DOI] [PubMed] [Google Scholar]
- 93. [02/26/13];Single ascending doses of ggf2 in patients with left ventricular dysfunction and symptomatic heart failure (ggf2-1101-1) http://www.clinicaltrials.gov/ct2/show/NCT01258387.
- 94.Hill MF, Patel AV, Murphy A, Smith HM, Galindo CL, Pentassuglia L, Peng X, Lenneman CG, Odiete O, Friedman DB, Kronenberg MW, Zheng S, Zhao Z, Song Y, Harrell FE, Jr, Srinivas M, Ganguly A, Iaci J, Parry TJ, Caggiano AO, Sawyer DB. Intravenous glial growth factor 2 (ggf2) isoform of neuregulin-1beta improves left ventricular function, gene and protein expression in rats after myocardial infarction. PLoS One. 2013;8:e55741. doi: 10.1371/journal.pone.0055741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jay SM, Kurtagic E, Alvarez LM, de Picciotto S, Sanchez E, Hawkins JF, Prince RN, Guerrero Y, Treasure CL, Lee RT, Griffith LG. Engineered bivalent ligands to bias erbb receptor-mediated signaling and phenotypes. J Biol Chem. 2011;286:27729–27740. doi: 10.1074/jbc.M111.221093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jay SM, Murthy AC, Hawkins JF, Wortzel JR, Steinhauser ML, Alvarez LM, Gannon J, Macrae CA, Griffith LG, Lee RT. An engineered bivalent neuregulin protects against doxorubicin-induced cardiotoxicity with reduced proneoplastic potential. Circulation. 2013;128:152–161. doi: 10.1161/CIRCULATIONAHA.113.002203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ferrara N, Gerber HP, LeCouter J. The biology of vegf and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 98.Beenken A, Mohammadi M. The fgf family: Biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8:235–253. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Banai S, Jaklitsch MT, Casscells W, Shou M, Shrivastav S, Correa R, Epstein SE, Unger EF. Effects of acidic fibroblast growth factor on normal and ischemic myocardium. Circ Res. 1991;69:76–85. doi: 10.1161/01.res.69.1.76. [DOI] [PubMed] [Google Scholar]
- 100.Banai S, Jaklitsch MT, Shou M, Lazarous DF, Scheinowitz M, Biro S, Epstein SE, Unger EF. Angiogenic-induced enhancement of collateral blood flow to ischemic myocardium by vascular endothelial growth factor in dogs. Circulation. 1994;89:2183–2189. doi: 10.1161/01.cir.89.5.2183. [DOI] [PubMed] [Google Scholar]
- 101.Pearlman JD, Hibberd MG, Chuang ML, Harada K, Lopez JJ, Gladstone SR, Friedman M, Sellke FW, Simons M. Magnetic resonance mapping demonstrates benefits of vegf-induced myocardial angiogenesis. Nat Med. 1995;1:1085–1089. doi: 10.1038/nm1095-1085. [DOI] [PubMed] [Google Scholar]
- 102.Simons M, Annex BH, Laham RJ, Kleiman N, Henry T, Dauerman H, Udelson JE, Gervino EV, Pike M, Whitehouse MJ, Moon T, Chronos NA. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: Double-blind, randomized, controlled clinical trial. Circulation. 2002;105:788–793. doi: 10.1161/hc0802.104407. [DOI] [PubMed] [Google Scholar]
- 103.Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, Shah PK, Willerson JT, Benza RL, Berman DS, Gibson CM, Bajamonde A, Rundle AC, Fine J, McCluskey ER. The viva trial: Vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation. 2003;107:1359–1365. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- 104.Ortega S, Schaeffer MT, Soderman D, DiSalvo J, Linemeyer DL, Gimenez-Gallego G, Thomas KA. Conversion of cysteine to serine residues alters the activity, stability, and heparin dependence of acidic fibroblast growth factor. J Biol Chem. 1991;266:5842–5846. [PubMed] [Google Scholar]
- 105.Arakawa T, Horan TP, Narhi LO, Rees DC, Schiffer SG, Holst PL, Prestrelski SJ, Tsai LB, Fox GM. Production and characterization of an analog of acidic fibroblast growth factor with enhanced stability and biological activity. Protein Eng. 1993;6:541–546. doi: 10.1093/protein/6.5.541. [DOI] [PubMed] [Google Scholar]
- 106.Shireman PK, Xue L, Maddox E, Burgess WH, Greisler HP. The s130k fibroblast growth factor-1 mutant induces heparin-independent proliferation and is resistant to thrombin degradation in fibrin glue. J Vasc Surg. 2000;31:382–390. doi: 10.1016/s0741-5214(00)90168-x. [DOI] [PubMed] [Google Scholar]
- 107.Brych SR, Blaber SI, Logan TM, Blaber M. Structure and stability effects of mutations designed to increase the primary sequence symmetry within the core region of a beta-trefoil. Protein Sci. 2001;10:2587–2599. doi: 10.1110/ps.ps.34701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zakrzewska M, Krowarsch D, Wiedlocha A, Otlewski J. Design of fully active fgf-1 variants with increased stability. Protein Eng Des Sel. 2004;17:603–611. doi: 10.1093/protein/gzh076. [DOI] [PubMed] [Google Scholar]
- 109.Sinha Roy R, Soni S, Harfouche R, Vasudevan PR, Holmes O, de Jonge H, Rowe A, Paraskar A, Hentschel DM, Chirgadze D, Blundell TL, Gherardi E, Mashelkar RA, Sengupta S. Coupling growth-factor engineering with nanotechnology for therapeutic angiogenesis. Proc Natl Acad Sci U S A. 2010;107:13608–13613. doi: 10.1073/pnas.1006007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dubey VK, Lee J, Somasundaram T, Blaber S, Blaber M. Spackling the crack: Stabilizing human fibroblast growth factor-1 by targeting the n and c terminus beta-strand interactions. J Mol Biol. 2007;371:256–268. doi: 10.1016/j.jmb.2007.05.065. [DOI] [PubMed] [Google Scholar]
- 111.Zhang J, Ding L, Zhao Y, Sun W, Chen B, Lin H, Wang X, Zhang L, Xu B, Dai J. Collagen-targeting vascular endothelial growth factor improves cardiac performance after myocardial infarction. Circulation. 2009;119:1776–1784. doi: 10.1161/CIRCULATIONAHA.108.800565. [DOI] [PubMed] [Google Scholar]
- 112.Hsieh PC, Davis ME, Gannon J, Macgillivray C, Lee RT. Controlled delivery of pdgf-bb, an endothelial-derived cardiomyocyte survival factor, for myocardial protection using injectable self-assembling peptide nanofibers. J Clin Invest. 2005 doi: 10.1172/JCI25878. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hsieh PC, Davis ME, Gannon J, MacGillivray C, Lee RT. Controlled delivery of pdgf-bb for myocardial protection using injectable self-assembling peptide nanofibers. J Clin Invest. 2006;116:237–248. doi: 10.1172/JCI25878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hsieh PC, MacGillivray C, Gannon J, Cruz FU, Lee RT. Local controlled intramyocardial delivery of platelet-derived growth factor improves postinfarction ventricular function without pulmonary toxicity. Circulation. 2006;114:637–644. doi: 10.1161/CIRCULATIONAHA.106.639831. [DOI] [PubMed] [Google Scholar]
- 115.Segers VF, Lee RT. Local delivery of proteins and the use of self-assembling peptides. Drug Discov Today. 2007;12:561–568. doi: 10.1016/j.drudis.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 116.Kuhn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, Keating MT. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med. 2007;13:962–969. doi: 10.1038/nm1619. [DOI] [PubMed] [Google Scholar]
- 117.Loffredo FS, Steinhauser ML, Jay SM, Gannon J, Pancoast JR, Yalamanchi P, Sinha M, Dall’Osso C, Khong D, Shadrach JL, Miller CM, Singer BS, Stewart A, Psychogios N, Gerszten RE, Hartigan AJ, Kim M, Serwold T, Wagers AJ, Lee RT. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013 doi: 10.1016/j.cell.2013.04.015. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Timmers L, Henriques JP, de Kleijn DP, Devries JH, Kemperman H, Steendijk P, Verlaan CW, Kerver M, Piek JJ, Doevendans PA, Pasterkamp G, Hoefer IE. Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J Am Coll Cardiol. 2009;53:501–510. doi: 10.1016/j.jacc.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 119. [02/26/13];Myocardial protection of exenatide in ami (empire) http://www.clinicaltrials.gov/ct2/show/NCT01580514.
- 120.DeWire SM, Violin JD. Biased ligands for better cardiovascular drugs: Dissecting g-protein-coupled receptor pharmacology. Circ Res. 2011;109:205–216. doi: 10.1161/CIRCRESAHA.110.231308. [DOI] [PubMed] [Google Scholar]
- 121.Smith CC, Yellon DM. Adipocytokines, cardiovascular pathophysiology and myocardial protection. Pharmacol Ther. 2010;129:206–219. doi: 10.1016/j.pharmthera.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 122.Mattu HS, Randeva HS. Role of adipokines in cardiovascular disease. J Endocrinol. 2013;216:T17–36. doi: 10.1530/JOE-12-0232. [DOI] [PubMed] [Google Scholar]
- 123.Nussinovitch U, Shoenfeld Y. Intravenous immunoglobulin - indications and mechanisms in cardiovascular diseases. Autoimmun Rev. 2008;7:445–452. doi: 10.1016/j.autrev.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 124. [02/26/13];Effect of intravenous immunglobulin (ivig) after myocardial infarction. http://www.clinicaltrials.gov/ct2/show/NCT00430885.
- 125.Amir S, Binder CJ. Experimental immunotherapeutic approaches for atherosclerosis. Clin Immunol. 2010;134:66–79. doi: 10.1016/j.clim.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lambert G, Sjouke B, Choque B, Kastelein JJ, Hovingh GK. The pcsk9 decade. J Lipid Res. 2012;53:2515–2524. doi: 10.1194/jlr.R026658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rothe A, Hosse RJ, Power BE. In vitro display technologies reveal novel biopharmaceutics. FASEB J. 2006;20:1599–1610. doi: 10.1096/fj.05-5650rev. [DOI] [PubMed] [Google Scholar]
- 128.Winter G, Griffiths AD, Hawkins RE, Hoogenboom HR. Making antibodies by phage display technology. Annu Rev Immunol. 1994;12:433–455. doi: 10.1146/annurev.iy.12.040194.002245. [DOI] [PubMed] [Google Scholar]
- 129.Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279:377–380. doi: 10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- 130.Rothenfluh DA, Bermudez H, O’Neil CP, Hubbell JA. Biofunctional polymer nanoparticles for intra-articular targeting and retention in cartilage. Nat Mater. 2008;7:248–254. doi: 10.1038/nmat2116. [DOI] [PubMed] [Google Scholar]
- 131.Boder ET, Midelfort KS, Wittrup KD. Directed evolution of antibody fragments with monovalent femtomolar antigen-binding affinity. Proc Natl Acad Sci U S A. 2000;97:10701–10705. doi: 10.1073/pnas.170297297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Silverman AP, Levin AM, Lahti JL, Cochran JR. Engineered cystine-knot peptides that bind alpha(v)beta(3) integrin with antibody-like affinities. J Mol Biol. 2009;385:1064–1075. doi: 10.1016/j.jmb.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Willmann JK, Kimura RH, Deshpande N, Lutz AM, Cochran JR, Gambhir SS. Targeted contrast-enhanced ultrasound imaging of tumor angiogenesis with contrast microbubbles conjugated to integrin-binding knottin peptides. J Nucl Med. 2010;51:433–440. doi: 10.2967/jnumed.109.068007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hanes J, Pluckthun A. In vitro selection and evolution of functional proteins by using ribosome display. Proc Natl Acad Sci U S A. 1997;94:4937–4942. doi: 10.1073/pnas.94.10.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Roberts RW, Szostak JW. Rna-peptide fusions for the in vitro selection of peptides and proteins. Proc Natl Acad Sci U S A. 1997;94:12297–12302. doi: 10.1073/pnas.94.23.12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Binz HK, Amstutz P, Pluckthun A. Engineering novel binding proteins from nonimmunoglobulin domains. Nat Biotechnol. 2005;23:1257–1268. doi: 10.1038/nbt1127. [DOI] [PubMed] [Google Scholar]
- 137.Wurch T, Pierre A, Depil S. Novel protein scaffolds as emerging therapeutic proteins: From discovery to clinical proof-of-concept. Trends Biotechnol. 2012;30:575–582. doi: 10.1016/j.tibtech.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 138.Tolcher AW, Sweeney CJ, Papadopoulos K, Patnaik A, Chiorean EG, Mita AC, Sankhala K, Furfine E, Gokemeijer J, Iacono L, Eaton C, Silver BA, Mita M. Phase i and pharmacokinetic study of ct-322 (bms-844203), a targeted adnectin inhibitor of vegfr-2 based on a domain of human fibronectin. Clin Cancer Res. 2011;17:363–371. doi: 10.1158/1078-0432.CCR-10-1411. [DOI] [PubMed] [Google Scholar]
- 139.Gebauer M, Skerra A. Anticalins small engineered binding proteins based on the lipocalin scaffold. Methods Enzymol. 2012;503:157–188. doi: 10.1016/B978-0-12-396962-0.00007-0. [DOI] [PubMed] [Google Scholar]
- 140.Girard TJ, Warren LA, Novotny WF, Likert KM, Brown SG, Miletich JP, Broze GJ., Jr Functional significance of the kunitz-type inhibitory domains of lipoprotein-associated coagulation inhibitor. Nature. 1989;338:518–520. doi: 10.1038/338518a0. [DOI] [PubMed] [Google Scholar]
- 141.Stumpp MT, Amstutz P. Darpins: A true alternative to antibodies. Curr Opin Drug Discov Devel. 2007;10:153–159. [PubMed] [Google Scholar]
- 142.Silverman J, Liu Q, Bakker A, To W, Duguay A, Alba BM, Smith R, Rivas A, Li P, Le H, Whitehorn E, Moore KW, Swimmer C, Perlroth V, Vogt M, Kolkman J, Stemmer WP. Multivalent avimer proteins evolved by exon shuffling of a family of human receptor domains. Nat Biotechnol. 2005;23:1556–1561. doi: 10.1038/nbt1166. [DOI] [PubMed] [Google Scholar]
- 143.Levy RJ, Lumry WR, McNeil DL, Li HH, Campion M, Horn PT, Pullman WE. Edema4: A phase 3, double-blind study of subcutaneous ecallantide treatment for acute attacks of hereditary angioedema. Ann Allergy Asthma Immunol. 2010;104:523–529. doi: 10.1016/j.anai.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 144.Xie J, Schultz PG. A chemical toolkit for proteins--an expanded genetic code. Nat Rev Mol Cell Biol. 2006;7:775–782. doi: 10.1038/nrm2005. [DOI] [PubMed] [Google Scholar]
- 145.Deiters A, Cropp TA, Summerer D, Mukherji M, Schultz PG. Site-specific pegylation of proteins containing unnatural amino acids. Bioorg Med Chem Lett. 2004;14:5743–5745. doi: 10.1016/j.bmcl.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 146.Walensky LD, Kung AL, Escher I, Malia TJ, Barbuto S, Wright RD, Wagner G, Verdine GL, Korsmeyer SJ. Activation of apoptosis in vivo by a hydrocarbon-stapled bh3 helix. Science. 2004;305:1466–1470. doi: 10.1126/science.1099191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Muir TW, Sondhi D, Cole PA. Expressed protein ligation: A general method for protein engineering. Proc Natl Acad Sci U S A. 1998;95:6705–6710. doi: 10.1073/pnas.95.12.6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schellenberger V, Wang CW, Geething NC, Spink BJ, Campbell A, To W, Scholle MD, Yin Y, Yao Y, Bogin O, Cleland JL, Silverman J, Stemmer WP. A recombinant polypeptide extends the in vivo half-life of peptides and proteins in a tunable manner. Nat Biotechnol. 2009;27:1186–1190. doi: 10.1038/nbt.1588. [DOI] [PubMed] [Google Scholar]
- 149.Wang A, Winblade Nairn N, Johnson RS, Tirrell DA, Grabstein K. Processing of n-terminal unnatural amino acids in recombinant human interferon-beta in escherichia coli. Chembiochem. 2008;9:324–330. doi: 10.1002/cbic.200700379. [DOI] [PubMed] [Google Scholar]
- 150.Nairn NW, Shanebeck KD, Wang A, Graddis TJ, VanBrunt MP, Thornton KC, Grabstein K. Development of copper-catalyzed azide-alkyne cycloaddition for increased in vivo efficacy of interferon beta-1b by site-specific pegylation. Bioconjug Chem. 2012;23:2087–2097. doi: 10.1021/bc300295x. [DOI] [PubMed] [Google Scholar]
- 151.Kim CH, Axup JY, Dubrovska A, Kazane SA, Hutchins BA, Wold ED, Smider VV, Schultz PG. Synthesis of bispecific antibodies using genetically encoded unnatural amino acids. J Am Chem Soc. 2012;134:9918–9921. doi: 10.1021/ja303904e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Munch RC, Janicki H, Volker I, Rasbach A, Hallek M, Buning H, Buchholz CJ. Displaying high-affinity ligands on adeno-associated viral vectors enables tumor cell-specific and safe gene transfer. Mol Ther. 2013;21:109–118. doi: 10.1038/mt.2012.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nagahara H, Vocero-Akbani AM, Snyder EL, Ho A, Latham DG, Lissy NA, Becker-Hapak M, Ezhevsky SA, Dowdy SF. Transduction of full-length tat fusion proteins into mammalian cells: Tat-p27kip1 induces cell migration. Nat Med. 1998;4:1449–1452. doi: 10.1038/4042. [DOI] [PubMed] [Google Scholar]
- 154.Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: Delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 155.Ignatovich IA, Dizhe EB, Pavlotskaya AV, Akifiev BN, Burov SV, Orlov SV, Perevozchikov AP. Complexes of plasmid DNA with basic domain 47–57 of the hiv-1 tat protein are transferred to mammalian cells by endocytosis-mediated pathways. J Biol Chem. 2003;278:42625–42636. doi: 10.1074/jbc.M301431200. [DOI] [PubMed] [Google Scholar]
- 156.Boado RJ, Hui EK, Lu JZ, Pardridge WM. Igg-enzyme fusion protein: Pharmacokinetics and anti-drug antibody response in rhesus monkeys. Bioconjug Chem. 2013;24:97–104. doi: 10.1021/bc3005123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pardridge WM. Molecular trojan horses for blood-brain barrier drug delivery. Curr Opin Pharmacol. 2006;6:494–500. doi: 10.1016/j.coph.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 158.Won YW, McGinn AN, Lee M, Bull DA, Kim SW. Targeted gene delivery to ischemic myocardium by homing peptide-guided polymeric carrier. Mol Pharm. 2013;10:378–385. doi: 10.1021/mp300500y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Scott RC, Crabbe D, Krynska B, Ansari R, Kiani MF. Aiming for the heart: Targeted delivery of drugs to diseased cardiac tissue. Expert Opin Drug Deliv. 2008;5:459–470. doi: 10.1517/17425247.5.4.459. [DOI] [PubMed] [Google Scholar]
- 160.Muller-Ehmsen J, Whittaker P, Kloner RA, Dow JS, Sakoda T, Long TI, Laird PW, Kedes L. Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J Mol Cell Cardiol. 2002;34:107–116. doi: 10.1006/jmcc.2001.1491. [DOI] [PubMed] [Google Scholar]
- 161.Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, Murry CE. Cardiomyocyte grafting for cardiac repair: Graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001;33:907–921. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- 162.Neff T, Blau CA. Pharmacologically regulated cell therapy. Blood. 2001;97:2535–2540. doi: 10.1182/blood.v97.9.2535. [DOI] [PubMed] [Google Scholar]
- 163.Kikuchi K, Poss KD. Cardiac regenerative capacity and mechanisms. Annu Rev Cell Dev Biol. 2012;28:719–741. doi: 10.1146/annurev-cellbio-101011-155739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Choi WY, Poss KD. Cardiac regeneration. Curr Top Dev Biol. 2012;100:319–344. doi: 10.1016/B978-0-12-387786-4.00010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Martin-Puig S, Fuster V, Torres M. Heart repair: From natural mechanisms of cardiomyocyte production to the design of new cardiac therapies. Ann N Y Acad Sci. 2012;1254:71–81. doi: 10.1111/j.1749-6632.2012.06488.x. [DOI] [PubMed] [Google Scholar]
- 166.Steinhauser ML, Lee RT. Regeneration of the heart. EMBO Mol Med. 2011;3:701–712. doi: 10.1002/emmm.201100175. [DOI] [PMC free article] [PubMed] [Google Scholar]