Abstract
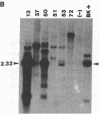
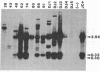
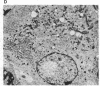
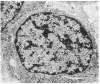
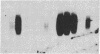
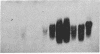
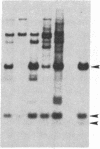
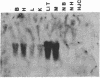
JC virus and BK virus are ubiquitous human viruses that share sequence and structural homology with simian virus 40. To characterize tissue-specific expression of these viruses and to establish model systems for the study of human viral-induced disease, transgenic mice containing early regions of each of the viruses were produced. The viral sequences induced tumors in a distinct and tissue-specific manner that was similar to their tissue tropism in humans. Ten JC virus-containing founder mice were produced, of which 5 survived to maturity. Four of them developed adrenal neuroblastomas, which metastasized to several other tissues. JC virus tumor-antigen RNA was detected at high levels in the tumor tissues and at low levels in the normal tissues of these mice. One of the three BK virus-containing mice was abnormally shaped and died at 2 weeks of age. The other two BK virus-containing mice developed primary hepatocellular carcinomas and renal tumors and died at 8-10 months of age. BK virus tumor-antigen RNA was expressed in tumor tissues of both mice. Since each of the viruses retained the general tissue tropism that it exhibits in humans, these data suggest that transgenic mice harboring human viruses will be useful as animal models for viral-induced diseases.
Full text
PDF
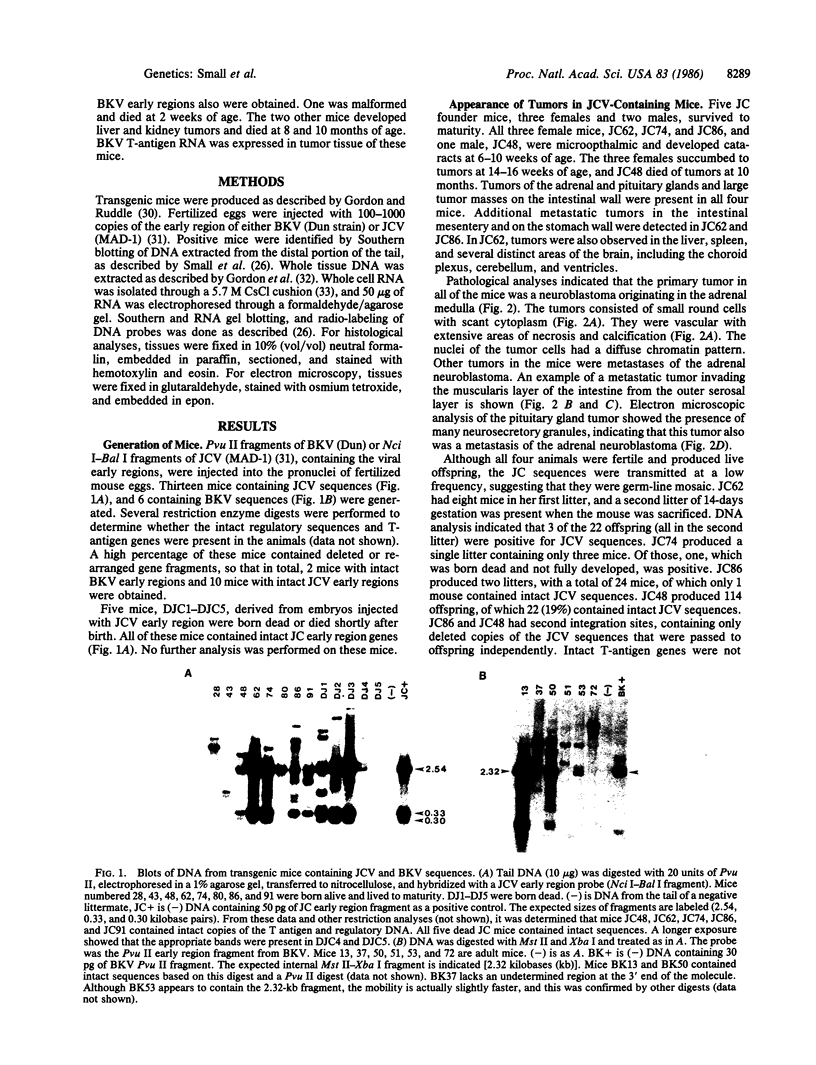
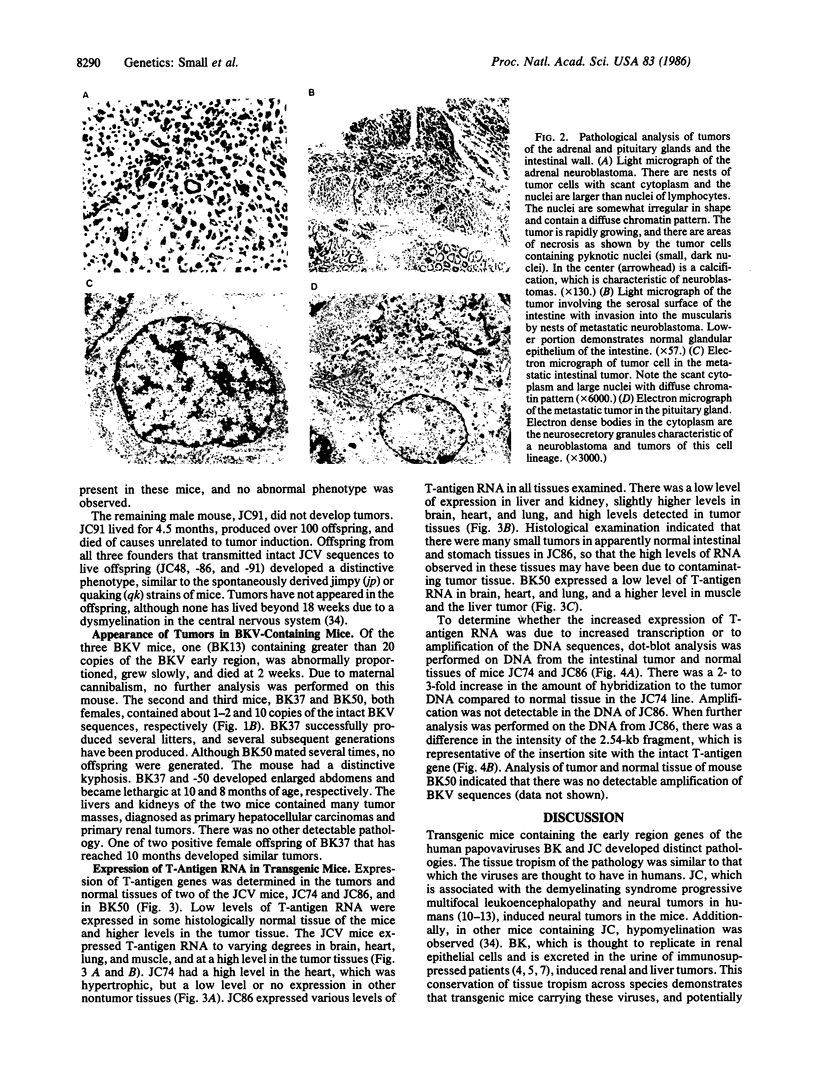


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramczuk J., Pan S., Maul G., Knowles B. B. Tumor induction by simian virus 40 in mice is controlled by long-term persistence of the viral genome and the immune response of the host. J Virol. 1984 Feb;49(2):540–548. doi: 10.1128/jvi.49.2.540-548.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster R. L., Chen H. Y., Messing A., van Dyke T., Levine A. J., Palmiter R. D. Transgenic mice harboring SV40 T-antigen genes develop characteristic brain tumors. Cell. 1984 Jun;37(2):367–379. doi: 10.1016/0092-8674(84)90367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaigne P., Rondot P., Escourolle R., Ribadeau dumas J. L., Cathala F., Hauw J. J. Leucoencéphalopathie multifocale progressive et "gliomes" multiples. Rev Neurol (Paris) 1974 Sep-Oct;130(9-10):379–392. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Coleman D. V., Wolfendale M. R., Daniel R. A., Dhanjal N. K., Gardner S. D., Gibson P. E., Field A. M. A prospective study of human polyomavirus infection in pregnancy. J Infect Dis. 1980 Jul;142(1):1–8. doi: 10.1093/infdis/142.1.1. [DOI] [PubMed] [Google Scholar]
- Corallini A., Altavilla G., Cecchetti M. G., Fabris G., Grossi M. P., Balboni P. G., Lanza G., Barbanti-Brodano G. Ependymomas, malignant tumors of pancreatic islets, and osteosarcomas induced in hamsters by BK virus, a human papovavirus. J Natl Cancer Inst. 1978 Sep;61(3):875–883. [PubMed] [Google Scholar]
- Costa J., Yee C., Tralka T. S., Rabson A. S. Hamster ependymomas produced by intracerebral inoculation of a human papovavirus (MMV). J Natl Cancer Inst. 1976 Apr;56(4):863–864. doi: 10.1093/jnci/56.4.863. [DOI] [PubMed] [Google Scholar]
- Frisque R. J., Bream G. L., Cannella M. T. Human polyomavirus JC virus genome. J Virol. 1984 Aug;51(2):458–469. doi: 10.1128/jvi.51.2.458-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner S. D., Field A. M., Coleman D. V., Hulme B. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet. 1971 Jun 19;1(7712):1253–1257. doi: 10.1016/s0140-6736(71)91776-4. [DOI] [PubMed] [Google Scholar]
- Gardner S. D., MacKenzie E. F., Smith C., Porter A. A. Prospective study of the human polyomaviruses BK and JC and cytomegalovirus in renal transplant recipients. J Clin Pathol. 1984 May;37(5):578–586. doi: 10.1136/jcp.37.5.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner S. D. Prevalence in England of antibody to human polyomavirus (B.k.). Br Med J. 1973 Jan 13;1(5845):77–78. doi: 10.1136/bmj.1.5845.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GiaRusso M. H., Koeppen A. H. Atypical progressive multifocal leukoencephalopathy and primary cerebral malignant lymphoma. J Neurol Sci. 1978 Feb;35(2-3):391–398. doi: 10.1016/0022-510x(78)90019-9. [DOI] [PubMed] [Google Scholar]
- Gordon J. W., Ruddle F. H. Gene transfer into mouse embryos: production of transgenic mice by pronuclear injection. Methods Enzymol. 1983;101:411–433. doi: 10.1016/0076-6879(83)01031-9. [DOI] [PubMed] [Google Scholar]
- Gordon J. W., Scangos G. A., Plotkin D. J., Barbosa J. A., Ruddle F. H. Genetic transformation of mouse embryos by microinjection of purified DNA. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7380–7384. doi: 10.1073/pnas.77.12.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Heritable formation of pancreatic beta-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature. 1985 May 9;315(6015):115–122. doi: 10.1038/315115a0. [DOI] [PubMed] [Google Scholar]
- Hargis B. J., Malkiel S. Sarcomas induced by injection of simian virus 40 into neonatal CFW mice. J Natl Cancer Inst. 1979 Oct;63(4):965–968. [PubMed] [Google Scholar]
- Hogan T. F., Borden E. C., McBain J. A., Padgett B. L., Walker D. L. Human polyomavirus infections with JC virus and BK virus in renal transplant patients. Ann Intern Med. 1980 Mar;92(3):373–378. doi: 10.7326/0003-4819-92-3-373. [DOI] [PubMed] [Google Scholar]
- Lecatsas G., van Wyk J. A. DNA viruses in urine after renal transplantation. S Afr Med J. 1978 May 20;53(20):787–788. [PubMed] [Google Scholar]
- Mann R. S., Carroll R. B. Cross-reaction of BK virus large T antigen with monoclonal antibodies directed against SV40 large T antigen. Virology. 1984 Oct 30;138(2):379–385. doi: 10.1016/0042-6822(84)90365-9. [DOI] [PubMed] [Google Scholar]
- McCance D. J., Mims C. A. Transplacental transmission of polyoma virus in mice. Infect Immun. 1977 Oct;18(1):196–202. doi: 10.1128/iai.18.1.196-202.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing A., Chen H. Y., Palmiter R. D., Brinster R. L. Peripheral neuropathies, hepatocellular carcinomas and islet cell adenomas in transgenic mice. Nature. 1985 Aug 1;316(6027):461–463. doi: 10.1038/316461a0. [DOI] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L. Prevalence of antibodies in human sera against JC virus, an isolate from a case of progressive multifocal leukoencephalopathy. J Infect Dis. 1973 Apr;127(4):467–470. doi: 10.1093/infdis/127.4.467. [DOI] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L., ZuRhein G. M., Varakis J. N. Differential neurooncogenicity of strains of JC virus, a human polyoma virus, in newborn Syrian hamsters. Cancer Res. 1977 Mar;37(3):718–720. [PubMed] [Google Scholar]
- Palmiter R. D., Chen H. Y., Messing A., Brinster R. L. SV40 enhancer and large-T antigen are instrumental in development of choroid plexus tumours in transgenic mice. Nature. 1985 Aug 1;316(6027):457–460. doi: 10.1038/316457a0. [DOI] [PubMed] [Google Scholar]
- RICHARDSON E. P., Jr Progressive multifocal leukoencephalopathy. N Engl J Med. 1961 Oct 26;265:815–823. doi: 10.1056/NEJM196110262651701. [DOI] [PubMed] [Google Scholar]
- Rosenthal N., Kress M., Gruss P., Khoury G. BK viral enhancer element and a human cellular homolog. Science. 1983 Nov 18;222(4625):749–755. doi: 10.1126/science.6314501. [DOI] [PubMed] [Google Scholar]
- Small J. A., Blair D. G., Showalter S. D., Scangos G. A. Analysis of a transgenic mouse containing simian virus 40 and v-myc sequences. Mol Cell Biol. 1985 Apr;5(4):642–648. doi: 10.1128/mcb.5.4.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small J. A., Scangos G. A., Cork L., Jay G., Khoury G. The early region of human papovavirus JC induces dysmyelination in transgenic mice. Cell. 1986 Jul 4;46(1):13–18. doi: 10.1016/0092-8674(86)90855-x. [DOI] [PubMed] [Google Scholar]
- Takemoto K. K., Martin M. A. Transformation of hamster kidney cells by BK papovavirus DNA. J Virol. 1975 Jan;17(1):247–253. doi: 10.1128/jvi.17.1.247-253.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K. K., Mullarkey M. F. Human papovavirus, BK strain: biological studies including antigenic relationship to simian virus 40. J Virol. 1973 Sep;12(3):625–631. doi: 10.1128/jvi.12.3.625-631.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K. K., Rabson A. S., Mullarkey M. F., Blaese R. M., Garon C. F., Nelson D. Isolation of papovavirus from brain tumor and urine of a patient with Wiskott-Aldrich syndrome. J Natl Cancer Inst. 1974 Nov;53(5):1205–1207. doi: 10.1093/jnci/53.5.1205. [DOI] [PubMed] [Google Scholar]
- Varakis J., ZuRhein G. M., Padgett B. L., Walker D. L. Induction of peripheral neuroblastomas in Syrian hamsters after injection as neonates with JC virus, a human polyoma virus. Cancer Res. 1978 Jun;38(6):1718–1722. [PubMed] [Google Scholar]
- Walker D. L., Padgett B. L., ZuRhein G. M., Albert A. E., Marsh R. F. Human papovavirus (JC): induction of brain tumors in hamsters. Science. 1973 Aug 17;181(4100):674–676. doi: 10.1126/science.181.4100.674. [DOI] [PubMed] [Google Scholar]
- Watanabe S., Yoshiike K., Nozawa A., Yuasa Y., Uchida S. Viable deletion mutant of human papovavirus BK that induces insulinomas in hamsters. J Virol. 1979 Dec;32(3):934–942. doi: 10.1128/jvi.32.3.934-942.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]