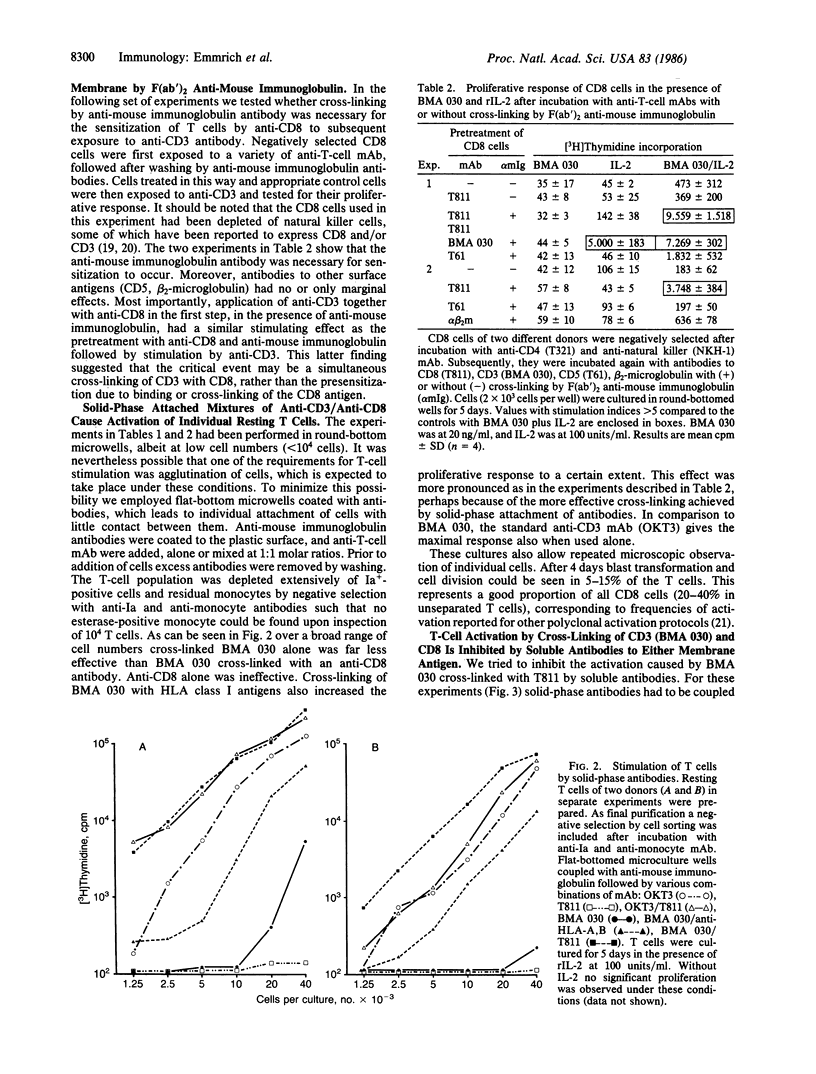
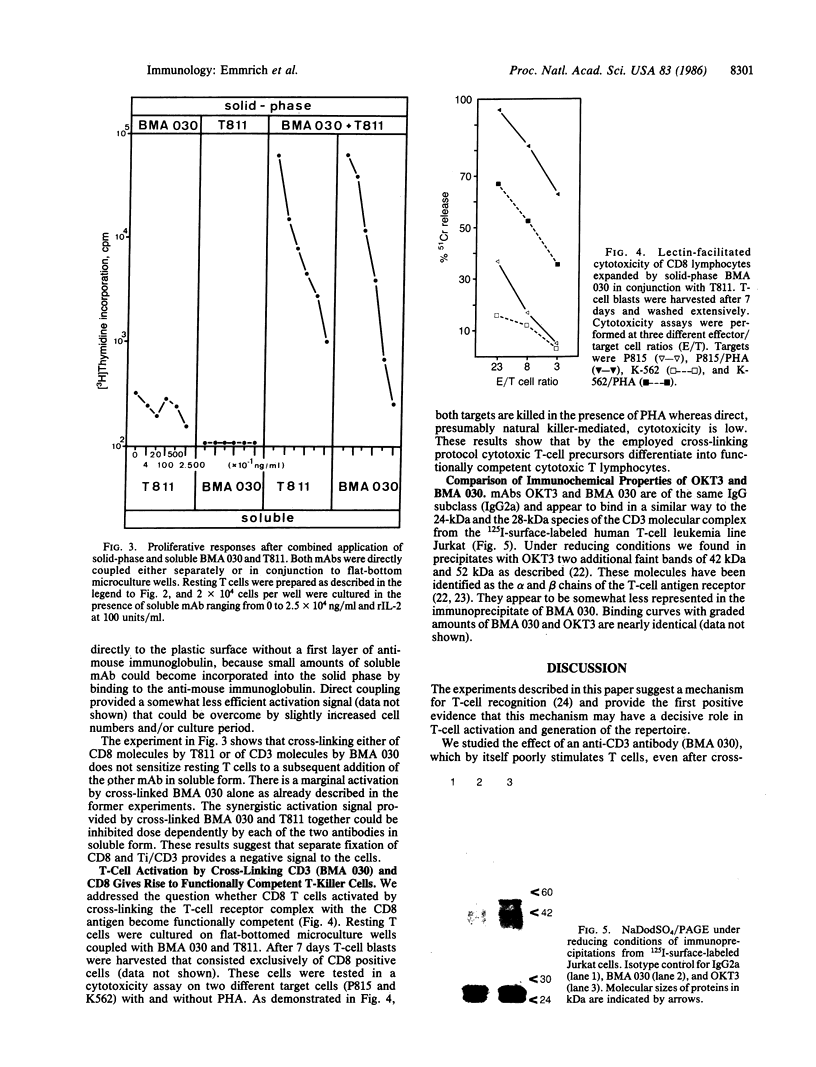
Abstract
Resting human T cells can be activated and induced to proliferate by cross-linking the T-cell receptor complex (Ti/CD3) with anti-CD3 (T3) antibodies, such as OKT3, together with interleukin 2. Here we describe functional properties of another monoclonal anti-CD3 antibody (BMA 030) that, cross-linked in various ways, only weakly stimulates accessory-cell-depleted T-cell cultures. However, when cross-linked to anti-CD4 or anti-CD8 antibodies a markedly enhanced proliferation of the corresponding subpopulation is observed. We have concentrated on the analysis of CD8 cells and have found that BMA 030, when cross-linked together with anti-CD8 (T811), induced proliferation more than 100-fold greater than BMA 030 alone, whereas cross-linking with antibodies to other T-cell membrane antigens (HLA-A, B, or CD5) provided no or marginal synergistic signals. There was no synergistic effect when only one of the two antibodies, BMA 030 or T811, was cross-linked and the other was applied in soluble form. In contrast, each of the two antibodies alone, when applied in soluble form, inhibited activation induced by the cross-linked antibodies. The T-cell differentiation antigen CD8 has been implicated in the major histocompatibility complex (MHC) class I restricted specificity of CD8 T cells. In previous work from other laboratories only the negative influences of soluble anti-CD8 antibodies have been noted. In contrast, our results suggest that cross-linking between Ti/CD3 and CD8 may be a critical event in the activation of mature CD8 cells. We hypothesize that, in antigen-induced T-cell activation, CD8 and Ti/CD3 become cross-linked by their simultaneous binding to class I-associated structures. Such a mechanism, if required for proliferation in early T-cell ontogeny, could generate a selective pressure for CD8 cells to recognize class I-associated antigens.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bank I., Chess L. Perturbation of the T4 molecule transmits a negative signal to T cells. J Exp Med. 1985 Oct 1;162(4):1294–1303. doi: 10.1084/jem.162.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst J., Alexander S., Elder J., Terhorst C. The T3 complex on human T lymphocytes involves four structurally distinct glycoproteins. J Biol Chem. 1983 Apr 25;258(8):5135–5141. [PubMed] [Google Scholar]
- Brenner M. B., Trowbridge I. S., Strominger J. L. Cross-linking of human T cell receptor proteins: association between the T cell idiotype beta subunit and the T3 glycoprotein heavy subunit. Cell. 1985 Jan;40(1):183–190. doi: 10.1016/0092-8674(85)90321-6. [DOI] [PubMed] [Google Scholar]
- Eichmann K., Falk I., Melchers I., Simon M. M. Quantitative studies on T cell diversity. I. Determination of the precursor frequencies for two types of streptococcus A-specific helper cells in nonimmune, polyclonally activated splenic T cells. J Exp Med. 1980 Sep 1;152(3):477–492. doi: 10.1084/jem.152.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmrich F., Andreesen R. Monoclonal antibodies against differentiation antigens on human macrophages. Immunol Lett. 1985;9(6):321–324. doi: 10.1016/0165-2478(85)90056-2. [DOI] [PubMed] [Google Scholar]
- Engleman E. G., Benike C. J., Grumet F. C., Evans R. L. Activation of human T lymphocyte subsets: helper and suppressor/cytotoxic T cells recognize and respond to distinct histocompatibility antigens. J Immunol. 1981 Nov;127(5):2124–2129. [PubMed] [Google Scholar]
- Engleman E. G., Benike C. J., Metzler C., Gatenby P. A., Evans R. L. Blocking of human T lymphocyte functions by anti-Leu-2 and anti-Leu-3 antibodies: differential inhibition of proliferation and suppression. J Immunol. 1983 Jun;130(6):2623–2628. [PubMed] [Google Scholar]
- Evans R. L., Wall D. W., Platsoucas C. D., Siegal F. P., Fikrig S. M., Testa C. M., Good R. A. Thymus-dependent membrane antigens in man: inhibition of cell-mediated lympholysis by monoclonal antibodies to TH2 antigen. Proc Natl Acad Sci U S A. 1981 Jan;78(1):544–548. doi: 10.1073/pnas.78.1.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathings W. E., Lawton A. R., Cooper M. D. Immunofluorescent studies of the development of pre-B cells, B lymphocytes and immunoglobulin isotype diversity in humans. Eur J Immunol. 1977 Nov;7(11):804–810. doi: 10.1002/eji.1830071112. [DOI] [PubMed] [Google Scholar]
- Gullberg M., Pobor G., Bandeira A., Larsson E. L., Coutinho A. Differential requirements for activation and growth of unprimed cytotoxic and helper T lymphocytes. Eur J Immunol. 1983 Sep;13(9):719–725. doi: 10.1002/eji.1830130906. [DOI] [PubMed] [Google Scholar]
- Imboden J. B., Stobo J. D. Transmembrane signalling by the T cell antigen receptor. Perturbation of the T3-antigen receptor complex generates inositol phosphates and releases calcium ions from intracellular stores. J Exp Med. 1985 Mar 1;161(3):446–456. doi: 10.1084/jem.161.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruisbeek A. M., Mond J. J., Fowlkes B. J., Carmen J. A., Bridges S., Longo D. L. Absence of the Lyt-2-,L3T4+ lineage of T cells in mice treated neonatally with anti-I-A correlates with absence of intrathymic I-A-bearing antigen-presenting cell function. J Exp Med. 1985 May 1;161(5):1029–1047. doi: 10.1084/jem.161.5.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Cooper D. A., Hodgdon J. C., Hussey R. E., Fitzgerald K. A., Schlossman S. F., Reinherz E. L. Identification of the receptor for antigen and major histocompatibility complex on human inducer T lymphocytes. Science. 1983 Dec 16;222(4629):1239–1242. doi: 10.1126/science.6606228. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Schlossman S. F., Reinherz E. L. Clonal analysis of human cytotoxic T lymphocytes: T4+ and T8+ effector T cells recognize products of different major histocompatibility complex regions. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4395–4399. doi: 10.1073/pnas.79.14.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perussia B., Fanning V., Trinchieri G. A human NK and K cell subset shares with cytotoxic T cells expression of the antigen recognized by antibody OKT8. J Immunol. 1983 Jul;131(1):223–231. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody with selective reactivity with functionally mature human thymocytes and all peripheral human T cells. J Immunol. 1979 Sep;123(3):1312–1317. [PubMed] [Google Scholar]
- Reinherz E. L., Meuer S. C., Fitzgerald K. A., Hussey R. E., Hodgdon J. C., Acuto O., Schlossman S. F. Comparison of T3-associated 49- and 43-kilodalton cell surface molecules on individual human T-cell clones: evidence for peptide variability in T-cell receptor structures. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4104–4108. doi: 10.1073/pnas.80.13.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab R., Crow M. K., Russo C., Weksler M. E. Requirements for T cell activation by OKT3 monoclonal antibody: role of modulation of T3 molecules and interleukin 1. J Immunol. 1985 Sep;135(3):1714–1718. [PubMed] [Google Scholar]
- Thomas Y., Rogozinski L., Chess L. Relationship between human T cell functional heterogeneity and human T cell surface molecules. Immunol Rev. 1983;74:113–128. doi: 10.1111/j.1600-065x.1983.tb01086.x. [DOI] [PubMed] [Google Scholar]
- Tite J. P., Sloan A., Janeway C. A., Jr The role of L3T4 in T cell activation: L3T4 may be both an Ia-binding protein and a receptor that transduces a negative signal. J Mol Cell Immunol. 1986;2(4):179–190. [PubMed] [Google Scholar]
- Weiss A., Stobo J. D. Requirement for the coexpression of T3 and the T cell antigen receptor on a malignant human T cell line. J Exp Med. 1984 Nov 1;160(5):1284–1299. doi: 10.1084/jem.160.5.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]



