Abstract
This review is intended to provide a broad outline of the biological and molecular functions of MYC as well as of the larger protein network within which MYC operates. We present a view of MYC as a sensor that integrates multiple cellular signals to mediate a broad transcriptional response controlling many aspects of cell behavior. We also describe the larger transcriptional network linked to MYC with emphasis on the MXD family of MYC antagonists. Last, we discuss evidence that the network has evolved for millions of years, dating back to the emergence of animals.
MYC integrates multiple cellular signals to mediate a broad transcriptional response that controls many aspects of cell behavior. Its deregulation contributes to a wide range of human cancers.
A BRIEF HISTORY OF MYC
Retroviral Origins
The history of MYC parallels the discovery of other major oncogenes in that it arose from studies on retroviruses associated with animal cancers. The experiments of Ellermann and Bang, and of Rous at the turn of the 20th century, demonstrating that chicken leukemias and sarcomas are transmissible through cell-free filtrates, were largely dismissed by the scientific community (Ellermann and Bang 1908; Rous 1911). Yet over the next 50 years, continuing reports of cell-free tumor transmission, as well as the direct isolation of viruses from tumors, eventually established the principle that many high-incidence animal tumors arise subsequent to viral infection (for a review, see Weiss et al. 1982). During the 1960s and 1970s four distinct retroviruses (MH-2, MC29, CMII, and OK10) were isolated from avian neoplasms and shown to be capable of transforming monocytes/macrophages in vitro, and inducing myelocytomas, endotheliomas, and kidney and liver tumors in chickens (Mladenov et al. 1967; Graf and Beug 1978). The grouping of these four viruses based on their transforming properties turned out to be propitious in that molecular analyses eventually revealed that they possess a common genetic element closely correlated with cell transformation, but not related to virus structural genes nor present in other transforming retroviruses (Sheiness et al. 1978; Bister and Duesberg 1979; Duesberg and Vogt 1979; Hu et al. 1979). Furthermore, deletions within this element crippled the transforming activity of the retrovirus (Ramsay et al. 1980; Bister et al. 1982). This unique viral oncogene was called v-myc, for myelocytomatosis (contending names were mcv and mac), and was shown to be acquired from a highly conserved cellular gene denoted c-myc (referred to here as MYC) (Roussel et al. 1979; Sheiness and Bishop 1979).
Association of MYC with Tumorigenesis
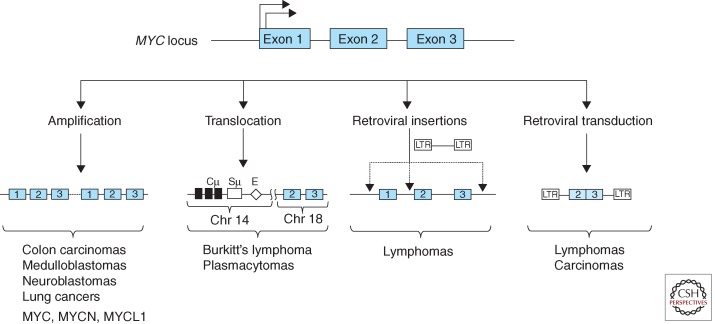
The link between MYC and cancer was greatly strengthened by the discovery that avian leukosis virus (ALV)-induced B-cell lymphomas consistently contained retroviral insertions in the vicinity of the MYC gene (Hayward et al. 1981). Unlike the v-myc-containing retroviruses described above, the ALV genome lacks any oncogenic sequences. However in a small subset of ALV integration sites within the host genome, the retroviral enhancer/promoter was found to be inserted proximal to the MYC locus, resulting in MYC overexpression and deregulation (Fig. 1) (Payne et al. 1982). Therefore, the oncogenic properties of MYC are not only manifested by the retroviral-transduced v-myc but can also occur as a consequence of viral perturbation of the cellular MYC gene. Within the following year it became clear that the cellular MYC gene is complicit in neoplasms that lack any retroviral involvement. Consistent chromosomal translocations involving immunoglobulin (Ig) genes had been previously reported in both mineral oil plasmacytomas in mice and in human Burkitt’s lymphomas. These translocations were then shown to juxtapose rearranged Ig sequences with non-Ig sequences at the translocation breakpoints, and it was quickly established that the non-Ig sequences originated from a rearranged MYC locus (Dalla-Favera et al. 1982a; Shen-Ong et al. 1982; Taub et al. 1982). That MYC is crucial for the genesis of B-cell lymphomas was shown through the production of transgenic mice carrying an Ig enhancer linked to MYC (Eμ-MYC mice) that rapidly develop aggressive B-cell lymphomas with high penetrance (Adams et al. 1985).
Figure 1.
Genetic rearrangements associated with the MYC locus in diverse cancers (partial list).
The consistent association uncovered in the 1980s between hematopoietic neoplasms and MYC gene alterations owing to retroviruses and chromosomal translocations turned out to only be the tip of the iceberg. MYC gene amplification had initially been reported in a myeloid leukemia cell line (Collins and Groudine 1982; Dalla-Favera et al. 1982b) and was soon shown to also occur in colon carcinomas (Alitalo et al. 1983). Moreover, analysis of neuroblastomas, a frequent childhood solid tumor arising from the peripheral nervous system, revealed gene amplifications in a MYC paralog that was designated N-myc (herein MYCN) (Kohl et al. 1983; Schwab et al. 1983). The amplification of MYCN in neuroblastomas was associated with poor clinical outcome (Brodeur et al. 1984). Another MYC family member, L-myc (MYCL1), was found to be amplified in small cell lung carcinomas (Nau et al. 1985). Therefore all three vertebrate MYC family genes (MYC, MYCN, and MYCL1) are linked to the etiology of human cancers. Figure 1 summarizes several modes of MYC gene family deregulation.
The initial findings during the 1980s of tumor-related genetic alterations in MYC gene family members opened the gates to a veritable flood of further studies that have served to firmly establish an extraordinarily pervasive link between MYC functions and the generation, progression, and maintenance of a wide range of neoplasms (for reviews, see Nesbit et al. 1999; Vita and Henriksson 2006; Beroukhim et al. 2010). Arguably, deregulation of MYC family genes underlies the etiology of all cancers. The predominant alterations, such as viral insertional events, chromosomal translocations, and gene amplifications that occur in tumor-associated MYC rarely disrupt or mutate its protein- coding sequences (Fig. 1). This is in contrast to many other oncogenes such as Src, Ras, and Abl in which mutations or deletions within autoinhibitory protein domains alleviate inhibition and trigger oncogenic activity. The overarching theme in MYC deregulation appears to be events that uncouple MYC expression from its normal regulatory constraints, frequently resulting in high levels of MYC expression coupled with an inability to modulate this expression in response to normal cellular and extracellular signals. It is important to note that, in addition to the dramatic cancer-associated rearrangements occurring directly at the MYC locus, polymorphisms in DNA sequences distal to MYC are known to exert long-range effects on MYC regulation (Wasserman et al. 2010; Wright et al. 2010; Sur et al. 2012; see Cole 2014). Moreover, aberrant MYC expression can result from defects in signal-transduction pathways that activate or repress MYC family gene expression at the transcriptional and posttranscriptional levels (Fig. 2). Among the pathways that heighten MYC expression and are frequently mutated in cancers are Wnt-β-catenin, Sonic hedgehog-Gli, and Notch. For reviews on the role of MYC family genes in neoplasia, see Huang and Weiss (2013), Kuzyk and Mai (2014), Roussel and Robinson (2013), Gabay et al. (2014), and Schmitz et al. (2014). Therapeutic approaches to cancer through inhibition of MYC activity are reviewed in Bradner (2014), Gabay et al. (2014), and Cermelli et al. (2014).
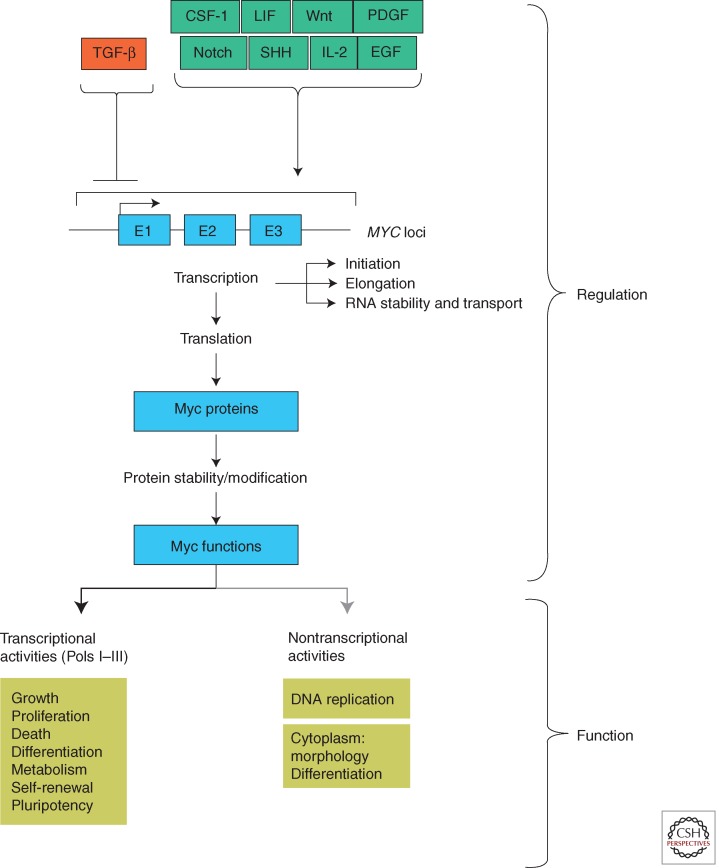
Figure 2.
The MYC pathway. Diagrammed is a partial list of environmental signals that lead to changes in MYC expression. The several levels at which MYC, RNA, and MYC protein are known to be regulated are indicated. Cellular readouts related to transcriptional and nontranscriptional activities ascribed to MYC protein are listed.
MYC-ENCODED PROTEINS
In 1977, an avian cell line transformed by the v-myc-containing retrovirus MC29 was found to produce an unusual viral-related protein of 110,000 kDa (Bister et al. 1977). This protein represented the fusion of a truncated retrovirus core protein precursor with the MYC protein (Mellon et al. 1978; Rettenmier et al. 1979). At the time, all retroviral oncoproteins were known to be localized to the cytoplasm or the plasma membrane. Therefore, it was something of a surprise to find the MC29 virus MYC-containing protein predominantly localized to the cell nucleus (Abrams et al. 1982; Donner et al. 1982; Hann et al. 1983). Later work showed that all cellular MYC family proteins are also nuclear. These early studies suggested MYC to be rather unique among retroviral oncoproteins and pointed to a potentially direct involvement in gene regulation and nuclear function. Subsequently, a large number of distinct oncoproteins were discovered to be localized to the nucleus and, like MYC, involved in transcription (e.g., MYB, FOS, and JUN).
MYC Protein Organization
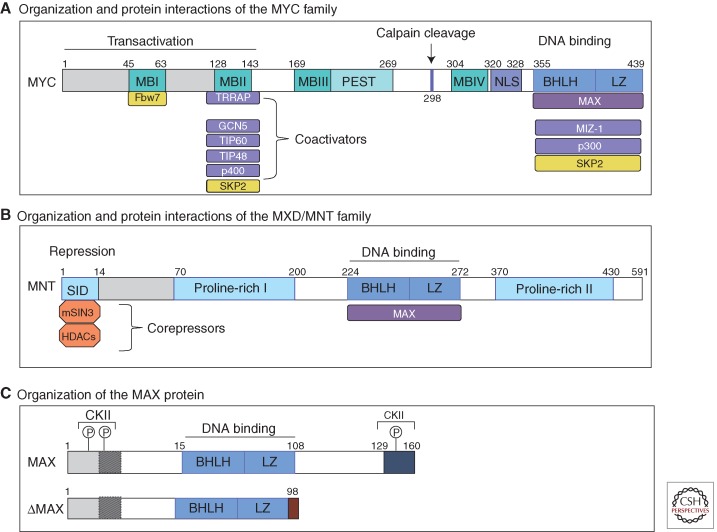
The overall organization of MYC proteins is similar among MYC paralogs and, to a lesser extent, its orthologs throughout evolution. As diagrammed in Figure 3A for human MYC, the 439 amino acid protein sequence contains several highly conserved regions that are functionally important. These regions are organized in roughly the same way in the MYCN and MYCL1 proteins, whereas many of the sequences outside of the conserved regions are divergent among the three paralogs. In broad terms, MYC proteins can be thought of as possessing (1) a large unstructured amino-terminal region containing the conserved regions known as MYC boxes (MBI, MBII) involved in transcriptional activation; (2) a middle segment rich in proline, glutamic acid, threonine, and proline residues (PEST) as well as two conserved MYC boxes (MBIII and MBIV), and a nuclear localization sequence; and (3) an ∼100-amino-acid carboxy-terminal region comprising the basic helix-loop-helix leucine zipper (bHLHZ) domain. MYC family proteins and the other proteins in the extended network (see Fig. 5) form a related subgroup within the much larger class of bHLHZ transcription factors (see below) (Lassar et al. 1989; Murre et al. 1989; Skinner et al. 2010).
Figure 3.
MYC, MNT, and MAX protein organization. Schematic representation of human: (A) MYC protein with its major domains and interacting partners. In blue are major functionally characterized transcriptional-binding partners of MYC, and in yellow major E3 ligases involved in MYC turnover. (B) MNT, as a representative of MXD family proteins. (C) MAX protein. Alternative splicing generates several MAX isoforms. The predominantly expressed MAX proteins (151 and 160 residues in length) differ by a nine-amino-acid segment proximal to the amino terminus (shaded box). In addition, in ΔMAX, the carboxy-terminal 61 amino acids (including the last leucine in the HLHZip) are replaced by five residues before terminating within an alternative exon. Also indicated are casein kinase II (CKII) phosphorylation sites that block Max homodimerization, but not heterodimerization with MYC. MB, MYC boxes; SID, SIN3-interacting domain (see O’Shea and Ayer 2013 for organization of MondoA).
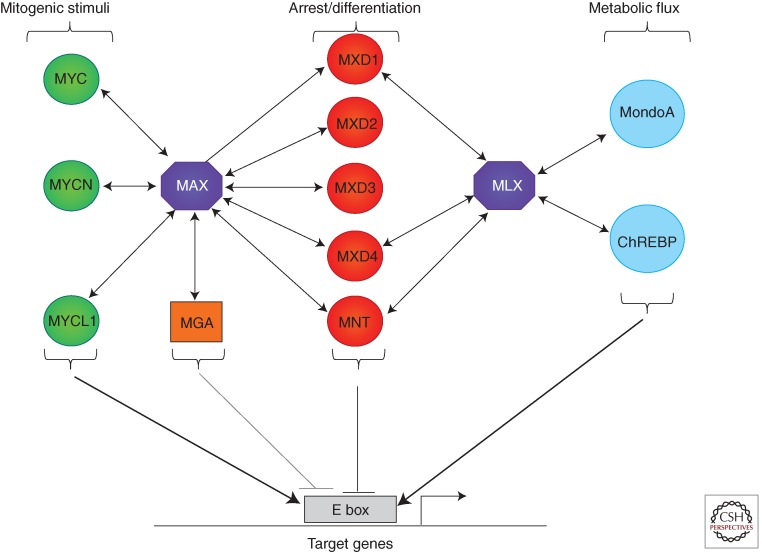
Figure 5.
Diagram of the extended MAX–MLX network. Double-headed arrows indicate individual interactions between network components.
MYC Heterodimerization and DNA Binding
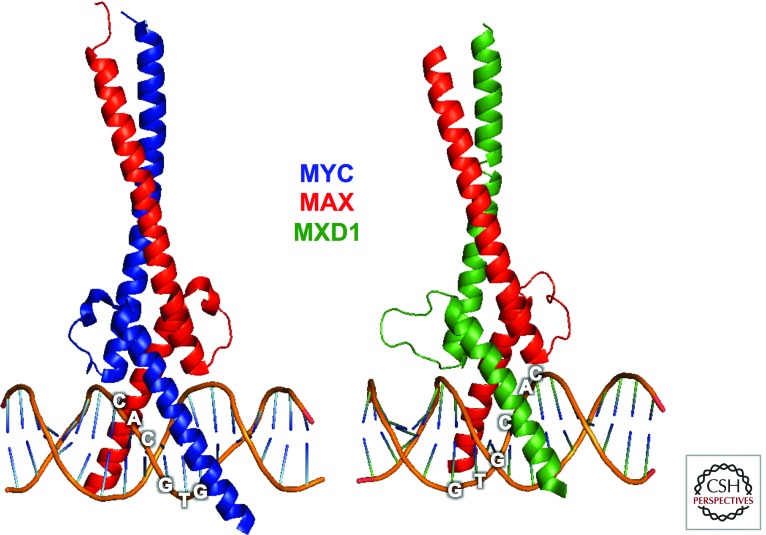
Dimerization among proteins of the bHLHZ class is typically mediated through the two HLHZ interfaces, which interact to form a stable four-helix bundle. The resulting dimer specifically binds DNA through formation of induced-fit helices by the basic regions that straddle the DNA double helix and make specific base contacts within the major groove of DNA (Ferre-D’Amare et al. 1993, 1994). In the case of MYC, homodimerization does not occur under physiological conditions, but a highly specific interaction with the small bHLHZ protein named MAX results in stable heterodimer formation with specific DNA-binding activity (Figs. 3C and 4) (Blackwood and Eisenman 1991; Blackwood et al. 1992; Nair and Burley 2003). Heterodimerization with MAX is essential for MYC association with E-box DNA sequences (5′-CACGTG-3′) and stimulation of transcription at promoter-proximal E boxes (Kretzner et al. 1992; Amati et al. 1993). Furthermore, the MYC–MAX dimeric HLHZ region presents a large solvent-accessible surface area (∼1000 Å) forming a platform for binding by other factors, such as Miz-1 and SKP2 (Peukert et al. 1997; Cheng et al. 1999; Nair and Burley 2003; von der Lehr et al. 2003). The structural studies indicate that MYC–MAX bHLHZ dimers can oligomerize to form tetramers (Nair and Burley 2003) but the physiological relevance of this higher-order form has not been unambiguously validated (Walhout et al. 1997; Vervoorts and Luscher 1999; Lebel et al. 2007).
Figure 4.
X-ray structures of (left) MYC–MAX (PDB: 1NKP), and (right) MXD1–MAX (PDB: 1NLW) bHLHZ dimers bound to E-box (5′-CACGTG-3′) DNA sequences at 19-nm and 20-nm resolution, respectively (Nair and Burley 2003). (Image created with the PyMOL Molecular Graphics System, Version 1.5.0.4, Schrödinger, LLC.)
MAX also homodimerizes, albeit weakly, relative to its heterodimerization with MYC. Moreover phosphorylation of MAX, while not interfering with its heterodimerization with MYC, inhibits MAX homodimerization in vivo (Berberich and Cole 1992), a finding consistent with the result that enforced MAX expression blocks MYC biological activity, probably through competition for E-box-binding sites (Lindeman et al. 1995; Canelles et al. 1997). Several alternatively spliced forms of MAX are expressed, one of which, ΔMAX, lacks the 62-residue carboxy-terminal region but retains the entire bHLHZ except for the last leucine of the Zipper (Fig. 3C) (Blackwood and Eisenman 1991; Makela et al. 1992). Induction of an alternative splicing factor, following activating EGFR mutation in glioblastoma, generates ΔMAX, which dimerizes with MYC and augments MYC- transforming activity (Makela et al. 1992; Babic et al. 2013). The mechanism underlying the elevated MYC activity is unknown but we surmise that the carboxyl terminus of MAX normally permits association of a negative regulatory factor with the heterodimer.
Although many biological functions of MYC family proteins appear to be dependent on their interaction with MAX, there is considerable evidence for MAX-independent activities of MYC (Hopewell and Ziff 1995; Steiger et al. 2008; Gallant 2013). Furthermore, MYC proteins, with or without MAX, can be detected at non-E-box DNA sequences through interaction with other DNA-binding proteins including NF-Y, and subunits of RNA polymerase III (Izumi et al. 2001; Gomez-Roman et al. 2003; Steiger et al. 2008; Sabò and Amati 2014). Perhaps such MAX-independent activities of MYC are the basis for the connection between MAX loss-of-function mutations and human pheochromocytomas (Comino-Mendez et al. 2011).
MYC Box Functions
Of the highly conserved MYC box regions within MYC family proteins, MBI and MBII are the best characterized (Fig. 3A). MBI serves as a phosphodegron and is involved in the ubiquitylation and proteasomal degradation of MYC. MYC proteins are very unstable with half-lives of 20–30 min in many normal cells (Hann and Eisenman 1984). However, the exact half-lives of MYC family proteins are dependent on physiological context, and, in many tumors, stabilization of MYC contributes to its deregulation (Salghetti et al. 1999; Gregory and Hann 2000; Sears et al. 2000; Cartwright et al. 2005). Multiple ubiquitin ligases have recently been shown to control MYC stability (see Farrell and Sears 2014). One of these ubiquitin ligases, FBW7, regulates MYC and MYCN stability in response to phosphorylation of Serine 62 and Threonine 58 within MBI (Welcker et al. 2004a,b; Yada et al. 2004). Interestingly many human B-cell lymphomas contain point mutations in MBI that block FBW7 binding and augment MYC stability (Bahram et al. 2000). MBI mutations have been shown to increase oncogenicity in several tumor models (Hemann et al. 2005; Wang et al. 2011b; B Freie and RN Eisenman, unpubl.). Conversely, tumorigenic phenotypes associated with Fbw7 mutations have been linked, for example in lymphoid and myeloid leukemias, to increased MYC protein stability. However, this ligase has many regulatory proteins as substrates and it is unclear to what extent they also contribute to Fbw7 oncogenicity (Welcker and Clurman 2008; King et al. 2013).
MBII, the most studied region within the MYC transactivation domain (TAD), functions as a hub for binding to multiple key interactors including components of histone acetyltransferase (HAT) complexes such as TRAPP-GCN5, Tip60, and Tip48 (McMahon et al. 1998) to promote histone acetylation and gene activation. MBII is important for most known MYC activities (Stone et al. 1987). Moreover, MBII is involved in MYC protein turnover because it is a docking site for SKP2, one of several E3 ligases, in addition to Fbw7, involved in the degradation of MYC (Kim et al. 2003; von der Lehr et al. 2003) (see Farrell and Sears 2014 for a detailed discussion of mechanisms underlying MYC degradation).
The MBI-MBII TAD region is also involved in association with other effectors of MYC activity such as the bromodomain protein BRD4 and the P-TEFb (cyclin T1, CDK9) transcriptional pause-release complex (Eberhardy and Farnham 2002; Kanazawa et al. 2003; Gargano et al. 2007; Wu et al. 2013; Rahl and Young 2014). In addition to MBI and MBII, there are conserved sequences within the central regions of MYC that are considered to be functionally important (Fig. 3). These include a nuclear localization signal (NLS), as well as MBIII and MBIV implicated in MYC cellular-transforming activity, transcription, and apoptosis (Herbst et al. 2004, 2005; Cowling et al. 2006).
MYC as a Sensor and Effector of Cellular Information
MYC normally functions as a sensor, integrating multiple cellular signals and mediating a transcriptional response that drives cell growth and proliferation and impacts differentiation, survival, and pluripotency. This concept of MYC function derives from extensive research relating to, first, how the abundance of the MYC protein is controlled, and second, the molecular functions of the MYC protein (outlined in Fig. 2) (see Levens 2013). Control of MYC gene expression and the production and fate of MYC protein occurs at nearly every level known to molecular biology. As mentioned above, activation of MYC transcription is an end point for a broad range of signal-transduction pathways. Transcription factors harnessed by these pathways bind to the MYC promoter to regulate transcription initiation and elongation, dependent on cellular context and chromatin conformation (Liu and Levens 2006; Wierstra and Alves 2008). Other factors appear to control MYC mRNA stability, export, and translation. At the level of the MYC protein, further regulation is exerted through posttranslational modification (Hann 2006) as well as multiple ubiquitin ligases, which together act as arbiters of MYC stability (see Farrell and Sears 2014). Simply put, MYC is under extraordinarily tight regulation by the cell. A corollary of this is that defects in regulation can, and do, occur at many levels, leading to the increased abundance and inappropriate expression of MYC typical of many cancers (see Huang and Weiss 2013; Roussel and Robinson 2013; Gabay et al. 2014; Schmitz et al. 2014). Our increasing knowledge of key regulatory events has led, and will continue to lead, to therapeutic approaches aimed at subverting MYC production (Gustafson and Weiss 2010; Dawson et al. 2011; Delmore et al. 2011; Mertz et al. 2011; Zuber et al. 2011; Loven et al. 2013; Puissant et al. 2013; Bradner 2014).
Once formed, MYC proteins function predominantly in transcriptional regulation. Nonetheless, MYC transcriptional activity in standard reporter assays is considerably weaker than many other well-studied transcription factors. Initial studies focused on identifying what were expected to be a small number of MYC–MAX regulated genes. Over time, the number of these MYC “target genes” continued to grow awkwardly large. Although most targets are activated, a substantial fraction were shown to be repressed by MYC. Eventually, application of methods to detect sequences directly bound by MYC in mammalian and Drosophila cells led to identification of >15% of genomic loci as MYC targets (Fernandez et al. 2003; Orian et al. 2003). A disproportionate number of bound genes appear to be involved in cell growth (i.e., translation, ribosome biogenesis, and metabolic processes) (Zeller et al. 2006). This fit well with microarray expression profiling studies, with genetic analyses, and with other work showing that MYC, in addition to regulating RNA polymerase II transcribed genes, is directly involved in RNA polymerase I and RNA polymerase III transcription (see Campbell and White 2014). Several classes of microRNAs were also found to be regulated by MYC (see Psathas and Thomas-Tikhonenko 2014). The notion emerged of a MYC “signature” encompassing groups of target genes devoted to growth and pluripotency (Kim et al. 2010; Ji et al. 2011). Analysis of target genes indicates that MYC binding results in hyperacetylation of histones, consistent with MYC’s recruitment of HATs (Martinato et al. 2008). However MYC–MAX interacts with a bewildering variety of other factors with diverse activities (chromatin remodelers, demethylases, antipausing factors, as well as other transcription factors such as MIZ-1 and the estrogen receptor) (see Hann 2014). One implication of this is that MYC’s precise molecular function in transcriptional activation and repression may be dependent on the particular factors recruited by MYC, the constellation of other transcription factors proximal to the binding site, and the chromatin context of the target gene (Cheng et al. 2006; Guccione et al. 2006; Eilers and Eisenman 2008).
Recent studies have challenged this polyfunctional view of MYC, as well as the concept of a restricted MYC signature. This work provides evidence that MYC is bound at every active gene in a given cell type and functions to increase transcription at these loci by recruitment of a complex that abrogates transcriptional pausing downstream from the transcription start site (TSS) and thereby promotes transcriptional elongation (Rahl et al. 2010; Lin et al. 2012; Nie et al. 2012). In this view, MYC acts solely as an amplifier of ongoing gene expression, and the apparent repression of target genes is owing to the normalization procedure used or to events occurring as an indirect or secondary response to MYC’s stimulation of elongation (Loven et al. 2012). Not surprisingly, this readjusted concept of MYC function has evoked considerable debate (see Levens 2013; Wiese et al. 2013; Rahl and Young 2014; Sabò and Amati 2014). Many other reviews in the literature examine MYC biological function in the light of these contrasting views.
Although MYC clearly has a major function in transcriptional regulation it is important to note that several distinct nontranscriptional activities of MYC have been reported. MYC was found to directly promote DNA replication by recruiting licensing factors to origins of replication and collaborating with the Werner DNA helicase to accelerate S-phase entry (Dominguez-Sola et al. 2007; Robinson et al. 2009; Dominguez-Sola and Gautier 2014). Another nontranscriptional activity of MYC derives from the cleavage of full-length MYC protein by calpain protease to remove the NLS and the entire bHLHZ domain (Fig. 3A). MYC-Nick, the resulting large amino-terminal segment of MYC, is predominantly cytoplasmic and influences cell morphology, differentiation, and survival at least in part through acetyltransferases bound to MBII (Conacci-Sorrell et al. 2010; M Conacci-Sorrell and RN Eisenman, unpubl.).
BEYOND MYC: THE EXTENDED MAX–MLX NETWORK
The heterodimeric interactions between the bHLHZ domains of MYC family proteins and MAX are striking in terms of both their specificity and evolutionary conservation (Blackwood and Eisenman 1991; Gallant et al. 1996; Nair and Burley 2003) (and see below). However, although MYC does not participate in dimerization with bHLHZ proteins other than MAX, there is considerable evidence that MAX dimerizes with another group of bHLHZ proteins: the MXD family and MGA. Moreover, a MAX-like bHLHZ protein known as MLX, specifically heterodimerizes with MondoA (MLXIP) and ChREBP (MondoB or MLXIPL) as well as with a subset of MXD family proteins. Taken together, the multiple interactions of MAX and MLX appear to constitute an extended network through which MYC, MXD, and Mondo gene families mediate a broad transcriptional response to mitogenic, growth arrest, and metabolic signals (diagrammed in Fig. 5) (see O’Shea and Ayer 2013).
The MXD Protein Family: Antagonists and Enablers of MYC Function
The MXD (originally called MAD) proteins were initially identified in protein interaction screens aimed at discovering novel dimerization partners for MAX (Ayer et al. 1993; Zervos et al. 1993). This family of bHLHZ proteins comprises MXD1–MXD4 and the more distantly related MNT (Hurlin et al. 1995b, 1997; Meroni et al. 1997). In addition, MAX binds to MGA, the largest protein in the MAX network and perhaps the most unusual in that it possesses both T-domain and bHLHZ DNA-binding motifs (Hurlin et al. 1999).
MXD Proteins Interact with the mSin3 Corepressor
In several respects the MXD proteins mirror the MYC family in that they do not homodimerize or bind DNA as monomers, whereas as heterodimers with MAX they specifically bind E-box sequences (Fig. 4). However, unlike MYC–MAX, which generally stimulates transcription, the binding of MXD–MAX heterodimers to promoter-proximal E boxes results in transcriptional repression. The transcriptional repression activity of MXD proteins is derived from their ability to bind the large corepressor complex known as mSIN3 (Ayer et al. 1995; Hurlin et al. 1995b; Schreiber-Agus et al. 1995; Hurlin et al. 1997). All MXD family proteins possess a conserved amino acid sequence (the mSin3 interaction domain, SID) near their amino termini that directly interacts with one of four paired amphipathic α-helical (PAH) domains within mSIN3 (Fig. 3B). The solution structure of the mSin3-PAH2:MXD1-SID interface has been solved and has provided a model for the specificity of this critical interaction (Brubaker et al. 2000; Cowley et al. 2004). There is some evidence that this interaction is regulated. For example, in the case of MNT, phosphorylation through the ERK pathway has been reported to block its association with mSIN3 (Popov et al. 2005).
mSIN3 acts as a scaffold that interacts with numerous factors, including class I histone deacetylases (HDAC1 and HDAC2), whose ability to deacetylate histones H3 and H4 in active chromatin is frequently associated with transcriptional silencing (Hassig et al. 1997; Laherty et al. 1997; Zhang et al. 1997). The functions of other proteins recruited by the mSin3 corepressor complex are less clear, but several of these have also been linked to repression and may further regulate the activities of the MXD family (Alland et al. 1997; Heinzel et al. 1997; Nomura et al. 1999; Shiio et al. 2006).
Antagonism between MYC and MXD
Recruitment of the Sin3-HDAC corepressor by the MXD family contrasts with MYC’s association with the TRRAP-GCN5 coactivator, and other HATs, and suggests that MYC and MXD possess opposing functions. In principle, MYC and MXD may act as antagonists at three levels: (1) competition for available MAX to form heterodimers, (2) competition between heterodimers for E-box-binding sites, and (3) activation versus repression at bound genes. There is considerable biological evidence from overexpression studies that MYC and MXD family proteins are functionally antagonistic. Overexpression of MYC drives growth and proliferation in a wide range of cell types, whereas enforced expression of MXD family members generally arrests growth and proliferation in normal and MYC-transformed cells (Lahoz et al. 1994; Chen et al. 1995; Hurlin et al. 1995b, 1997; Koskinen et al. 1995; Roussel et al. 1996; Iritani et al. 2002; Marcotte et al. 2003). Murine lymphoid cells provide a good example of the opposing effects of MYC and MXD on growth. In primary T cells, MYC is required for the growth and proliferation of immature thymocytes and for antigenic activation (Dose et al. 2006; Wang et al. 2011a). These events are significantly inhibited by ectopic MXD1 expression, as is the expression of a large number of MYC-induced growth-related genes (Iritani et al. 2002). Moreover, overexpression of the sole Drosophila MXD ortholog dMnt inhibits the growth and proliferation of cells in the wing-imaginal disc, functions known to be linked to dMyc activity (Johnston et al. 1999; Loo et al. 2005; Gallant 2013). To date there has been only limited analysis of genomic-binding sites occupied by MXD proteins; however, in both Drosophila and vertebrate cells there appears to be considerable overlap between MYC and MNT sites (Orian et al. 2003; Toyo-oka et al. 2006). Therefore, MXD proteins, at least when overexpressed, appear to possess the capacity to block MYC activity at shared binding sites. It will be interesting to determine whether, and how, MXD antagonism is exerted on MYC’s activity as a transcriptional amplifier.
Regulation of MYC and MXD Expression
An interesting issue is how the apparent antagonism between MYC and MXD is manifested during normal and tumor cell growth. Importantly, the expression patterns of MYC and the different MXD family members are dependent on cell cycle and differentiation status (Queva et al. 1998) (for a summary, see a review by Hooker and Hurlin 2006). Although MYC levels are nearly undetectable during quiescence, MYC RNA and proteins are rapidly induced upon cell-cycle entry and remain relatively constant during cell-cycle progression (Kelly et al. 1983; Hann et al. 1985). In contrast, MXD1, 2, and 4 are present in resting cells and decrease upon mitogen-induced cell-cycle entry, when MYC is induced. Importantly, MYC is down-regulated in many cell types during terminal differentiation, a time during which MXD1, 2, and 4 proteins are expressed. Thus, MYC is largely associated with proliferation, whereas MXD1, 2, and 4 expression is characteristic of nonproliferating cells consistent with findings from the overexpression studies mentioned above (e.g., Lahoz et al. 1994; Hurlin et al. 1995a; Iritani et al. 2002). MXD3 and MNT are interesting exceptions to this inverse pattern of MYC and MXD expression in that they are both expressed during cell proliferation. MNT is present, coincident with MYC, throughout the cell cycle and persists following differentiation, whereas MXD3 expression is restricted to cells in S phase (Queva et al. 2001; Yun et al. 2007).
Differentiation
Induction of MXD1 occurs during terminal differentiation in a wide range of cell types including myeloid, muscle, epidermal, and neuronal cells (Ayer and Eisenman 1993; Larsson et al. 1994; Hurlin et al. 1995a; Queva et al. 1998; Loo et al. 2005). During the transition from proliferation to differentiation, MYC–MAX heterodimers are replaced by MXD1–MAX complexes (Ayer and Eisenman 1993; Hurlin et al. 1995a; Xu et al. 2001). Because both heterodimer pairs specifically bind E-box DNA sequences, it has been surmised that the heterodimer switch during differentiation results in a switch from activation to repression of MYC target genes, an idea supported by findings of decreased histone acetylation and down-regulation of gene expression at several gene promoters known to be regulated by MYC (Bouchard et al. 2001; Xu et al. 2001; Iritani et al. 2002). However, as a systematic study of MXD family binding to genomic DNA has not yet been performed, the full extent to which the widespread stimulation of gene expression mediated by MYC is actually suppressed by MXD1 (or the other MXD family members) during differentiation remains to be determined.
Genetic deletion studies support the view that MXD1 and MXD2 restrain MYC activity. Targeted deletion of MXD1 in mice produced a surprisingly mild phenotype. Overall embryonic and adult development was normal but an increased frequency of immature granulocyte progenitors was apparent (Foley et al. 1998). This was owing to impaired cell-cycle exit of granulocytic precursors resulting in delayed onset of terminal differentiation. The precursors were additionally found to be more sensitive to apoptosis induced upon cytokine removal, enabling the mice to retain nearly normal levels of mature granulocytes. Mice with MXD2 (originally called Mxi1) deletions also developed normally but displayed a hyperplastic phenotype in multiple tissues and ectopic proliferation within several organs as well as a marked sensitivity to neoplasia following chemical carcinogen treatment (Schreiber-Agus et al. 1998; for a review, see Foley and Eisenman 1999). MXD3 null mice are phenotypically normal, although several tissues show an enhanced sensitivity to apoptotic stimuli (Queva et al. 2001). The relatively minor effects of the MXD1 and MXD2 single gene deletions on embryogenesis may be owing to the fact that they are normally expressed during a period (i.e., differentiation) when MYC is strongly down-regulated. Therefore, they may not directly oppose MYC activity but rather function along with other differentiation factors to repress growth and proliferation genes that are stimulated by MYC before differentiation. In contrast, constitutive deletion of MNT, which is induced by mitogenic stimuli and coexpressed with MYC, results in early postnatal lethality (Toyo-oka et al. 2004; Link et al. 2012). Redundancy among MXD paralogs may also contribute to the mild phenotypes of the single-gene MXD1–4 knockouts. However, null mutation of the single MXD ortholog in Drosophila also displayed only a modest effect on growth (Loo et al. 2005) suggesting redundancy cannot fully explain the mild single knockout phenotypes.
Oncogenesis
Because deregulated MYC is fundamental to the establishment and maintenance of many tumor types, it seemed likely that MXD family members could act as tumor suppressors by antagonizing MYC function. Perhaps the most likely contender for tumor suppression activity is MXD2, as its constitutive deletion in mice results in widespread hyperplasia and a tumor-prone phenotype (Schreiber-Agus et al. 1998). MXD2 is located on human chromosome 10q24, a region deleted in a broad spectrum of human tumors and that also contains the PTEN tumor suppressor. Loss of heterozygosity in the MXD2 region, and potentially inactivating mutations in the remaining MXD2 allele, were reported in a subpopulation of prostate cancer cells (Eagle et al. 1995; Prochownik et al. 1998). However, a series of extensive follow-up studies were unable to corroborate these findings (Bartsch et al. 1996; Edwards et al. 1997; Kuczyk et al. 1998). To date there is no compelling evidence that MXD1–4 act as tumor suppressors. Perhaps the loss of MXD proteins that are normally expressed during arrest and differentiation is simply irrelevant in the context of the high levels of deregulated MYC that drive tumor initiation. This may not be the case for MGA and MNT, which appear to act as tumor suppressors.
Potentially inactivating mutations in MGA have been recurrently detected in chronic lymphocytic leukemia (Edelmann et al. 2012; De Paoli et al. 2013) and recent studies on MNT show that it functions as both an antagonist and enabler of MYC’s oncogenic activities. There have been few studies on MGA, but it has been found associated with two different repression complexes (Ogawa et al. 2002; Tahiliani et al. 2007), and in zebrafish the MGA ortholog regulates bmp2b/swirl during gastrulation (ST Dougan, pers. comm.). MNT, as mentioned above, is generally coexpressed with MYC in proliferating cells and MNT null mice display developmental defects and die soon after birth (Toyo-oka et al. 2004). Murine embryonic fibroblasts (MEFs) derived from these mice, or in which MNT has been depleted, resemble cells with activated MYC in that they show marked increases in proliferation rate, enhanced expression of known MYC-regulated genes, and are prone to apoptosis (Hurlin et al. 2003; Nilsson et al. 2004). Mice with conditional MNT deletions in mammary epithelium or T cells show hyperproliferation of these cell types and frequently develop mammary adenocarcinomas and T-cell lymphomas, respectively, late in life (Dezfouli et al. 2006; Toyo-oka et al. 2006). Additionally, in metastatic tumor cells under hypoxic conditions, hypoxia-inducible factors (HIF-1α and HIF-2α) induce a microRNA (miR-210), resulting in MNT down-regulation and an MYC-dependent bypass of cell-cycle arrest (Zhang et al. 2009).
The data described above are consistent with the notion that MNT opposes MYC function. Yet that antagonism acts to favor MYC-induced tumorigenesis because the dominant physiological activity of MNT is to oppose the proapoptotic activity elicited by MYC (Link et al. 2012). In biological settings where MYC protein levels are elevated, cell survival becomes increasingly dependent on MNT as shown by the fact that even a small increase in MYC abundance compromises cell survival in the absence of MNT. The lowered threshold for MYC-induced apoptosis caused by loss of MNT is tied to the aberrant accumulation of reactive oxygen species (ROS) (Link et al. 2012). Therefore, as proposed for MYC-induced neoplasia (Vafa et al. 2002), tumors generated as a consequence of MNT deletion may arise owing to ROS-induced oxidative damage to DNA and mutations that suppress apoptosis (see Kuzyk and Mai 2014). These studies suggest that although MNT can antagonize both MYC-stimulated proliferation and apoptosis, its prosurvival function is critical for MYC-driven tumorigenesis. Comprehensive analysis of genomic binding by MYC and MXD family proteins will be required to more fully understand the functional relationships among these transcription factors.
Expanding the Network: MLX and Its Dimerization Partners
MLX is a MAX-like bHLHZ class protein initially discovered as a dimerization partner for a subset of MXD family proteins, namely, MXD1, MXD4, and MNT (Billin et al. 1999; Meroni et al. 2000). MXD–MLX heterodimers interact with mSIN3, bind E-box DNA sequences, and repress transcription, apparently acting similarly to MXD–MAX dimers. MLX does not appear to associate with MAX or MYC family proteins; however, further analysis revealed two other MLX dimerization partners: MondoA and ChREBP (Fig. 5) (Billin et al. 2000). Both MondoA and ChREBP are cytoplasmic-nuclear shuttling proteins whose nuclear accumulation is triggered by glucose-derived metabolites (Stoltzman et al. 2008; Peterson et al. 2010). MondoA-MLX and ChREBP-MLX dimers bind E-box sequences and regulate genes involved in glucose and glutamine metabolism, processes important for the biology of normal and cancer cells. Therefore, MondoA and ChREBP heterodimers with MLX act as nutrient-sensing transcription factors, and there is considerable evidence indicating that they are critical regulators of cell metabolism. For a detailed discussion of these proteins and their functions see O’Shea and Ayer (2013).
Implications of an Extended MYC Network
The existence of an extended MYC network (Fig. 5) has potentially important implications for understanding MYC functions, the most obvious of which is that MYC does not act alone but within the context of a larger protein interactome. It is also predicted that changes in the abundance of individual network components will have an impact on the activity of all of the network factors through competition for available MAX and MLX as well as for DNA-binding sites. Although initial studies suggested that MAX, a highly stable protein, is present in excess relative to MYC (Blackwood et al. 1992), more recent work indicates that, at least in some biological settings, MYC and MNT compete for binding to limiting amounts of MAX (Walker et al. 2005). MAX availability is further modulated by the turnover of MXD family proteins, which are regulated through ubiquitin-mediated proteasomal degradation and display short half-lives (Zhu et al. 2008). In contrast, MondoA and ChREBP are stable proteins and tight regulation of their transcriptional activity occurs through their nuclear accumulation in response to changes in metabolic flux (see O’Shea and Ayer 2013). Moreover, not all network members are equal when it comes to dimerization with MAX. A live cell bimolecular fluorescence complementation analysis reported different apparent binding affinities for MAX among MXD proteins compared with MYC and differences in subnuclear localization patterns between MYC–MAX and MXD–MAX heterodimers (Grinberg et al. 2004). Although the consequences of these differences in binding and localization are unknown, variation in binding efficiencies are nonetheless compatible with the idea that modulations in the levels of individual family members may have distinct effects on network activity. The possibility that even relatively small changes in the abundance of individual factors may have network-wide consequences perhaps accounts in part for the extraordinary degree of regulation of MYC expression shown at transcriptional, posttranscriptional, and posttranslational levels (Fig. 2) (see Levens 2013; Farrell and Sears 2014).
There is also evidence for regulatory cross talk among network members. To begin with, it has long been known that MYC negatively autoregulates its own expression. This appears to occur through an evolutionarily conserved circuitry involving the Polycomb complex that can be abrogated during tumorigenesis (Grignani et al. 1990; Penn et al. 1990; Goodliffe et al. 2005; Kaur and Cole 2013). Cross-regulation may also extend to other MYC family members as well as other network components (Rosenbaum et al. 1989). MYC, complexed with MIZ-1, has been shown to repress MXD4 in erythroleukemia cells (Kime and Wright 2003). MYC also appears to up-regulate MondoA and ChREBP, factors that in turn influence MYC-driven metabolic reprogramming during tumor progression (Lin et al. 2009; Kaadige et al. 2010; Sloan and Ayer 2010; P Carroll, D Diolaiti, L McFerrin et al., unpubl.). Increased abundance of MondoA and ChREBP would be expected to sequester MLX, potentially blocking the formation of MXD–MLX dimers. At present, there is no information as to whether MXD–MLX and MXD–MAX heterodimers differ functionally. However, recruitment of MLX into MondoA and ChREBP heterodimers would likely increase competition between MYC and coexpressed MXD proteins for available MAX. Perhaps it is an imbalance in the network that is responsible for the hereditary pheochromocytomas that arise in association with inactivating mutations in MAX (Comino-Mendez et al. 2011). We are clearly still at an early stage in understanding the ramifications of network function. We expect that genetic studies involving alterations in the levels of network members combined with chromatin immunoprecipitation analyses of heterodimer binding to DNA will be required to understand the dynamics of the integrated MYC network in greater detail.
EVOLUTION OF A METAZOAN GENE NETWORK
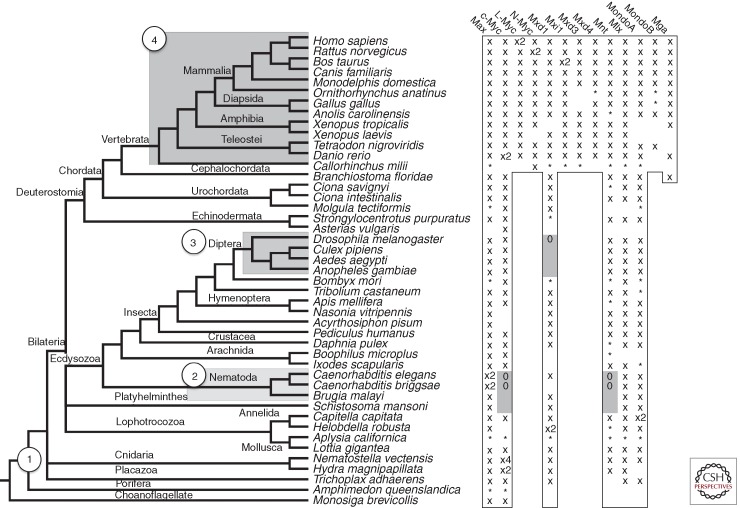
The MAX and MLX networks have been primarily characterized in mice, humans, and Drosophila. Annotation of these proteins indicates that network orthologs have overlapping functions (e.g., mammalian MYC regulates growth and proliferation, whereas Drosophila dMyc predominantly drives growth) (for reviews, see Brown et al. 2008; Bellosta and Gallant 2010; Gallant 2013). Despite this functional overlap, these species differ considerably in complexity and number of members in the extended network. A survey of the animal kingdom reveals these networks all radiated from a common core set of proteins that emerged before animal divergence (Fig. 6) (McFerrin and Atchley 2011). Strikingly, MAX and MLX network members span all known Metazoan lineages, emphasizing the importance of these ancient transcription factors.
Figure 6.
MAX and MLX network protein distribution based on conservation of bHLHZ domains. (Left) Species tree representing organism divergence. Circled numbers correspond to the emergence of the four major networks. (Right) Outlined and gray cells indicate proteins that are expected to be present or absent, respectively, through identifiable conservation of the homologous bHLHZ domain. “X” and “*”, respectively, indicate the full or partial bHLHZ sequence was found, whereas “0” marks where the protein is known to be absent. Note the species tree is based on the relationship among species listed and is not derived from the network proteins listed on the right.
The origin of the MAX and MLX networks dates to over 500 million years ago, with the protein and DNA interactions of these transcription factors predating the origin of animals. In the choanoflagellate Monosiga brevicollis, an ancestor of animals that can grow as a uni- or multicellular organism, MYC and MAX heterodimerize, localize to the nucleus, and bind E boxes (Young et al. 2011). MYC and MAX similarly heterodimerize and target E boxes in the early diploblastic cnidarian Hydra, where Myc shows oncogenic potential and is specifically activated in all proliferating cell types (Hartl et al. 2010). MAX, MYC, MXD, MLX, and Mondo sequences have also been identified in the Placazoan Trichoplax adhaerens, considered the simplest animal with the smallest known genome.
Network Divergence
Within the animal kingdom, lineage-specific radiation and deletion of MAX and MLX network components (based on conservation of bHLHZ domains) gives rise to four main network configurations: Core, Diptera, Nematode, and Vertebrate (numbered 1–4 in Fig. 6). The core network, consisting of MYC, MAX, MNT, MXD, MLX, and Mondo proteins, is the basis from which other animal networks evolved. Nematodes show extensive divergence, presumably owing to a massive gene reduction and rearrangement (Witherspoon and Robertson 2003; Denver et al. 2004; Coghlan 2005), and contain two MAX orthologs (Mxl-1 and Mxl-3), a single MLX ortholog (Mxl-2), a MXD-like protein (MDL-1), and a MYC and Mondo-like protein (MML-1). Mxl-1 and Mxl-3 apparently heterodimerize with MDL-1, whereas Mxl-2 binds MML-1 (Yuan et al. 1998; Gallant 2006; Pickett et al. 2007). Hence, the MLX network is conserved in nematodes, but it is not known if the antagonistic transcriptional regulation characteristic of MYC and MNT is performed by MDL-1 and MML-1.
The Diptera lineage, including fruit flies and mosquitoes, possesses a minimal network in that these organisms contain single orthologs of MYC, MAX, MNT, MLX, and Mondo (Mio) (Gallant et al. 1996; Peyrefitte et al. 2001; Loo et al. 2005; Billin and Ayer 2006). A Drosophila mutation originally designated diminutive (dm), resulting in abnormally small body size and female sterility (Bridges 1935), was later shown to correspond to the locus encoding the MYC ortholog (Gallant et al. 1996; Schreiber-Agus et al. 1997). Extensive studies in Drosophila have shown that dMyc is closely linked to multiple signaling pathways and acts as an essential regulator of growth, proliferation, and apoptosis, whereas dMnt antagonizes dMyc to negatively regulate cell growth and body size (Johnston et al. 1999; Loo et al. 2005; Bellosta and Gallant 2010; Gallant 2013; Johnston 2014). Furthermore, a homologous ubiquitin ligase system in flies and mammals regulates dMyc abundance in response to phosphorylation of a conserved degron similar to vertebrate MBI (Moberg et al. 2004; Welcker et al. 2004b; Yada et al. 2004; Galletti et al. 2009). dMlx and its MondoA/ChREBP-like dimerization partner Mio have been identified in Drosophila and recently shown to regulate sugar sensing and utilization (Havula et al. 2013; Musselman et al. 2013). The existence of this pared-down MAX–MLX network in Drosophila will continue to provide an excellent model for dissecting basic network functions (see Gallant 2013; Johnston 2014).
In contrast, two whole genome duplication (WGD) events, occurring either before or during vertebrate divergence (Dehal and Boore 2005), formed the MYC (MYC, MYCN, MYCL1), MXD (MXD1–4, MNT), and Mondo (MondoA, MondoB/ChREBP) protein families. Only a single copy of MAX and MLX exists in vertebrates despite multiple duplication events, suggesting that the regulation of these proteins is highly controlled by natural selection. Another MAX network member, MGA, also arose during vertebrate divergence and is predicted to be a fourth MYC family member because of its bHLHZ domain similarity (Hurlin et al. 1999; McFerrin and Atchley 2011). In addition, MYC has experienced subsequent and independent duplication events. Old world primates, but not prosimians, show a duplication of MYCL1 denoted MYCL2 (DePinho et al. 1987; Morton et al. 1989; Arnason et al. 1998) that is intronless and presumably arose through a reverse transcription event. Similarly, the murine lineage has an additional MYC family member, S-MYC, presumably formed by a MYCN cDNA sequence reintegrating into the genome (Sugiyama et al. 1989, 1999; Doskocil 1996). Another MYC homolog, B-MYC, exists in the mammalian lineage and lacks the carboxy-terminal bHLHZ sequence (Ingvarsson et al. 1988; Asker et al. 1995). Although B-MYC cannot dimerize with MAX or bind DNA, it appears to associate with other MYC amino-terminal-binding proteins (Burton et al. 2006). Targeted deletion of B-MYC in mice leads to increased MYC levels accompanied by apoptosis and decreased spermatogenesis (Turunen et al. 2012).
Based on the homologous bHLHZ domain that defines the interaction among network members, each of the MAX, MLX, MYC, MNT, MXD, Mondo, and MGA orthologous protein groups distinctly cluster, with paralogs forming distinguishable subgroups and orthologous sequences generally reflecting species divergence (Fig. 6). Nematodes are the exception, with more divergent but clearly related bHLHZ sequences. This indicates that the protein and DNA interactions of network members are largely conserved in animals. Moreover, other functional domains including the MYC boxes (MBI-IV) and Mondo conserved regions (see O’Shea and Ayer 2013) have been preserved throughout animal evolution (McFerrin and Atchley 2012). The level of sequence and functional conservation of network members, even in the most primitive animals, implies that the MAX and MLX networks are involved in fundamental cellular functions dating back millions of years to the emergence of animals.
ACKNOWLEDGMENTS
We are grateful for endless discussions of MYC to members, past and present, of the Eisenman Laboratory. We also thank Peter Hurlin and Don Ayer for critical readings of the manuscript. Research from our laboratory mentioned in this review is supported by grants to R.N.E. from the National Institutes of Health/National Cancer Institute (RO1 CA20525; R37 CA57138).
Footnotes
Editors: Chi V. Dang and Robert N. Eisenman
Additional Perspectives on MYC and the Pathway to Cancer available at www.perspectivesinmedicine.org
REFERENCES
*Reference is also in this collection.
- Abrams H, Rohrschneider L, Eisenman RN 1982. Nuclear location of the putative transforming protein of avian myelocytomatosis virus. Cell 29: 427–439 [DOI] [PubMed] [Google Scholar]
- Adams JM, Harris AW, Pinkert CA, Corcoran LM, Alexander WS, Cory S, Palmiter RD, Brinster RL 1985. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature 318: 533–538 [DOI] [PubMed] [Google Scholar]
- Alitalo K, Schwab M, Lin CC, Varmus HE, Bishop JM 1983. Homogeneously staining chromosomal regions contain amplified copies of an abundantly expressed cellular oncogene (c-myc) in malignant neuroendocrine cells from a human colon carcinoma. Proc Natl Acad Sci 80: 1707–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alland L, Muhle R, Hou H Jr, Potes J, Chin L, Schreiber-Agus N, DePinho RA 1997. Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature 387: 49–55 [DOI] [PubMed] [Google Scholar]
- Amati B, Brooks MW, Levy N, Littlewood TD, Evan GI, Land H 1993. Oncogenic activity of the c-Myc protein requires dimerization with Max. Cell 72: 233–245 [DOI] [PubMed] [Google Scholar]
- Arnason U, Gullberg A, Janke A 1998. Molecular timing of primate divergences as estimated by two nonprimate calibration points. J Mol Evol 47: 718–727 [DOI] [PubMed] [Google Scholar]
- Asker CE, Magnusson KP, Piccoli SP, Andersson K, Klein G, Cole MD, Wiman KG 1995. Mouse and rat B-myc share amino acid sequence homology with the c-myc transcriptional activator domain and contain a B-myc specific carboxy terminal region. Oncogene 11: 1963–1969 [PubMed] [Google Scholar]
- Ayer DE, Eisenman RN 1993. A switch from Myc:Max to Mad:Max heterocomplexes accompanies monocyte/macrophage differentiation. Genes Dev 7: 2110–2119 [DOI] [PubMed] [Google Scholar]
- Ayer DE, Kretzner L, Eisenman RN 1993. Mad: A heterodimeric partner for Max that antagonizes Myc transcriptional activity. Cell 72: 211–222 [DOI] [PubMed] [Google Scholar]
- Ayer DE, Lawrence QA, Eisenman RN 1995. Mad–Max transcriptional repression is mediated by ternary complex formation with mammalian homologs of yeast repressor Sin3. Cell 80: 767–776 [DOI] [PubMed] [Google Scholar]
- Babic I, Anderson ES, Tanaka K, Guo D, Masui K, Li B, Zhu S, Gu Y, Villa GR, Akhavan D, et al. 2013. EGFR mutation-induced alternative splicing of Max contributes to growth of glycolytic tumors in brain cancer. Cell Metab 17: 1000–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahram F, von der Lehr N, Cetinkaya C, Larsson LG 2000. c-Myc hot spot mutations in lymphomas result in inefficient ubiquitination and decreased proteasome-mediated turnover. Blood 95: 2104–2110 [PubMed] [Google Scholar]
- Bartsch D, Peiffer SL, Kaleem Z, Wells SAJ, Goodfellow PJ 1996. Mxi1 tumor suppressor gene is not mutated in primary pancreatic adenocarcinoma. Cancer Lett 102: 73–76 [DOI] [PubMed] [Google Scholar]
- Bellosta P, Gallant P 2010. Myc function in Drosophila. Genes Cancer 1: 542–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berberich SJ, Cole MD 1992. Casein kinase II inihibits the DNA-binding activity of Max homodimers but not Myc/Max heterodimers. Genes Dev 6: 166–176 [DOI] [PubMed] [Google Scholar]
- Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, Barretina J, Boehm JS, Dobson J, Urashima M, et al. 2010. The landscape of somatic copy-number alteration across human cancers. Nature 463: 899–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billin AN, Ayer DE 2006. The Mlx network: Evidence for a parallel Max-like transcriptional network that regulates energy metabolism. Curr Top Microbiol Immunol 302: 255–278 [DOI] [PubMed] [Google Scholar]
- Billin AN, Eilers AL, Queva C, Ayer DE 1999. Mlx, a novel Max-like BHLHZip protein that interacts with the Max network of transcription factors. J Biol Chem 274: 36344–36350 [DOI] [PubMed] [Google Scholar]
- Billin AN, Eilers AL, Coulter KL, Logan JS, Ayer DE 2000. MondoA, a novel basic helix-loop-helix-leucine zipper transcriptional activator that constitutes a positive branch of a Max-like network. Mol Cell Biol 20: 8845–8854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bister K, Duesberg PH 1979. Genetic structure of avian acute leukemia viruses. Cold Spring Harb Symp Quant Biol 44: 801–822 [DOI] [PubMed] [Google Scholar]
- Bister K, Hayman M, Vogt PK 1977. Defectiveness of MC29: Isolation of long-term non-producer cultures and analysis of viral polypeptide synthesis. Virology 82: 431–448 [DOI] [PubMed] [Google Scholar]
- Bister K, Ramsay GN, Hayman MJ 1982. Deletions within the transformation-specific RNA sequences of acute leukemia virus MC29 give rise to partially transformation-defective mutants. J Virol 41: 754–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwood EM, Eisenman RN 1991. Max: A helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc. Science 251: 1211–1217 [DOI] [PubMed] [Google Scholar]
- Blackwood EM, Luscher B, Eisenman RN 1992. Myc and Max associate in vivo. Genes Dev 6: 71–80 [DOI] [PubMed] [Google Scholar]
- Bouchard C, Dittrich O, Kiermaier A, Dohmann K, Menkel A, Eilers M, Luscher B 2001. Regulation of cyclin D2 gene expression by the Myc/Max/Mad network: Myc-dependent TRRAP recruitment and histone acetylation at the cyclin D2 promoter. Genes Dev 15: 2042–2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Bradner J 2014. Inhibiting MYC. Cold Spring Harb Perspect Med 10.1101/cshperspect.a014266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CB 1935. Drosophila melanogaster: Legend for symbols, mutants, valuations. Drosoph Inf Serv 3: 5–19 [Google Scholar]
- Brodeur GM, Seeger RC, Schwab M, Varmus HE, Bishop JM 1984. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science 224: 1121–1124 [DOI] [PubMed] [Google Scholar]
- Brown SJ, Cole MD, Erives AJ 2008. Evolution of the holozoan ribosome biogenesis regulon. BMC Genomics 9: 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker K, Cowley SM, Huang K, Loo L, Yochum GS, Ayer DE, Eisenman RN, Radhakrishnan I 2000. Solution structure of the interacting domains of the Mad-Sin3 complex: Implications for recruitment of a chromatin-modifying complex. Cell 103: 655–665 [DOI] [PubMed] [Google Scholar]
- Burton RA, Mattila S, Taparowsky EJ, Post CB 2006. B-myc: N-terminal recognition of myc-binding proteins. Biochemistry 45: 9857–9865 [DOI] [PubMed] [Google Scholar]
- *.Campbell KJ, White RJ 2014. MYC regulation of cell growth through control of transcription by RNA polymerases I and III. Cold Spring Harb Perspect Med 10.1101/cshperspect.a018408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canelles M, Delgado MD, Hyland KM, Lerga A, Richard C, Dang CV, Leon J 1997. Max and inhibitory c-Myc mutants induce erythroid differentiation and resistance to apoptosis in human myeloid leukemia cells. Oncogene 14: 1315–1327 [DOI] [PubMed] [Google Scholar]
- Cartwright P, McLean C, Sheppard A, Rivett D, Jones K, Dalton S 2005. LIF/STAT3 controls ES cell self-renewal and pluripotency by a Myc-dependent mechanism. Development 132: 885–896 [DOI] [PubMed] [Google Scholar]
- *.Cermelli S, Jang IS, Bernard B, Grandori C 2014. Synthetic lethal screens as a means to understand and treat MYC-driven cancers. Cold Spring Harb Perspect Med 10.1101/cshperspect.a014209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Willingham T, Margraf LR, Schreiber-Agus N, DePinho RA, Nisen PD 1995. Effects of the MYC oncogene antagonist, MAD, on proliferation, cell cycling and the malignant phenotype of human brain tumor cells. Nat Med 1: 638–643 [DOI] [PubMed] [Google Scholar]
- Cheng S-WG, Davies KP, Yung E, Beltran RJ, Yu J, Kalpana GV 1999. c-MYC interacts with INI1/hSNF5 and requires the SWI/SNF complex for activation function. Nat Genet 22: 102–105 [DOI] [PubMed] [Google Scholar]
- Cheng AS, Jin VX, Fan M, Smith LT, Liyanarachchi S, Yan PS, Leu YW, Chan MW, Plass C, Nephew KP, et al. 2006. Combinatorial analysis of transcription factor partners reveals recruitment of c-MYC to estrogen receptor-α responsive promoters. Mol Cell 21: 393–404 [DOI] [PubMed] [Google Scholar]
- Coghlan A 2005. Nematode genome evolution. WormBook 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Cole MD 2014. MYC association with cancer risk and a new model of MYC-mediated repression. Cold Spring Harb Perspect Med 10.1101/cshperspect.a014316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S, Groudine M 1982. Amplification of endogenous myc-related DNA sequences in a human myeloid leukaemia cell line. Nature 298: 679–681 [DOI] [PubMed] [Google Scholar]
- Comino-Mendez I, Gracia-Aznarez FJ, Schiavi F, Landa I, Leandro-Garcia LJ, Leton R, Honrado E, Ramos-Medina R, Caronia D, Pita G, et al. 2011. Exome sequencing identifies MAX mutations as a cause of hereditary pheochromocytoma. Nat Genet 43: 663–667 [DOI] [PubMed] [Google Scholar]
- Conacci-Sorrell M, Ngouenet C, Eisenman RN 2010. Myc-Nick: A cytoplasmic cleavage product of Myc that promotes α-tubulin acetylation and cell differentiation. Cell 142: 480–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley SM, Kang RS, Frangioni JV, Yada JJ, DeGrand AM, Radhakrishnan I, Eisenman RN 2004. Functional analysis of the Mad1-mSin3A repressor–corepressor interaction reveals determinants of specificity, affinity, and transcriptional response. Mol Cell Biol 24: 2698–2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowling VH, Chandriani S, Whitfield ML, Cole MD 2006. A conserved Myc protein domain, MBIV, regulates DNA binding, apoptosis, transformation, and G2 arrest. Mol Cell Biol 26: 4226–4239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla-Favera R, Bregni M, Erikson J, Patterson D, Gallo RC, Croce CM 1982a. Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc Natl Acad Sci 79: 7824–7827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla-Favera R, Wong-Staal F, Gallo RC 1982b. Onc gene amplification in promyelocytic leukaemia cell line HL-60 and primary leukaemic cells of the same patient. Nature 299: 61–63 [DOI] [PubMed] [Google Scholar]
- Dawson MA, Prinjha RK, Dittmann A, Giotopoulos G, Bantscheff M, Chan WI, Robson SC, Chung CW, Hopf C, Savitski MM, et al. 2011. Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Nature 478: 529–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehal P, Boore JL 2005. Two rounds of whole genome duplication in the ancestral vertebrate. PLoS Biol 3: e314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmore JE, Issa GC, Lemieux ME, Rahl PB, Shi J, Jacobs HM, Kastritis E, Gilpatrick T, Paranal RM, Qi J, et al. 2011. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 146: 904–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denver DR, Morris K, Lynch M, Thomas WK 2004. High mutation rate and predominance of insertions in the Caenorhabditis elegans nuclear genome. Nature 430: 679–682 [DOI] [PubMed] [Google Scholar]
- De Paoli L, Cerri M, Monti S, Rasi S, Spina V, Bruscaggin A, Greco M, Ciardullo C, Fama R, Cresta S, et al. 2013. MGA, a suppressor of MYC, is recurrently inactivated in high risk chronic lymphocytic leukemia. Leuk Lymphoma 54: 1087–1090 [DOI] [PubMed] [Google Scholar]
- DePinho R, Mitsock L, Hatton K, Ferrier P, Zimmerman K, Legouy E, Tesfaye A, Collum R, Yancopoulos G, Nisen P, et al. 1987. Myc family of cellular oncogenes. J Cell Biochem 33: 257–266 [DOI] [PubMed] [Google Scholar]
- Dezfouli S, Bakke A, Huang J, Wynshaw-Boris A, Hurlin PJ 2006. Inflammatory disease and lymphomagenesis caused by deletion of the Myc antagonist Mnt in T cells. Mol Cell Biol 26: 2080–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Dominguez-Sola D, Gautier J 2014. MYC and the control of DNA replication. Cold Spring Harb Perspect Med 10.1101/cshperspect.a014423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Sola D, Ying CY, Grandori C, Ruggiero L, Chen B, Li M, Galloway DA, Gu W, Gautier J, Dalla-Favera R 2007. Non-transcriptional control of DNA replication by c-Myc. Nature 448: 445–451 [DOI] [PubMed] [Google Scholar]
- Donner P, Greiser-Wilke I, Moelling K 1982. Nuclear localization and DNA binding of the transforming gene product of avian myelocytomatosis virus. Nature 296: 262–266 [DOI] [PubMed] [Google Scholar]
- Dose M, Khan I, Guo Z, Kovalovsky D, Krueger A, von Boehmer H, Khazaie K, Gounari F 2006. c-Myc mediates pre-TCR-induced proliferation but not developmental progression. Blood 108: 2669–2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doskocil J 1996. The amplification of oligonucleotide themes in the evolution of the myc protooncogene family. J Mol Evol 42: 512–524 [DOI] [PubMed] [Google Scholar]
- Duesberg PH, Vogt PK 1979. Avian acute leukemia viruses MC29 and MH2 share specific RNA sequences: Evidence for a second class of transforming genes. Proc Natl Acad Sci 76: 1633–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle LR, Yin X, Brothman AR, Williams BJ, Atkin NB, Prochownik EV 1995. Mutation of the MXI1 gene in prostate cancer. Nat Genet 9: 249–255 [DOI] [PubMed] [Google Scholar]
- Eberhardy SR, Farnham PJ 2002. Myc recruits P-TEFb to mediate the final step in the transcriptional activation of the cad promoter. J Biol Chem 277: 40156–40162 [DOI] [PubMed] [Google Scholar]
- Edelmann J, Holzmann K, Miller F, Winkler D, Buhler A, Zenz T, Bullinger L, Kuhn MW, Gerhardinger A, Bloehdorn J, et al. 2012. High-resolution genomic profiling of chronic lymphocytic leukemia reveals new recurrent genomic alterations. Blood 120: 4783–4794 [DOI] [PubMed] [Google Scholar]
- Edwards SM, Dearnaley DP, Ardern-Jones A, Hamoudi RA, Easton DF, Ford D, Shearer R, Dowe A 1997. No germline mutations in the dimerization domain of MXI1 in prostate cancer clusters. Brit J Cancer 76: 992–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers M, Eisenman RN 2008. Myc’s broad reach. Genes Dev 22: 2755–2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellermann V, Bang O 1908. Experimentelle leukamie bei hühnern [Experimental leukemia in chickens]. Fizentralbl Bakteril 46: 595–609 [Google Scholar]
- *.Farrell AS, Sears RC 2014. MYC degradation. Cold Spring Harb Perspect Med 10.1101/cshperspect.a014365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez PC, Frank SR, Wang L, Schroeder M, Liu S, Greene J, Cocito A, Amati B 2003. Genomic targets of the human c-Myc protein. Genes Dev 17: 1115–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre-D’Amare AR, Prendergast GC, Ziff EB, Burley SK 1993. Recognition of Max by its cognate DNA through a dimeric b/HLH/Z domain. Nature 363: 38–46 [DOI] [PubMed] [Google Scholar]
- Ferre-D’Amare AR, Pognonec P, Roeder RG, Burley SK 1994. Structure and function of the b/HLH/Z domain of USF. EMBO J 13: 180–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley KP, Eisenman RN 1999. Two Mad tails: What the recent knockouts of Mad1 and Mxi1 tell us about the MYC/MAX/MAD network. Biochim Biophys Acta Bio 1423: M37–M47 [DOI] [PubMed] [Google Scholar]
- Foley KP, McArthur GA, Quéva C, Hurlin PJ, Soriano P, Eisenman RN 1998. Targeted disruption of Mad1 inhibits cell cycle exit during granulocyte differentiation. EMBO J 17: 774–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Gabay M, Li Y, Felsher DW 2014. MYC activation is a hallmark of cancer initiation and maintenance. Cold Spring Harb Perspect Med 10.1101/cshperspect.a014241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant P 2006. Myc/Max/Mad in invertebrates. The evolution of the Max network. Curr Top Micro Immunol 302: 235–253 [DOI] [PubMed] [Google Scholar]
- *.Gallant P 2013. Myc function in Drosophila. Cold Spring Harb Perspect Med 3: a014324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant P, Shiio Y, Cheng PF, Parkhurst S, Eisenman RN 1996. Myc and Max homologs in Drosophila. Science 274: 1523–1527 [DOI] [PubMed] [Google Scholar]
- Galletti M, Riccardo S, Parisi F, Lora C, Saqcena MK, Rivas L, Wong B, Serra A, Serras F, Grifoni D, et al. 2009. Identification of domains responsible for ubiquitin-dependent degradation of dMyc by glycogen synthase kinase 3β and casein kinase 1 kinases. Mol Cell Biol 29: 3424–3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargano B, Amente S, Majello B, Lania L 2007. P-TEFb is a crucial co-factor for Myc transactivation. Cell Cycle 6: 2031–2037 [DOI] [PubMed] [Google Scholar]
- Gomez-Roman N, Grandori C, Eisenman RN, White RJ 2003. Direct activation of RNA polymerase III transcription by c-Myc. Nature 421: 290–294 [DOI] [PubMed] [Google Scholar]
- Goodliffe JM, Wieschaus E, Cole MD 2005. Polycomb mediates Myc autorepression and its transcriptional control of many loci in Drosophila. Genes Dev 19: 2941–2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf T, Beug H 1978. Avian leukemia viruses: Interaction with their target cells in vitro and in vivo. Biochim Biophys Acta 516: 269–299 [DOI] [PubMed] [Google Scholar]
- Gregory MA, Hann SR 2000. c-Myc proteolysis by the ubiquitin-proteasome pathway: Stabilization of c-Myc in Burkitt’s lymphoma cells. Mol Cell Biol 20: 2423–2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grignani F, Lombardi L, Inghirami G, Sternas L, Cechova K, Dalla-Favera R 1990. Negative autoregulation of c-myc gene expression is inactivated in transformed cells. EMBO J 9: 3913–3922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinberg AV, Hu CD, Kerppola TK 2004. Visualization of Myc/Max/Mad family dimers and the competition for dimerization in living cells. Mol Cell Biol 24: 4294–4308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guccione E, Martinato F, Finocchiaro G, Luzi L, Tizzoni L, Dall’Olio V, Zardo G, Nervi C, Bernard L, Amati B 2006. Myc-binding-site recognition in the human genome is determined by chromatin context. Nat Cell Biol 8: 764–770 [DOI] [PubMed] [Google Scholar]
- Gustafson WC, Weiss WA 2010. Myc proteins as therapeutic targets. Oncogene 29: 1249–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann SR 2006. Role of post-translational modifications in regulating c-Myc proteolysis, transcriptional activity and biological function. Semin Cancer Biol 16: 288–302 [DOI] [PubMed] [Google Scholar]
- Hann SR, Eisenman RN 1984. Proteins encoded by the human c-myc oncogene: Differential expression in neoplastic cells. Mol Cell Biol 4: 2486–2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann SR, Abrams HD, Rohrschneider LR, Eisenman RN 1983. Proteins encoded by v-myc and c-myc oncogenes: Identification and localization in acute leukemia virus transformants and bursal lymphoma cell lines. Cell 34: 789–798 [DOI] [PubMed] [Google Scholar]
- Hann SR, Thompson CB, Eisenman RN 1985. c-myc Oncogene protein synthesis is independent of the cell cycle in human and avian cells. Nature 314: 366–369 [DOI] [PubMed] [Google Scholar]
- *.Hann SR 2014. Myc protein interactions. Cold Spring Harb Perspect Med 10.1101/cshperspect.a014399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl M, Mitterstiller AM, Valovka T, Breuker K, Hobmayer B, Bister K 2010. Stem cell-specific activation of an ancestral myc protooncogene with conserved basic functions in the early metazoan Hydra. Proc Natl Acad Sci 107: 4051–4056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassig CA, Fleischer TC, Billin AN, Schreiber SL, Ayer DE 1997. Histone deacetylase activity is required for full transcriptional repression by mSin3A. Cell 89: 341–347 [DOI] [PubMed] [Google Scholar]
- Havula E, Teesalu M, Hyotylainen T, Seppala H, Hasygar K, Auvinen P, Oresic M, Sandmann T, Hietakangas V 2013. Mondo/ChREBP-Mlx-regulated transcriptional network is essential for dietary sugar tolerance in Drosophila. PLoS Genet 9: e1003438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward WS, Neel BG, Astrin SM 1981. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature 290: 475–480 [DOI] [PubMed] [Google Scholar]
- Heinzel T, Lavinsky RM, Mullen T-M, Söderström M, Laherty CD, Torchia J, Yang W-M, Brard G, Ngo SG, Davie JR, et al. 1997. N-CoR, mSin3, and histone deacetylase-containing complexes mediates transcriptional repression. Nature 387: 43–48 [DOI] [PubMed] [Google Scholar]
- Hemann MT, Bric A, Teruya-Feldstein J, Herbst A, Nilsson JA, Cordon-Cardo C, Cleveland JL, Tansey WP, Lowe SW 2005. Evasion of the p53 tumour surveillance network by tumour-derived MYC mutants. Nature 436: 807–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst A, Salghetti SE, Kim SY, Tansey WP 2004. Multiple cell-type-specific elements regulate Myc protein stability. Oncogene 23: 3863–3871 [DOI] [PubMed] [Google Scholar]
- Herbst A, Hemann MT, Tworkowski KA, Salghetti SE, Lowe SW, Tansey WP 2005. A conserved element in Myc that negatively regulates its proapoptotic activity. EMBO Rep 6: 177–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker CW, Hurlin PJ 2006. Of Myc and Mnt. J Cell Sci 119: 208–216 [DOI] [PubMed] [Google Scholar]
- Hopewell R, Ziff EB 1995. The nerve growth factor-responsive PC12 cell line does not express the Myc dimerization partner Max. Mol Cell Biol 15: 3470–3478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu SS, Lai MM, Vogt PK 1979. Genome of avian myelocytomatosis virus MC29: Analysis by heteroduplex mapping. Proc Natl Acad Sci 76: 1265–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Huang M, Weiss WA 2013. Neuroblastoma and MYCN. Cold Spring Harb Perspect Med 3: a014415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlin PJ, Foley KP, Ayer DE, Eisenman RN, Hanahan D, Arbeit JM 1995a. Regulation of Myc and Mad during epidermal differentiation and HPV-associated tumorigenesis. Oncogene 11: 2487–2501 [PubMed] [Google Scholar]
- Hurlin PJ, Quéva C, Koskinen PJ, Steingrímsson E, Ayer DE, Copeland NG, Jenkins NA, Eisenman RN 1995b. Mad3 and Mad4: Novel Max-interacting transcriptional repressors that suppress c-Myc-dependent transformation and are expressed during neural and epidermal differentiation. EMBO J 14: 5646–5659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlin P, Queva C, Eisenman RN 1997. Mnt, a novel Max-interacting protein is coexpressed with Myc in proliferating cells and mediates repression at Myc-binding sites. Genes Dev 11: 44–58 [DOI] [PubMed] [Google Scholar]
- Hurlin PJ, Steingrimsson E, Copeland NG, Jenkins NA, Eisenman RN 1999. Mga, a dual-specificity trasncription factor that interacts with Max and contains a T-domain DNA-binding motif. EMBO J 18: 7019–7028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlin PJ, Zhou ZQ, Toyo-Oka K, Ota S, Walker WL, Hirotsune S, Wynshaw-Boris A 2003. Deletion of Mnt leads to disrupted cell cycle control and tumorigenesis. EMBO J 22: 4584–4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingvarsson S, Asker C, Axelson H, Klein G, Sumegi J 1988. Structure and expression of B-myc, a new member of the myc gene family. Mol Cell Biol 8: 3168–3174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iritani BM, Delrow J, Grandori C, Gomez I, Klacking M, Carlos LS, Eisenman RN 2002. Modulation of T-lymphocyte development, growth and cell size by the Myc antagonist and transcriptional repressor Mad1. EMBO J 21: 4820–4830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi H, Molander C, Penn LZ, Ishisaki A, Kohno K, Funa K 2001. Mechanism for the transcriptional repression by c-Myc on PDGF β-receptor. J Cell Sci 114: 1533–1544 [DOI] [PubMed] [Google Scholar]
- Ji H, Wu G, Zhan X, Nolan A, Koh C, De Marzo A, Doan HM, Fan J, Cheadle C, Fallahi M, et al. 2011. Cell-type independent MYC target genes reveal a primordial signature involved in biomass accumulation. PLoS ONE 6: e26057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Johnston LA 2014. Socializing with Myc: Cell competition in development and as a model for premalignant cancer. Cold Spring Harb Perspect Med 10.1101/cshperspect.a014274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LA, Prober DA, Edgar BA, Eisenman RN, Gallant P 1999. Drosophila myc regulates cellular growth during development. Cell 98: 779–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaadige MR, Elgort MG, Ayer DE 2010. Coordination of glucose and glutamine utilization by an expanded Myc network. Transcription 1: 36–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa S, Soucek L, Evan G, Okamoto T, Peterlin BM 2003. c-Myc recruits P-TEFb for transcription, cellular proliferation and apoptosis. Oncogene 22: 5707–5711 [DOI] [PubMed] [Google Scholar]
- Kaur M, Cole MD 2013. MYC acts via the PTEN tumor suppressor to elicit autoregulation and genome-wide gene repression by activation of the Ezh2 methyltransferase. Cancer Res 73: 695–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K, Cochran BH, Stiles CD, Leder P 1983. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell 35: 603–610 [DOI] [PubMed] [Google Scholar]
- Kim SY, Herbst A, Tworkowski KA, Salghetti SE, Tansey WP 2003. Skp2 regulates myc protein stability and activity. Mol Cell 11: 1177–1188 [DOI] [PubMed] [Google Scholar]
- Kim J, Woo AJ, Chu J, Snow JW, Fujiwara Y, Kim CG, Cantor AB, Orkin SH 2010. A Myc network accounts for similarities between embryonic stem and cancer cell transcription programs. Cell 143: 313–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kime L, Wright SC 2003. Mad4 is regulated by a transcriptional repressor complex that contains Miz-1 and c-Myc. Biochem J 370: 291–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King B, Trimarchi T, Reavie L, Xu L, Mullenders J, Ntziachristos P, Aranda-Orgilles B, Perez-Garcia A, Shi J, Vakoc C, et al. 2013. The ubiquitin ligase FBXW7 modulates leukemia-initiating cell activity by regulating MYC stability. Cell 153: 1552–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl NE, Kanda N, Schreck RR, Bruns G, Latt SA, Gilbert F, Alt FW 1983. Transposition and amplification of oncogene-related sequences in human neuroblastomas. Cell 35: 359–367 [DOI] [PubMed] [Google Scholar]
- Koskinen PJ, Ayer DE, Eisenman RN 1995. Repression of Myc-Ras co-transformation by Mad is mediated by multiple protein-protein interactions. Cell Growth Differ 6: 623–629 [PubMed] [Google Scholar]
- Kretzner L, Blackwood EM, Eisenman RN 1992. The Myc and Max proteins possess distinct transcriptional activities. Nature 359: 426–429 [DOI] [PubMed] [Google Scholar]
- Kuczyk MA, Serth J, Bokemeter C, Schwede J, Herrmann R, Machtens S, Grünewald V, Jonas U 1998. The MXI1 tumor suppressor gene is not mutated in primary prostate cancer. Oncol Rep 5: 213–216 [PubMed] [Google Scholar]
- *.Kuzyk A, Mai S 2014. c-MYC-induced genomic instability. Cold Spring Harb Perspect Med 10.1101/cshperspect.a014373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laherty CD, Yang W-M, Sun J-M, Davie JR, Seto E, Eisenman RN 1997. Histone deacetylases associated with the mSin3 corepressor mediate Mad transcriptional repression. Cell 89: 349–356 [DOI] [PubMed] [Google Scholar]
- Lahoz EG, Xu L, Schreiber-Agus N, DePinho RA 1994. Suppression of Myc, but not E1a, transformation activity by Max-associated proteins, Mad and Mxi1. Proc Natl Acad Sci 91: 5503–5507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L-G, Pettersson M, Öberg F, Nilsson K, Luscher B 1994. Expression of mad, mxi1, max, and c-myc during induced differentiation of hematopoietic cells: Opposite regulation of mad and c-myc. Oncogene 9: 1247–1252 [PubMed] [Google Scholar]
- Lassar AB, Buskin JN, Lockshon D, Davis RL, Apone S, Hauschka SD, Weintraub H 1989. MyoD is a sequence-specific DNA-binding protein requiring a region of myc homology to bind to the muscle creatine kinase enhancer. Cell 58: 823–831 [DOI] [PubMed] [Google Scholar]
- Lebel R, McDuff FO, Lavigne P, Grandbois M 2007. Direct visualization of the binding of c-Myc/Max heterodimeric b-HLH-LZ to E-box sequences on the hTERT promoter. Biochemistry 46: 10279–10286 [DOI] [PubMed] [Google Scholar]
- *.Levens D 2013. Cellular MYCro economics: Balancing MYC function with MYC expression. Cold Spring Harb Perspect Med 3: a014233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Lin C, Tanaka H, Fero ML, Eisenman RN 2009. Gene regulation and epigenetic remodeling in murine embryonic stem cells by c-Myc. PLoS ONE 4: e7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY, Loven J, Rahl PB, Paranal RM, Burge CB, Bradner JE, Lee TI, Young RA 2012. Transcriptional amplification in tumor cells with elevated c-Myc. Cell 151: 56–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeman GJ, Harris AW, Bath ML, Eisenman RN, Adams JM 1995. Overexpressed max is not oncogenic and attenuates myc-induced lymphoproliferation and lymphomagenesis in transgenic mice. Oncogene 10: 1013–1017 [PubMed] [Google Scholar]
- Link JM, Ota S, Zhou ZQ, Daniel CJ, Sears RC, Hurlin PJ 2012. A critical role for Mnt in Myc-driven T-cell proliferation and oncogenesis. Proc Natl Acad Sci 109: 19685–19690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Levens D 2006. Making Myc. Curr Top Microbiol Immunol 302: 1–32 [DOI] [PubMed] [Google Scholar]
- Loo LW, Secombe J, Little JT, Carlos LS, Yost C, Cheng PF, Flynn EM, Edgar BA, Eisenman RN 2005. The transcriptional repressor dMnt is a regulator of growth in Drosophila melanogaster. Mol Cell Biol 25: 7078–7091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loven J, Orlando DA, Sigova AA, Lin CY, Rahl PB, Burge CB, Levens DL, Lee TI, Young RA 2012. Revisiting global gene expression analysis. Cell 151: 476–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loven J, Hoke HA, Lin CY, Lau A, Orlando DA, Vakoc CR, Bradner JE, Lee TI, Young RA 2013. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell 153: 320–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makela TP, Koskinen PJ, Vastrik I, Alitalo K 1992. Alternative forms of Max as enhancers or suppressors of Myc-Ras cotransformation. Science 256: 373–377 [DOI] [PubMed] [Google Scholar]
- Marcotte R, Qian JF, Chen J, Wang E 2003. hMad4, c-Myc endogenous inhibitor, induces a replicative senescence-like state when overexpressed in human fibroblasts. J Cell Biochem 89: 576–588 [DOI] [PubMed] [Google Scholar]
- Martinato F, Cesaroni M, Amati B, Guccione E 2008. Analysis of Myc-induced histone modifications on target chromatin. PLoS ONE 3: e3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFerrin LG, Atchley WR 2011. Evolution of the Max and Mlx networks in animals. Genome Biol Evol 3: 915–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFerrin LG, Atchley WR 2012. A novel N-terminal domain may dictate the glucose response of Mondo proteins. PLoS ONE 7: e34803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon SB, Van Buskirk HA, Dugan KA, Copeland TD, Cole MD 1998. The novel ATM-related protein TRRAP is an essential cofactor for the c-Myc and E2F oncoproteins. Cell 94: 363–374 [DOI] [PubMed] [Google Scholar]
- Mellon P, Pawson A, Bister K, Martin GS, Duesberg PH 1978. Specific RNA sequences and gene products of MC29 avian acute leukemia virus. Proc Natl Acad Sci 75: 5874–5878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meroni G, Reymond A, Alcalay M, Borsani G, Tanigami A, Tonlorenzi R, Lo Nigro C, Messali S, Zollo M, Ledbetter DH, et al. 1997. Rox, a novel bHLHZip protein expressed in quiescent cells that heterodimerizes with Max, binds a non-canonical E box and acts as a transcriptional repressor. EMBO J 16: 2892–2906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meroni G, Cairo S, Merla G, Messali S, Brent R, Ballabio A, Reymond A 2000. Mlx, a new Max-like bHLHZip family member: The center stage of a novel transcription factors regulatory pathway? Oncogene 19: 3266–3277 [DOI] [PubMed] [Google Scholar]
- Mertz JA, Conery AR, Bryant BM, Sandy P, Balasubramanian S, Mele DA, Bergeron L, Sims RJ 3rd 2011. Targeting MYC dependence in cancer by inhibiting BET bromodomains. Proc Natl Acad Sci 108: 16669–16674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mladenov Z, Heine U, Beard D, Beard JW 1967. Strain MC29 avian leukosis virus. Myelocytoma, endothelioma, and renal growths: Pathomorphological and ultrastructural aspects. J Natl Cancer Inst 38: 251–285 [PubMed] [Google Scholar]
- Moberg KH, Mukherjee A, Veraksa A, Artavanis-Tsakonas S, Hariharan IK 2004. The Drosophila F box protein archipelago regulates dMyc protein levels in vivo. Curr Biol 14: 965–974 [DOI] [PubMed] [Google Scholar]
- Morton CC, Nussenzweig MC, Sousa R, Sorenson GD, Pettengill OS, Shows TB 1989. Mapping and characterization of an X-linked processed gene related to MYCL1. Genomics 4: 367–375 [DOI] [PubMed] [Google Scholar]
- Murre C, McCaw PS, Baltimore D 1989. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell 56: 777–783 [DOI] [PubMed] [Google Scholar]
- Musselman LP, Fink JL, Ramachandran PV, Patterson BW, Okunade AL, Maier E, Brent MR, Turk J, Baranski TJ 2013. Role of fat body lipogenesis in protection against the effects of caloric overload in Drosophila. J Biol Chem 288: 8028–8042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SK, Burley SK 2003. X-ray structures of myc-max and mad-max recognizing DNA. Molecular bases of regulation by proto-oncogenic transcription factors. Cell 112: 193–205 [DOI] [PubMed] [Google Scholar]
- Nau MM, Brooks BJ, Battey J, Sausville E, Gazdar AF, Kirsch IR, McBride OW, Bertness V, Hollis GF, Minna JD 1985. L-myc, a new myc-related gene amplified and expressed in human small cell lung cancer. Nature 318: 69–73 [DOI] [PubMed] [Google Scholar]
- Nesbit CE, Tersak JM, Prochownik EV 1999. MYC oncogenes and human neoplastic disease. Oncogene 18: 3004–3019 [DOI] [PubMed] [Google Scholar]
- Nie Z, Hu G, Wei G, Cui K, Yamane A, Resch W, Wang R, Green DR, Tessarollo L, Casellas R, et al. 2012. c-Myc is a universal amplifier of expressed genes in lymphocytes and embryonic stem cells. Cell 151: 68–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson JA, Maclean KH, Keller UB, Pendeville H, Baudino TA, Cleveland JL 2004. Mnt loss triggers myc transcription targets, proliferation, apoptosis, and transformation. Mol Cell Biol 24: 1560–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Khan MM, Kaul SC, Dong H-D, Wadhwa R, Colmenares C, Kohno I, Ishii S 1999. Ski is a component of the histone deacetylase complex required for transcriptional repression by Mad and thyroid hormone receptor. Genes Dev 13: 412–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H, Ishiguro K, Gaubatz S, Livingston DM, Nakatani Y 2002. A complex with chromatin modifiers that occupies E2F- and Myc-responsive genes in G0 cells. Science 296: 1132–1136 [DOI] [PubMed] [Google Scholar]
- Orian A, van Steensel B, Delrow J, Bussemaker HJ, Li L, Sawado T, Williams E, Loo LM, Cowley SM, Yost C, et al. 2003. Genomic binding by the Drosophila Myc, Max, Mad/Mnt transcription factor network. Genes Dev 17: 1101–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.O’Shea JM, Ayer DE 2013. Coordination of nutrient availability and utilization by MAX- and MLX-centered transcription networks. Cold Spring Harb Perspect Med 3: a014258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Psathas JN, Thomas-Tikhonenko A 2014. MYC and the art of microRNA maintenance. Cold Spring Harb Perspect Med 10.1101/cshperspect.a014175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne GS, Bishop JM, Varmus HE 1982. Multiple arrangements of viral DNA and an activated host oncogene in bursal lymphomas. Nature 295: 209–214 [DOI] [PubMed] [Google Scholar]
- Penn LJZ, Brooks MW, Laufer EM, Land H 1990. Negative autoregulation of c-myc transcription. EMBO J 9: 1113–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson CW, Stoltzman CA, Sighinolfi MP, Han KS, Ayer DE 2010. Glucose controls nuclear accumulation, promoter binding, and transcriptional activity of the MondoA-Mlx heterodimer. Mol Cell Biol 30: 2887–2895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peukert K, Staller P, Schneider A, Carmichael G, Hanel F, Eilers M 1997. An alternative pathway for gene regulation by Myc. EMBO J 16: 5672–5686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrefitte S, Kahn D, Haenlin M 2001. New members of the Drosophila Myc transcription factor subfamily revealed by a genome-wide examination for basic helix-loop-helix genes. Mech Dev 104: 99–104 [DOI] [PubMed] [Google Scholar]
- Pickett CL, Breen KT, Ayer DE 2007. A C. elegans Myc-like network cooperates with semaphorin and Wnt signaling pathways to control cell migration. Dev Biol 310: 226–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov N, Wahlstrom T, Hurlin PJ, Henriksson M 2005. Mnt transcriptional repressor is functionally regulated during cell cycle progression. Oncogene 24: 8326–8337 [DOI] [PubMed] [Google Scholar]
- Prochownik EV, Grove LE, Deubler D, Zhu XL, Stephenson RA, Rohr LR, Yin X, Brothman AR 1998. Commonly occurring loss and mutation of the MXI1 gene in prostate cancer. Genes Chromosomes Cancer 22: 295–304 [PubMed] [Google Scholar]
- Puissant A, Frumm SM, Alexe G, Bassil CF, Qi J, Chanthery YH, Nekritz EA, Zeid R, Gustafson WC, Greninger P, et al. 2013. Targeting MYCN in neuroblastoma by BET bromodomain inhibition. Cancer Discov 3: 308–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queva C, Hurlin PJ, Foley KP, Eisenman RN 1998. Sequential expression of the MAD family of transcriptional repressors during differentiation and development. Oncogene 16: 967–977 [DOI] [PubMed] [Google Scholar]
- Queva C, McArthur GA, Iritani BM, Eisenman RN 2001. Targeted deletion of the S-phase specific Myc antagonist Mad3 sensitizes neural and lymphoid cells to radiation-induced apoptosis. Mol Cell Biol 21: 703–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Rahl PB, Young RA 2014. Myc and transcription elongation. Cold Spring Harb Perspect Med 10.1101/cshperspect.a014340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahl PB, Lin CY, Seila AC, Flynn RA, McCuine S, Burge CB, Sharp PA, Young RA 2010. c-Myc regulates transcriptional pause release. Cell 141: 432–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay G, Graf T, Hayman MJ 1980. Mutants of avian myelocytomatosis virus with smaller gag gene-related proteins have an altered transforming ability. Nature 288: 170–172 [DOI] [PubMed] [Google Scholar]
- Rettenmier CW, Anderson SM, Riemen MW, Hanafusa H 1979. gag-Related polypeptides encoded by replication-defective avian oncoviruses. J Virol 32: 749–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson K, Asawachaicharn N, Galloway DA, Grandori C 2009. c-Myc accelerates S-phase and requires WRN to avoid replication stress. PLoS ONE 4: e5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum H, Webb E, Adams JM, Cory S, Harris AW 1989. N-myc transgene promotes B lymphoid proliferation, elicits lymphomas and reveals cross-regulation with c-myc. EMBO J 8: 749–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rous P 1911. Transmission of a malignant new growth by means of a cell-free filtrate. J Am Med Assoc 56: 198. [PubMed] [Google Scholar]
- *.Roussel MF, Robinson GW 2013. Role of MYC in medulloblastoma. Cold Spring Harb Perspect Med 3: a014308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel M, Saule S, Lagrou C, Rommens C, Beug H, Graf T, Stehelin D 1979. Three new types of viral oncogene of cellular origin specific for haematopoietic cell transformation. Nature 281: 452–455 [DOI] [PubMed] [Google Scholar]
- Roussel MF, Ashmun RA, Sherr CJ, Eisenman RN, Ayer DE 1996. Inhibition of cell proliferation by the Mad1 transcriptional repressor. Mol Cell Biol 16: 2796–2801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Sabò A, Amati B 2014. Genome recognition by Myc. Cold Spring Harb Perspect Med 10.1101/cshperspect.a014191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salghetti SE, Kim SY, Tansey WP 1999. Destruction of myc by ubiquitin-mediated proteolysis: Cancer-associated and transforming mutations stabilize myc. EMBO J 18: 717–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Schmitz R, Ceribelli M, Pittaluga S, Wright G, Staudt LM 2014. Oncogenic mechanisms in Burkitt lymphoma. Cold Spring Harb Perspect Med 10.1101/cshperspect.a014282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber-Agus N, Chin L, Chen K, Torres R, Rao G, Guida P, Skoultchi AI, DePinho RA 1995. An amino-terminal domain of Mxi1 mediates anti-Myc oncogenic activity and interacts with a homolog of the yeast repressor SIN3. Cell 80: 777–786 [DOI] [PubMed] [Google Scholar]
- Schreiber-Agus N, Stein D, Chen K, Goltz JS, Stevens L, DePinho RA 1997. Drosophila Myc is oncogenic in mammalian cells and plays a role in the diminutive phenotype. Proc Natl Acad Sci 94: 1235–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber-Agus N, Meng Y, Hoang T, Hou H Jr, Chen K, Greenberg R, Cordon-Cardo C, Lee H-W, DePinho RA 1998. Role of Mxi1 in ageing organ systems and the regulation of normal and neoplastic growth. Nature 393: 483–487 [DOI] [PubMed] [Google Scholar]
- Schwab M, Alitalo K, Klempnauer KH, Varmus HE, Bishop JM, Gilbert F, Brodeur G, Goldstein M, Trent J 1983. Amplified DNA with limited homology to myc cellular oncogene is shared by human neuroblastoma cell lines and a neuroblastoma tumour. Nature 305: 245–248 [DOI] [PubMed] [Google Scholar]
- Sears R, Nuckolls F, Haura E, Taya Y, Tamai K, Nevins JR 2000. Multiple ras-dependent phosphorylation pathways regulate myc protein stability. Genes Dev 14: 2501–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheiness D, Bishop JM 1979. DNA and RNA from uninfected vertebrate cells contain nucleotide sequences related to the putative transforming gene of avian myelocytomatosis virus. J Virol 31: 514–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheiness D, Fanshier L, Bishop JM 1978. Identification of nucleotide sequences which may encode the oncogenic capacity of the avian retrovirus MC29. J Virol 28: 600–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Ong GL, Keath EJ, Piccoli SP, Cole MD 1982. Novel myc oncogene RNA from abortive immunoglobulin-gene recombination in mouse plasmacytomas. Cell 31: 443–452 [DOI] [PubMed] [Google Scholar]
- Shiio Y, Rose DW, Donohoe S, Aebersold R, Eisenman RN 2006. Identification and characterization of SAP25, a novel component of the mSin3 corepressor complex. Mol Cell Biol 26: 1386–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MK, Rawls A, Wilson-Rawls J, Roalson EH 2010. Basic helix-loop-helix transcription factor gene family phylogenetics and nomenclature. Differentiation 80: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan E, Ayer DE 2010. Myc, Mondo, and metabolism. Genes Cancer 1: 587–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger D, Furrer M, Schwinkendorf D, Gallant P 2008. Max-independent functions of Myc in Drosophila melanogaster. Nat Genet 40: 1084–1091 [DOI] [PubMed] [Google Scholar]
- Stoltzman CA, Peterson CW, Breen KT, Muoio DM, Billin AN, Ayer DE 2008. Glucose sensing by MondoA:Mlx complexes: A role for hexokinases and direct regulation of thioredoxin-interacting protein expression. Proc Natl Acad Sci 105: 6912–6917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J, de Lange T, Ramsay G, Jakobovits E, Bishop JM, Varmus H, Lee W 1987. Definition of regions in human c-myc that are involved in transformation and nuclear localization. Mol Cell Biol 7: 1697–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama A, Kume A, Nemoto K, Lee SY, Asami Y, Nemoto F, Nishimura S, Kuchino Y 1989. Isolation and characterization of s-myc, a member of the rat myc gene family. Proc Natl Acad Sci 86: 9144–9148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama A, Noguchi K, Kitanaka C, Katou N, Tashiro F, Ono T, Yoshida MC, Kuchino Y 1999. Molecular cloning and chromosomal mapping of mouse intronless myc gene acting as a potent apoptosis inducer. Gene 226: 273–283 [DOI] [PubMed] [Google Scholar]
- Sur IK, Hallikas O, Vaharautio A, Yan J, Turunen M, Enge M, Taipale M, Karhu A, Aaltonen LA, Taipale J 2012. Mice lacking a Myc enhancer that includes human SNP rs6983267 are resistant to intestinal tumors. Science 338: 1360–1363 [DOI] [PubMed] [Google Scholar]
- Tahiliani M, Mei P, Fang R, Leonor T, Rutenberg M, Shimizu F, Li J, Rao A, Shi Y 2007. The histone H3K4 demethylase SMCX links REST target genes to X-linked mental retardation. Nature 447: 601–605 [DOI] [PubMed] [Google Scholar]
- Taub R, Kirsch I, Morton C, Lenoir G, Swan D, Tronick S, Aaronson S, Leder P 1982. Translocation of the c-myc gene into the immunoglobulin heavy chain locus in human Burkitt lymphoma and murine plasmacytoma cells. Proc Natl Acad Sci 79: 7837–7841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyo-oka K, Hirotsune S, Gambello MJ, Zhou ZQ, Olson L, Rosenfeld MG, Eisenman R, Hurlin P, Wynshaw-Boris A 2004. Loss of the Max-interacting protein Mnt in mice results in decreased viability, defective embryonic growth and craniofacial defects: Relevance to Miller-Dieker syndrome. Hum Mol Genet 13: 1057–1067 [DOI] [PubMed] [Google Scholar]
- Toyo-oka K, Bowen TJ, Hirotsune S, Li Z, Jain S, Ota S, Lozach LE, Bassett IG, Lozach J, Rosenfeld MG, et al. 2006. Mnt-deficient mammary glands exhibit impaired involution and tumors with characteristics of Myc overexpression. Cancer Res 66: 5565–5573 [DOI] [PubMed] [Google Scholar]
- Turunen HT, Sipila P, Strauss L, Bjorkgren I, Huhtaniemi I, Poutanen M 2012. Loss of Bmyc results in increased apoptosis associated with upregulation of Myc expression in juvenile murine testis. Reproduction 144: 495–503 [DOI] [PubMed] [Google Scholar]
- Vafa O, Wade M, Kern S, Beeche M, Pandita TK, Hampton GM, Wahl GM 2002. c-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function. A mechanism for oncogene-induced genetic instability. Mol Cell 9: 1031–1044 [DOI] [PubMed] [Google Scholar]
- Vervoorts J, Luscher B 1999. DNA binding of Myc/Max/Mad network complexes to oligonucleotides containing two E box elements: c-Myc/Max heterodimers do not bind DNA cooperatively. Biol Chem 380: 1121–1126 [DOI] [PubMed] [Google Scholar]
- Vita M, Henriksson M 2006. The Myc oncoprotein as a therapeutic target for human cancer. Semin Cancer Biol 16: 318–330 [DOI] [PubMed] [Google Scholar]
- von der Lehr N, Johansson S, Wu S, Bahram F, Castell A, Cetinkaya C, Hydbring P, Weidung I, Nakayama K, Nakayama KI, et al. 2003. The F-box protein Skp2 participates in c-Myc proteosomal degradation and acts as a cofactor for c-Myc-regulated transcription. Mol Cell 11: 1189–1200 [DOI] [PubMed] [Google Scholar]
- Walhout AJ, Gubbels JM, Bernards R, van der Vliet PC, Timmers HT 1997. C-myc/max heterodimers bind cooperatively to the e-box sequences located in the first intron of the rat ornithine decarboxylase (odc) gene. Nucleic Acids Res 25: 1493–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker W, Zhou ZQ, Ota S, Wynshaw-Boris A, Hurlin PJ 2005. Mnt-Max to Myc-Max complex switching regulates cell cycle entry. J Cell Biol 169: 405–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, McCormick LL, Fitzgerald P, Chi H, Munger J, et al. 2011a. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 35: 871–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Cunningham M, Zhang X, Tokarz S, Laraway B, Troxell M, Sears RC 2011b. Phosphorylation regulates c-Myc’s oncogenic activity in the mammary gland. Cancer Res 71: 925–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman NF, Aneas I, Nobrega MA 2010. An 8q24 gene desert variant associated with prostate cancer risk confers differential in vivo activity to a MYC enhancer. Genome Res 20: 1191–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R, Teich N, Varmus HE, Coffin J 1982. RNA tumor viruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- Welcker M, Clurman BE 2008. FBW7 ubiquitin ligase: A tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer 8: 83–93 [DOI] [PubMed] [Google Scholar]
- Welcker M, Orian A, Grim JA, Eisenman RN, Clurman BE 2004a. A nucleolar isoform of the Fbw7 ubiquitin ligase regulates c-Myc and cell size. Curr Biol 14: 1852–1857 [DOI] [PubMed] [Google Scholar]
- Welcker M, Orian A, Jin J, Grim JA, Harper JW, Eisenman RN, Clurman BE 2004b. The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proc Natl Acad Sci 101: 9085–9090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierstra I, Alves J 2008. The c-myc promoter: Still MysterY and challenge. Adv Cancer Res 99: 113–333 [DOI] [PubMed] [Google Scholar]
- *.Wiese K, Walz S, von Eyss B, Wolf E, Athineos D, Sansom O, Eilers M 2013. The role of Miz-1 and Myc-dependent tumorigenesis. Cold Spring Harb Perspect Med 10.1101/cshperspect.a014290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witherspoon DJ, Robertson HM 2003. Neutral evolution of ten types of mariner transposons in the genomes of Caenorhabditis elegans and Caenorhabditis briggsae. J Mol Evol 56: 751–769 [DOI] [PubMed] [Google Scholar]
- Wright JB, Brown SJ, Cole MD 2010. Upregulation of c-MYC in cis through a large chromatin loop linked to a cancer risk-associated single-nucleotide polymorphism in colorectal cancer cells. Mol Cell Biol 30: 1411–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SY, Lee AY, Lai HT, Zhang H, Chiang CM 2013. Phospho switch triggers Brd4 chromatin binding and activator recruitment for gene-specific targeting. Mol Cell 49: 843–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Popov N, Hou M, Wang Q, Bjorkholm M, Gruber A, Menkel AR, Henriksson M 2001. Switch from Myc/Max to Mad1/Max binding and decrease in histone acetylation at the telomerase reverse transcriptase promoter during differentiation of HL60 cells. Proc Natl Acad Sci 98: 3826–3831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yada M, Hatakeyama S, Kamura T, Nishiyama M, Tsunematsu R, Imaki H, Ishida N, Okumura F, Nakayama K, Nakayama KI 2004. Phosphorylation-dependent degradation of c-Myc is mediated by the F-box protein Fbw7. EMBO J 23: 2116–2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SL, Diolaiti D, Conacci-Sorrell M, Ruiz-Trillo I, Eisenman RN, King N 2011. Premetazoan ancestry of the Myc-Max network. Mol Biol Evol 28: 2961–2971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Tirabassi RS, Bush AB, Cole MD 1998. The C. elegans MDL-1 and MXL-1 proteins can functionally substitute for vertebrate MAD and MAX. Oncogene 17: 1109–1118 [DOI] [PubMed] [Google Scholar]
- Yun JS, Rust JM, Ishimaru T, Diaz E 2007. A novel role of the Mad family member Mad3 in cerebellar granule neuron precursor proliferation. Mol Cell Biol 27: 8178–8189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller KI, Zhao X, Lee CWH, Chiu KP, Yao F, Yustein JT, Ooi HS, Orlov YL, Shahab A, Yong HC, et al. 2006. Global mapping of c-Myc-binding sites and target gene networks in human B cells. Proc Natl Acad Sci 103: 17834–17839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zervos AS, Gyuris J, Brent R 1993. Mxi1, a protein that specifically interacts with Max to bind Myc-Max recognition sites. Cell 72: 223–232 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Iratni R, Erdjument-Bromage H, Tempst P, Reinberg D 1997. Histone deacetylases and SAP18, a novel polypeptide, are components of a human Sin3 complex. Cell 89: 357–364 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Sun H, Dai H, Walsh RM, Imakura M, Schelter J, Burchard J, Dai X, Chang AN, Diaz RL, et al. 2009. MicroRNA miR-210 modulates cellular response to hypoxia through the MYC antagonist MNT. Cell Cycle 8: 2756–2768 [DOI] [PubMed] [Google Scholar]
- Zhu J, Blenis J, Yuan J 2008. Activation of PI3K/Akt and MAPK pathways regulates Myc-mediated transcription by phosphorylating and promoting the degradation of Mad1. Proc Natl Acad Sci 105: 6584–6589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber J, Shi J, Wang E, Rappaport AR, Herrmann H, Sison EA, Magoon D, Qi J, Blatt K, Wunderlich M, et al. 2011. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature 478: 524–528 [DOI] [PMC free article] [PubMed] [Google Scholar]