Abstract
Computerized physician order entry (CPOE) has been promoted as an important component of patient safety, quality improvement, and modernization of medical practice. In practice, however, CPOE affects health care delivery in complex ways, with benefits as well as risks. Every implementation of CPOE is associated with both generally recognized and unique local factors that can facilitate or confound its rollout, and neurohospitalists will often be at the forefront of such rollouts. In this article, we review the literature on CPOE, beginning with definitions and proceeding to comparisons to the standard of care. We then proceed to discuss clinical decision support systems, negative aspects of CPOE, and cultural context of CPOE implementation. Before concluding, we follow the experiences of a Chief Medical Information Officer and neurohospitalist who rolled out a CPOE system at his own health care organization and managed the resulting workflow changes and setbacks.
Keywords: CPOE, EMR, implementation
Introduction
Computerized physician order entry (CPOE) has been promoted and championed as a component of health information technology by numerous political leaders1,2 and consumer groups such as Leapfrog,3 which incorporated CPOE as a core quality measure in 2000.4,5 The Health Information Technology for Economic and Clinical Health (HITECH) Act on February 17, 2009, specifically incentivized CPOE adoption with $19.2 billion in funds.6 The drive to implement CPOE primarily comes from its presumed benefit in reducing medical errors. CPOE is a complex intervention, however; its implementation does not always reduce medical errors and occasionally augments them. Because neurohospitalists will increasingly interact with CPOE and the closely related phenomenon of clinical decision support systems (CDSSs) and will likely be expected to lead and master the attendant workflow changes, here we review the literature about CPOE. We begin with definitions and meaningful use, discuss CPOE compared to the (still) standard of care, proceed to CPOE and CDSS, and talk briefly about the potential pitfalls of CPOE and about qualitative approaches to CPOE. We then review a neurohospitalist (TY)’s experiences in implementing CPOE before offering concluding remarks.
Computerized Physician Order Entry Definitions and Meaningful Use
Although CPOE as a concept has evolved over time, in practice the meaning has changed little. In 2003, Harvard researchers defined CPOE as “…a variety of computer-based systems that share the common features of automating the medication ordering process and that ensure standardized, legible, and complete orders.”7 In 2010, as one of the meaningful use criteria for implementing electronic health records, the Centers for Medicare and Medicaid Services (CMS) defined CPOE as “…the provider’s use of computer assistance to directly enter medication orders from a computer or mobile device. The order is also documented or captured in a digital, structured, and computable format for use in improving safety and organization.”8 These 2 definitions (which admittedly focus on medications rather than physician orders at large) share in common the following features:
physicians entering the orders directly (not through a unit secretary);
physicians working through a digital interface (no handwriting);
standardization/structure (for example, not through word processed documents).
The first 2 follow naturally from the name; the latter is a more abstract point that follows from preexisting auditability requirements and leads to the more comprehensive CMS requirement of a “computable format.” In practice, all CPOE systems included in the 2003 study would have met the CMS standard.
CPOE implementation is one of CMS’s criteria for electronic medical record (EMR)’s “meaningful use,” criteria meant to ensure not just the implementation of EMRs but their active incorporation into patient care and workflow. CPOE appears in both the “eligible provider” and the “hospitals” lists of core objectives for stage I meaningful use, defined as entering medication orders through CPOE for at least 30% of patients in the practice or admitted to the hospital, respectively.9 Although the first 2 years of meaningful use have elapsed as of this writing, providers are still eligible for incentive payments if they implement stage I meaningful use in 2013 or 2014.10 Prior to the meaningful use incentive, CPOE had limited uptake. According to 1 report, only 14% of all hospitals had achieved the meaningful use criteria mandated for CPOE as of 2010.11 The “standard of care”—a mix of paper orders and others—was routine, especially at smaller hospitals. It remains to be determined whether the meaningful use incentives have altered this trajectory, and when and how frequently neurohospitalists will interact with CPOE as it comes online.
Computerized Physician Order Entry Compared to the Standard of Care
There are 33 publications that appear in a PubMed search restricted to “clinical trials” of CPOE as of March 24, 2013.12 However, on review, only 2 of these publications are about the same randomized controlled trial of CPOE when compared to the standard of care. This paucity of the literature is unsurprising, as randomizing patients or even individual physicians to receive or deliver care through CPOE would be logistically challenging, would militate against a central principle of electronic workflow (ie, that information flow freely within the organization), and would likely not test the most theoretically beneficial components of CPOE (such as CDSS), which are often the last to be “rolled out.”13 For these reasons among others, there may be little incentive to study CPOE in an experimental fashion.
The singular trial to do so assessed physicians’ use of a minimalist “discharge software” system to generate discharge letters and medication reconciliation when compared to paper orders and usual discharge procedure. The first publication from this trial examined the effect of discharge software use by the randomized physicians on readmissions, emergency visits, and adverse drug events and found no difference compared to the standard of care group.14 The second assessed provider and patient attitudes toward the discharge process and found that patients were more prepared for discharge in the software group (but equally satisfied with it) and that outpatient providers rated the discharge quality higher but that inpatient providers found the process for discharge more onerous as well.15 These mixed results apply only to the specific CPOE system evaluated, and CPOE systems are so complex and variable that generalizing from this experience would be inappropriate, but they do remind us that CPOE’s theoretical benefits are not always attained in practice.
Nonrandomized designs, especially before/after studies comparing CPOE to pre-CPOE practice, are more common, but the conclusions from these studies are also mixed. One showed a 10-fold reduction in prescription errors themselves when CPOE was implemented,16 and another showed reduced preventable adverse drug events in the hospital after implementation of CPOE.17 However, the same trial showed an increase in all adverse drug events, while 3 additional trials showed reduced medication errors but not reduced adverse events.18–20 Another showed complex associations between CPOE and laboratory and radiographic test ordering, with CPOE appearing to increase the ordering of some tests and decreasing others.21 Another study examined provider attitudes toward CPOE and empowerment and found that CPOE implementation was associated with a general fall in regard to CPOE and feelings of professional disempowerment.22 One study did however find a reduction in mortality of approximately 20% after CPOE implementation at a pediatric hospital.23 These studies are summarized in Table 1. Taken together, they suggest neurohospitalists should question 1-sided portrayals of CPOE and be mindful of, and educate other providers about, CPOE’s impact on daily workflow and overall impact on outcomes (eg, preventable adverse events) rather than processes (eg, percent orders with complete information) alone.
Table 1.
| Author | Year | Design | Interventiona | Findings |
|---|---|---|---|---|
| Graumlich et al14 | 2009 | Randomized controlled trial | Discharge softwareb | No changes in patient satisfaction with discharge; improvement in outpatient provider perceptions of discharge quality; worsening of inpatient provider perception of time required to complete the discharge process |
| Graumlich et al15 | 2009 | Randomized controlled trial | Discharge softwareb | No change in hospital readmissions, emergency department visits, or adverse events |
| Mir et al16 | 2009 | Before/after analysis | CPOE | There was a 10-fold decrease in erroneous (missing, incomplete) prescription information |
| Leung et al17 | 2012 | Before/after analysis | CPOE | Decreased preventable adverse drug events by 33%, but increased overall adverse drug events |
| van Doormaal et al18 | 2009 | Before/after analysis | CPOE | Significant reduction in medication errors, but no change in preventable adverse drug events |
| King et al19 | 2003 | Before/after analysis | CPOE | Significant decrease in medication errors, but not in adverse drug events |
| Devine et al20 | 2010 | Before/after analysis | CPOE | Significant reduction in errors, especially of illegibility; no significant reduction in preventable adverse drug events |
| Collin et al21 | 2008 | Before/after analysis | CPOE | The ordering of some tests (full blood counts, urea and electrolytes, and chest roentgenograms) was reduced, while the ordering of other tests (computed tomography under some circumstances) increased |
| Bartos et al22 | 2008 | Before/after analysis | CPOE | All clinical providers—physicians, nurses, unit secretaries, physician extenders, and “other staff” who do not interact with CPOE directly—perceived themselves to be less powerful after CPOE implementation. All but the “other” category—all who interacted with CPOE—perceived it more negatively after implementation |
| Longhurst et al23 | 2010 | Before/after analysis | CPOE | Mortality in the children’s hospital decreased by 20% (0.8%-40%) following implementation of CPOE |
Abbreviations: CPOE, computerized physician order entry.
a These studies all compared CPOE to the pre-CPOE, handwritten orders standard of care (except for the first 2). Of note, almost all CPOE systems, especially the more recent ones, incorporate varying amounts of clinical decision support, whether by the structure of the order entry itself or by the provisioning of order sets, clinical alerts, and other cues to encourage or discourage certain kinds of orders.
b Both of these publications are about the same randomized trial. Of note, the intervention was a form of CPOE in that it generated a medication reconciliation, but not the comprehensive kind (ie, wherein providers can order any and all interventions from admission to discharge) commonly considered under the aegis of CPOE.
Computerized Physician Order Entry With CDSS
The above-mentioned studies paint a sobering view of CPOE, but neurohospitalists today are likely to interact with iteratively remodeled, progressively more sophisticated systems with significant decision support and customization. The literature on CPOE CDSS interventions is correspondingly more extensive with a myriad of end points.
Several studies have assessed CDSS interfaces themselves. One randomized trial tested a systematic process for designing order sets (which are CDSS components) and showed reduced physician cognitive burden when using the order set.24 Another trial tested the impact of interruptions on performance of complex and simple tasks on CPOE, finding that complex tasks were more likely to be complicated by error and that task interruption led not to increased error but to significantly longer times to task resumption and completion.25 Another found that principles of “user centered design” could improve time to order placement, completely eliminating the slowing of prior workflow induced by CPOE.26 Two more studies assessed “alerts” and found that their impact on clinical practice was minimal; they were often clicked-through (eg, ignored) at increasing rates over time27 and even when the alert required an action (rather than a simple “pop-up” warning).28
The CDSS’ impacts on processes and outcomes, particularly medication dosing and other interventions, have also been measured. Two randomized controlled trials29,30 and 1 before–after study in Australia31 all found that CDSS improved renal function-based medication dosing, with another finding the same specifically for aminoglycoside dosing.32 Another trial found that a multifaceted intervention including CPOE helped increase appropriate use of scheduled insulin among hospitalized patients and also decreased the length of stay.33 A subsequent trial by the same group found that a larger order set around glucose management reduced hyperglycemia without increasing hypoglycemia.34 A simulated study of CPOE for drip medications in the pediatric intensive care unit found that CPOE dramatically reduced order errors including dose miscalculations and also provider satisfaction.35 Studies have also been used to demonstrate improved dosing of medications among the elderly patients36 and improved transfusion guideline adherence.37 Of particular relevance to neurohospitalists, at least 1 study showed improved timeliness of thrombolysis for patients with stroke in the emergency department.38
Although CPOE and CDSS have primarily targeted medication ordering, CDSS can be used for other purposes as well. An early study at the Indiana University showed that CPOE with CDSS could improve the completeness of specific order sets such as scheduled partial thromboplastin time laboratory draws to accompany a heparin drip.39 The CPOE can also be used to better ration the use of specific medications (eg, vancomycin).40 The range of potential, testable interventions is theoretically limitless for CPOE and CDSS.
Negative Effects of, and Qualitative Approaches to, CPOE
Despite its range of potential benefits, the effects of CPOE are not all benign. One study found that CPOE actually caused errors ranging from wrong dosing to duplication, many stemming from a hybrid paper/CPOE workflow and from poor design decisions such as displaying pharmacy formulary availability rather than appropriate default dosing options.41 In this context, one study at the same location found mortality unexpectedly more than doubled in their children’s hospital after CPOE implementation, discussed subsequently.42 Beyond CPOE, CDSS itself is prone to unique pitfalls such as “alert fatigue”43 that can negate the recognition of significant clinical notifications and impede clinical workflow. The CDSS also occasionally fails to result in promised improvements in medication dosing and prevention of adverse events.44,45
It is in this context that qualitative research can provide deeper insights into CPOE and its implementation. In the study finding by Han et al., increased mortality was reported in the children’s hospital, and the investigators postulated that unacceptable delays, well meaningly programmed into the CPOE system as “safety” measures, prevented physicians from preordering medications before the arrival of sick children by air transport, prevented nurses from obtaining medications by manual override, locked physicians or nurses out of the same chart while pharmacists were editing a chart, and forced multiple providers to sit at terminals distant from their patient and often, from each other, to obey the CPOE system mandates.42 These ad hoc findings mirrored contemporaneous studies by Beuscart-Zephir et al,46,47 which explored the changes in workflow attending a transition from standard of care to CPOE: a dramatic drop in allowed ambiguity, an introduction of asynchrony between nurse and physician workflow, and a corresponding reduction in direct, face-to-face communication. These changes may also help to explain the generalized feelings of disempowerment found by Bartos et al previously.22 Another early study by Ash et al examined CPOE implementations over a period of 7 years and incorporated surveys, expert opinions, community beliefs, and other data. The conclusions of this study were complex and difficult to summarize. Among others it noted that the CPOE/EMR system took on an identity as “the Hub”—not merely a repository of data but an active member of the health care team acting to translate/make explicit communications between providers that had been previously implicit and contextualized.48
In a more recent review article, Greenhalgh and Swinglehurst argue that information and communication technologies (eg, CPOE) should be studied by ethnographers so as to better describe these complex systems in their even more complex context.49 The described ethnographies emphasize the importance of customizable views for different members of the team50; chances to repeatedly enter, copy, or manipulate important information and thus to “relocalize” it51; and considering a focus on less rather than more structured data fields, given the fact that data are often differentially interpreted by different providers in a way that facilitates rather than impedes clinical care.52,53 Neurohospitalists who are already working with CPOE and information systems will recognize the tradeoffs in all of the above-mentioned mandates, while those helping to choose or implement CPOE will benefit their practices and institutions enormously by considering the above prospectively.
Implementing CPOE: The EvergreenHealth Experience
CPOE has many advantages and disadvantages, and the full range of its potential and pitfalls was experienced or avoided by one neurohospitalist (TY) as he rolled out CPOE at EvergreenHealth (EH) in Seattle. The lessons from that roll out provide helpful context for the preceding discussion. EH implemented Cerner’s EMR in stages, beginning with laboratory viewing, then adding dictations, image viewing, basic charting, and ultimately moved toward CPOE on May 20, 2011.13 Despite preimplementation (“go-live”) training, physicians were often confused by the order structure and attendant mouse clicking mandated by CPOE. One neurologist, unable to recall the specific sequence of actions needed to order a brain MRI, considered the CPOE system “dumb” and “broken.” The CPOE slowed physician workflow in some situations; it mandated 5 mouse clicks for substituting a single nonformulary drug, meaning up to 20 clicks for an average patient needing 4 substitutions (a common scenario).
On the other hand, CPOE greatly sped up some orders, not just in writing but in execution; “stat” chest x-rays went from requiring 30 minutes for completion to requiring 10 minutes, while pharmacist verification of physician orders went from requiring 60 minutes for completion to requiring 20 minutes.
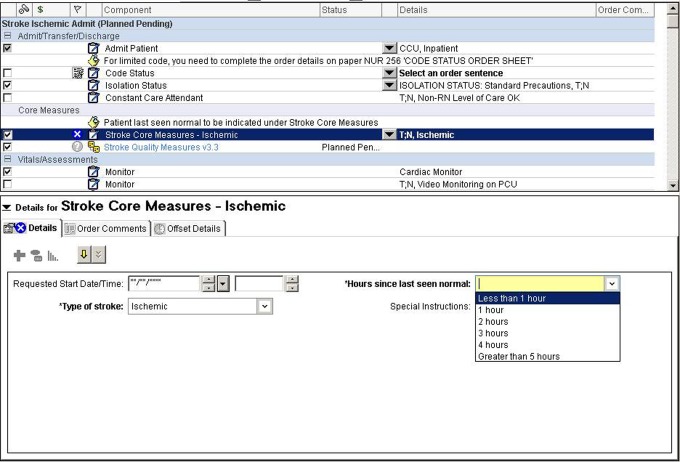
The EH adopted CDSS in the form of order sets into CPOE, building several of these specifically for individual services and with certain guidelines in mind. The ischemic stroke admission order set automatically activates routine, pertinent orders for patients with stroke, based on recognized quality measures54—stroke education, mechanical (but not pharmacological) venous thromboembolism prophylaxis, physical, occupational, and speech/swallow therapy consultation as well as documentation regarding symptom onset to guide thrombolysis decision making (Figure 1).
Figure 1.
Screenshot from Cerner computerized physician order entry (CPOE) ischemic stroke admission order set. This order set was designed to comply with national performance measures, such as the administration of thrombolytic therapy within 180 minutes of patients’ arrival. The blue circles with “X” in the middle represent “hardstops,” meaning the physician must fill out these specific fields before signing and activating the order set. To meet the relevant time cutoff, physicians must enter the “hours since last seen normal,” with the computer then calculating whether thrombolysis is indicated.
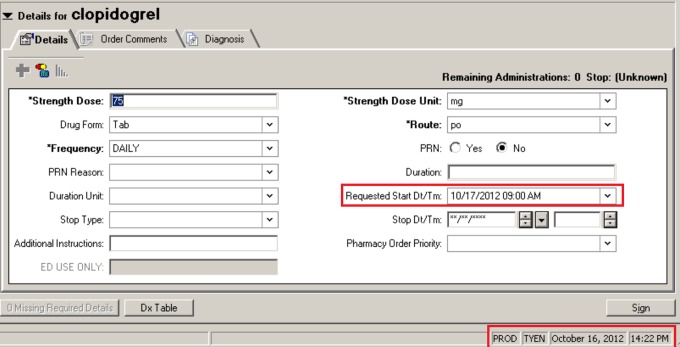
Physicians at EH noted a change in workflow following CPOE that drove increased responsibility for explication into the physicians’ hands.46,47 Where a physician formerly relied upon a nurse to clarify when a medication would first be given, he or she must now carefully read the “Next Dose Logic” screen to avoid unacceptably delaying medication administration. In the example shown in Figure 2, clopidogrel was ordered at 2:22 pm on December 16, 2012, and because this fell after the routine daily administration time of 9 am, unless otherwise specified by the physician the first dose would have been given the following morning rather than right away. Beneficial ambiguity has been abandoned,52,53 and the result can be occasionally unintended, previously uncommon errors of overrestriction and overinterpretation.41
Figure 2.
Screenshot from Cerner computerized physician order entry (CPOE) module for medication ordering. Note that the order is being completed at 2:22 pm on October 16 (boxed in red), but because it is to be a daily medication without modification the patient would receive a first dose on October 17 at 9 am, almost 19 hours later (also boxed in red).
Despite initial frustration, the acceptance of CPOE at EH was highlighted when CPOE became unavailable during a system outage, necessitating paper orders for 12 hours. All providers immediately recognized CPOE’s newly integral role in clinical effectiveness. Nurses recognized CPOE’s ability to render orders legibly; pharmacists recognized its power in processing and scheduling complex regimens; and even physicians saw how CDSS modules facilitated evidence-based decision making. All were relieved when the system returned to online status.
For neurohospitalists and other physicians at EH who use CPOE daily, repetition has reduced CPOE’s cognitive burden and imparted insights into the advantages, eccentricities, and pitfalls of the system and led to its acceptance, if not in all cases its embrace.
Conclusion
CPOE will become a progressively more important part of the inpatient landscape. Financial, quality, and safety incentives are driving CPOE adoption, not always because of (and sometimes in spite of) evidence, but as a means to disseminate standardized order sets, clinical alerts, and other CDSS avenues. Neurohospitalists dealing with acute and emergent life-threatening conditions will often be situated to use or misuse these systems early in their adoptions. We hope this review and contextualized experience at one health care system will help the readers to understand these systems as the highly variable, not uniformly benign, actively engaged “members” of the team they become almost immediately upon implementation. As CPOE systems interpret the care we seek to provide to our patients, we too must understand their idiosyncrasies and rules in order to best serve our patients.
Acknowledgments
We would like to thank Vanja Douglas, MD, for reading an earlier version of this manuscript and providing valuable insight and feedback into its revision.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.President Bush continues EHR push, sets national goals; April 26, 2004. , author. http://www.healthcareitnews.com/news/president-bush-continues-ehr-push-sets-national-goals Accessed August 27, 2012.
- 2. Obama's big idea: digital health records; 2009. http://money.cnn.com/2009/01/12/technology/stimulus_health_care/ Accessed Aug-ust 27, 2012.
- 3. Statement by the Leapfrog group on the final meaningful use rule: good first step, but grave concerns; 2009. http://www.leapfroggroup.org/news/leapfrog_news/4779269 Accessed August 27, 2012.
- 4. Leapfrog on the Bush administration’s new quality measurement program; 2006. http://www.leapfroggroup.org/news/leapfrog_news/2903476 Accessed August 27, 2012.
- 5. Leapfrog group actions will be felt throughout the health care system; 2001. http://www.managedcaremag.com/archives/0106/0106.leapfrog.html Accessed August 27, 2012.
- 6. The American Recovery and Reinvestment Act: HR1; 2009. http://frwebgate.access.gpo.gov/cgi-bin/getdoc.cgi?dbname=111_cong_bills&docid=f:h1enr.pdf Accessed May 24, 2012.
- 7. Kaushal R, Shojania KG, Bates DW. Effects of computerized physician order entry and clinical decision support systems on medication safety: a systematic review. Arch Intern Med. 2003;163(12):1409–1416 [DOI] [PubMed] [Google Scholar]
- 8. CPOE for medication orders; 2010. http://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/down-loads/1_CPOE_for_Medication_Orders.pdf Accessed August 27, 2012.
- 9. Meaningful use: stage I objectives; 2010. http://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/Downloads/MU_Stage1_ReqOverview.pdf Accessed March 23, 2013.
- 10. Marcotte L, Seidman J, Trudel K, et al. Achieving meaningful use of health information technology: a guide for physicians to the EHR incentive programs. Arch Intern Med. 2012;172(9):731–736 [DOI] [PubMed] [Google Scholar]
- 11. Traffic jams on the road to meaningful use; 2010. https://www.klasresearch.com/Store/ReportDetail.aspx?ProductID=565 Accessed August 27, 2012.
- 12.Pubmed Search: “CPOE[All Fields] AND (Randomized Controlled Trial[ptyp] OR Clinical Trial[ptyp])”, author. http://www.ncbi.nlm.nih.gov/pubmed/ Accessed March 24, 2013.
- 13.Electronic medical record adoption model (EMRAM), author http://www.himssanalytics.org/emram/index.aspx Accessed November 1, 2012.
- 14. Graumlich JF, Novotny NL, Stephen Nace G, et al. Patient readmissions, emergency visits, and adverse events after software-assisted discharge from hospital: cluster randomized trial. J Hosp Med. 2009;4(7):E11–E19 [DOI] [PubMed] [Google Scholar]
- 15. Graumlich JF, Novotny NL, Nace GS, Aldag JC. Patient and physician perceptions after software-assisted hospital discharge: cluster randomized trial. J Hosp Med. 2009;4(6):356–363 [DOI] [PubMed] [Google Scholar]
- 16. Mir C, Gadri A, Zelger GL, Pichon R, Pannatier A. Impact of a computerized physician order entry system on compliance with prescription accuracy requirements. Pharm World Sci. 2009;31(5):596–602 [DOI] [PubMed] [Google Scholar]
- 17. Leung AA, Keohane C, Amato M, et al. Impact of vendor computerized physician order entry in community hospitals. J Gen Intern Med. 2012;27(7):801–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Doormaal JE, van den Bemt PM, Zaal RJ, et al. The influence that electronic prescribing has on medication errors and preventable adverse drug events: an interrupted time-series study. J Am Med Inform Assoc. 2009;16(6):816–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. King WJ, Paice N, Rangrej J, Forestell GJ, Swartz R. The effect of computerized physician order entry on medication errors and adverse drug events in pediatric inpatients. Pediatrics. 2003;112(3 pt 1):506–509 [DOI] [PubMed] [Google Scholar]
- 20. Devine EB, Hansen RN, Wilson-Norton JL, et al. The impact of computerized provider order entry on medication errors in a multispecialty group practice. J Am Med Inform Assoc. 2010;17(1):78–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Collin S, Reeves BC, Hendy J, Fulop N, Hutchings A, Priedane E. Implementation of computerised physician order entry (CPOE) and picture archiving and communication systems (PACS) in the NHS: quantitative before and after study. BMJ. 2008;337:a939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bartos CE, Butler BS, Penrod LE, Fridsma DB, Crowley RS. Negative CPOE attitudes correlate with diminished power in the workplace. AMIA Annu Symp Proc. 2008;2008:36–40 [PMC free article] [PubMed] [Google Scholar]
- 23. Longhurst CA, Parast L, Sandborg CI, et al. Decrease in hospital-wide mortality rate after implementation of a commercially sold computerized physician order entry system. Pediatrics. 2010;126(1):14–21 [DOI] [PubMed] [Google Scholar]
- 24. Avansino J, Leu MG. Effects of CPOE on provider cognitive workload: a randomized crossover trial. Pediatrics. 2012;130(3):e547–e552 [DOI] [PubMed] [Google Scholar]
- 25. Magrabi F, Li SY, Day RO, Coiera E. Errors and electronic prescribing: a controlled laboratory study to examine task complexity and interruption effects. J Am Med Inform Assoc. 2010;17(5):575–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chan J, Shojania KG, Easty AC, Etchells EE. Does user-centred design affect the efficiency, usability and safety of CPOE order sets? J Ame Med Inform Assoc. 2011;18(3):276–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lin CP, Payne TH, Nichol WP, Hoey PJ, Anderson CL, Gennari JH. Evaluating clinical decision support systems: monitoring CPOE order check override rates in the department of veterans affairs' computerized patient record system. J Am Med Inform Assoc. 2008;15(5):620–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Strom BL, Schinnar R, Bilker W, Hennessy S, Leonard CE, Pifer E. Randomized clinical trial of a customized electronic alert requiring an affirmative response compared to a control group receiving a commercial passive CPOE alert: NSAID--warfarin co-prescribing as a test case. J Am Med Inform Assoc. 2010;17(4):411–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chertow GM, Lee J, Kuperman GJ, et al. Guided medication dosing for inpatients with renal insufficiency. JAMA. 2001;286(22):2839–2844 [DOI] [PubMed] [Google Scholar]
- 30. Terrell KM, Perkins AJ, Hui SL, Callahan CM, Dexter PR, Miller DK. Computerized decision support for medication dosing in renal insufficiency: a randomized, controlled trial. Ann Emerg Med. 2010;56(6):623–629 [DOI] [PubMed] [Google Scholar]
- 31. Roberts GW, Farmer CJ, Cheney PC, et al. Clinical decision support implemented with academic detailing improves prescribing of key renally cleared drugs in the hospital setting. J Am Med Inform Assoc. 2010;17(3):308–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cox ZL, Nelsen CL, Waitman LR, McCoy JA, Peterson JF. Effects of clinical decision support on initial dosing and monitoring of tobramycin and amikacin. Am J Health Syst Pharm. 2011;68(7):624–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schnipper JL, Ndumele CD, Liang CL, Pendergrass ML. Effects of a subcutaneous insulin protocol, clinical education, and computerized order set on the quality of inpatient management of hyperglycemia: results of a clinical trial. J Hosp Med. 2009;4(1):16–27 [DOI] [PubMed] [Google Scholar]
- 34. Schnipper JL, Liang CL, Ndumele CD, Pendergrass ML. Effects of a computerized order set on the inpatient management of hyperglycemia: a cluster-randomized controlled trial. Endocr Pract. 2010;16(2):209–218 [DOI] [PubMed] [Google Scholar]
- 35. Vaidya V, Sowan AK, Mills ME, Soeken K, Gaffoor M, Hilmas E. Evaluating the safety and efficiency of a CPOE system for continuous medication infusions in a pediatric ICU. AMIA Annu Symp Proc. 2006:1128 [PMC free article] [PubMed] [Google Scholar]
- 36. Peterson JF, Rosenbaum BP, Waitman LR, et al. Physicians' response to guided geriatric dosing: initial results from a randomized trial. Stud Health Technol Inform. 2007;129(pt 2):1037–1040 [PubMed] [Google Scholar]
- 37. Rothschild JM, McGurk S, Honour M, et al. Assessment of education and computerized decision support interventions for improving transfusion practice. Transfusion. 2007;47(2):228–239 [DOI] [PubMed] [Google Scholar]
- 38. Heo JH, Kim YD, Nam HS, et al. A computerized in-hospital alert system for thrombolysis in acute stroke. Stroke. 2010;41(9):1978–1983 [DOI] [PubMed] [Google Scholar]
- 39. Overhage JM, Tierney WM, Zhou XH, McDonald CJ. A randomized trial of “corollary orders” to prevent errors of omission. J Am Med Inform Assoc. 1997;4(5):364–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shojania KG, Yokoe D, Platt R, Fiskio J, Ma'luf N, Bates DW. Reducing vancomycin use utilizing a computer guideline: results of a randomized controlled trial. J Am Med Inform Assoc. 1998;5(6):554–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Koppel R, Metlay JP, Cohen A, et al. Role of computerized physician order entry systems in facilitating medication errors. JAMA. 2005;293(10):1197–1203 [DOI] [PubMed] [Google Scholar]
- 42. Han YY, Carcillo JA, Venkataraman ST, et al. Unexpected increased mortality after implementation of a commercially sold computerized physician order entry system. Pediatrics. 2005;116(6):1506–1512 [DOI] [PubMed] [Google Scholar]
- 43. Mack EH, Wheeler DS, Embi PJ. Clinical decision support systems in the pediatric intensive care unit. Pediatr Crit Care Med. 2009;10(1):23–28 [DOI] [PubMed] [Google Scholar]
- 44. Metzger J, Welebob E, Bates DW, Lipsitz S, Classen DC. Mixed results in the safety performance of computerized physician order entry. Health Aff (Millwood). 2010;29(4):655–663 [DOI] [PubMed] [Google Scholar]
- 45. Nebeker JR, Hoffman JM, Weir CR, Bennett CL, Hurdle JF. High rates of adverse drug events in a highly computerized hospital. Arch Intern Med. 2005;165(10):1111–1116 [DOI] [PubMed] [Google Scholar]
- 46. Beuscart-Zephir MC, Pelayo S, Degoulet P, Anceaux F, Guerlinger S, Meaux JJ. A usability study of CPOE's medication administration functions: impact on physician-nurse cooperation. Stud Health Technol Inform. 2004;107(pt 2):1018–1022 [PubMed] [Google Scholar]
- 47. Beuscart-Zephir MC, Pelayo S, Anceaux F, Meaux JJ, Degroisse M, Degoulet P. Impact of CPOE on doctor-nurse cooperation for the medication ordering and administration process. Int J Med Inform. 2005;74(7-8):629–641 [DOI] [PubMed] [Google Scholar]
- 48. Ash JS, Sittig DF, Seshadri V, Dykstra RH, Carpenter JD, Stavri PZ. Adding insight: a qualitative cross-site study of physician order entry. Int J Med Inform. 2005;74(7-8):623–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Greenhalgh T, Swinglehurst D. Studying technology use as social practice: the untapped potential of ethnography. BMC Med. 2011;9:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Reddy MC, Dourish P, Pratt W. Coordinating heterogeneous work: information and representation in medical care. In: Proceedings of European Conference on Computer Supported Cooperative Work (ECSCCW’01) 2001; 2001:239–258 [Google Scholar]
- 51. Osterlund C. Mapping medical work: documenting practices across multiple medical settings. J Cent Inf Stud. 2004;5:35–43 http://www.yc.tcu.ac.jp/~cisj/05/5_06.pdf [Google Scholar]
- 52. Monteiro E. Integrating health information systems: a critical appraisal. Methods Inf Med. 2003;42(4):428–432 [PubMed] [Google Scholar]
- 53. Ellingsen G, Monteiro E. Seamless integration: standardisation across multiple local settings. Comput Supported Cooperative Work. 2006;15(5-6):443–466 [Google Scholar]
- 54. Poisson S, Josephson SA. Quality measures in stroke. Neurohospitalist. 2011;1(2):71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]