Abstract
High-level T-cell expression of PD-1 during SIV infection is correlated with impaired proliferation and function. We evaluated the phenotype and distribution of T-cells and Tregs during antiretroviral therapy plus PD-1 modulation (using a B7-DC-Ig fusion protein) and post-ART. Chronically SIV-infected rhesus macaques received: 11 wk of ART (Group A); 11 wk of ART plus B7-DC-Ig (Group B); 11 wk of ART plus B7-DC-Ig, then 12 wk of B7-DC-Ig alone (Group C). Continuous B7-DC-Ig treatment (group C) decreased rebound viremia post-ART compared to pre-ART levels, associated with decreased PD-1hi expressing T-cells and Tregs in PBMCs, and PD-1hi Tregs in lymph nodes. It transiently decreased expression of Ki67 and α4β7 in PBMC CD4+ and CD8+ Tregs for up to 8 wk post-ART and maintained Ag-specific T-cell responses at low levels. Continued immune modulation targeting PD-1hi cells during and post-ART helps maintain lower viremia, keeps a favorable T-cell/Treg repertoire and modulates antigen-specific responses.
Keywords: PD-1, Treg, ART, immunomodulation, viremia
Introduction
T cell exhaustion, which leads to progressive loss of T cell antigen-specific function, is one of the major hurdles in the efficient treatment of chronic viral infections (1–3) and malignant neoplastic diseases (4, 5). Programmed death 1 (PD-1), an inhibitory surface co-receptor, is a member of the CD28/B7 family that is expressed on T cells, B cells and myeloid-derived cells (6, 7). There are two known ligands of PD-1: PD-L1 (B7-H1) and PD-L2 (B7-DC), which are expressed on the surface of a variety of hematopoietic (T cells, B cells, dendritic cells and macrophages) and non-hematopoietic cells (endothelial cells and fibroblasts) (8–12). Upon ligand-receptor interaction, PD-1 dampens TCR signaling, thus reducing cell proliferation and cytokine production, while favoring cell anergy and apoptosis (3, 6). PD-1 over-expression has been associated with T cell and Treg dysfunction in a variety of chronic viral infections such as HCV, HBV, HIV/SIV and LCMV (1, 13–20). Particularly in the case of HIV infection, increased PD-1 expression on antigen-specific CD4+ and CD8+ T cells has been associated with T cell exhaustion and disease progression (1), and has been identified as a unique marker for failing immune reconstitution in HIV patients undergoing antiretroviral therapy (ART) (21). Similarly, PD-1 expression levels have also been used as a reliable indicator of low-level HIV-1 and SIV replication (22). Although ART has decreased the morbidity and mortality associated with HIV infection globally (23), complete eradication of the viral reservoir (24, 25), as well as reduction of ART-associated side-effects, remain priorities in the development of new treatment strategies against HIV infection (26). Similarly, the identification of immunomodulatory therapies that might assist in viral reservoir eradication and improve Ag-specific immune responses is of great therapeutic interest.
Given that PD-1 over-expression has been extensively associated with T cell exhaustion, recent efforts have been directed towards improving T cell functionality during chronic viral infection and neoplastic malignancies by in vivo PD-1 blockade (2, 27–31). In the present study, we chose to target PD-1 by using B7-DC-Ig (Amplimmune, Inc.), a fusion protein consisting of the extracellular domain (ECD) of human B7-DC and the hinge and Fc domain of human IgG1. Murine B7-DC-Ig (ECD of murine B7-DC fused with the hinge and Fc domain of murine IgG2a) in combination with cyclophosphamide, has been previously shown to enhance vaccine-mediated Ag-specific immune responses in a murine TC-1 tumor model. Furthermore, the enhancement of Ag-specific immune responses was due to a decrease in the number of tumor-infiltrated Tregs and to an overall decrease in PDhi CD8+ T cells. Importantly, murine B7-DC-Ig does not block PD-1/PD-L1 interaction or PD-1 detection by flow cytometry, and acts only on cells expressing high amounts of PD-1 on the cell surface (32). In order to test the potential PD-1 immunomodulatory properties of B7-DC-Ig in the context of SIV infection, we used chronically SIVmac251-infected rhesus macaques and treated them with a triple cocktail of antiretroviral drugs with or without supplemental PD-1 immunomodulation (through the use of B7-DC-Ig). Concurrently, we also evaluated the impact of continuous PD-1 immunomodulation as a single therapeutic agent, by continuing B7-DC-Ig treatment for up to 12 wks post-ART release. Because of the well-documented effects of ART on Tregs and T-cells, throughout the study we evaluated the phenotype and distribution of both cell types in different tissue compartments and T-cell functionality. Our results suggest that prolonged in vivo targeting of PD-1 with B7-DC-Ig during and after ART favors lower systemic viral loads and helps maintain a favorable Treg and T cell repertoire.
Materials and Methods
Animals and sample collection
Fourteen naive and twenty-three SIVmac251-positive rhesus macaques, chronically infected for 23 to 65 weeks, were housed at Advanced BioScience Laboratories, Inc. (ABL; Kensington, MD) or the NIH Animal Center (Poolesville, MD). Animals were maintained according to Institutional Animal Care and Use Committee guidelines and the NIH Guide for the Care and Use of Laboratory Animals. Fifteen of the SIV-infected macaques, recycled from previous vaccine studies (33, 34) were divided into three treatment groups (Fig. 2A) based on their previous immunization status, VL and CD4+ T-cell counts. Two macaques in Groups A and B and one in Group C were Mamu A*01 positive; Group C also contained one B*08 positive macaque (Fig. 3B–D). ART composition and dosages were previously described (35). B7-DC-Ig fusion protein (Amplimmune, Inc; Gaithersburg, MD) was administered intravenously weekly at 10 mg/kg. B7-DC-Ig binding to macaque CD3+ T-cells was confirmed by direct staining of PBMC with APC-conjugated B7-DC-Ig (data not shown).
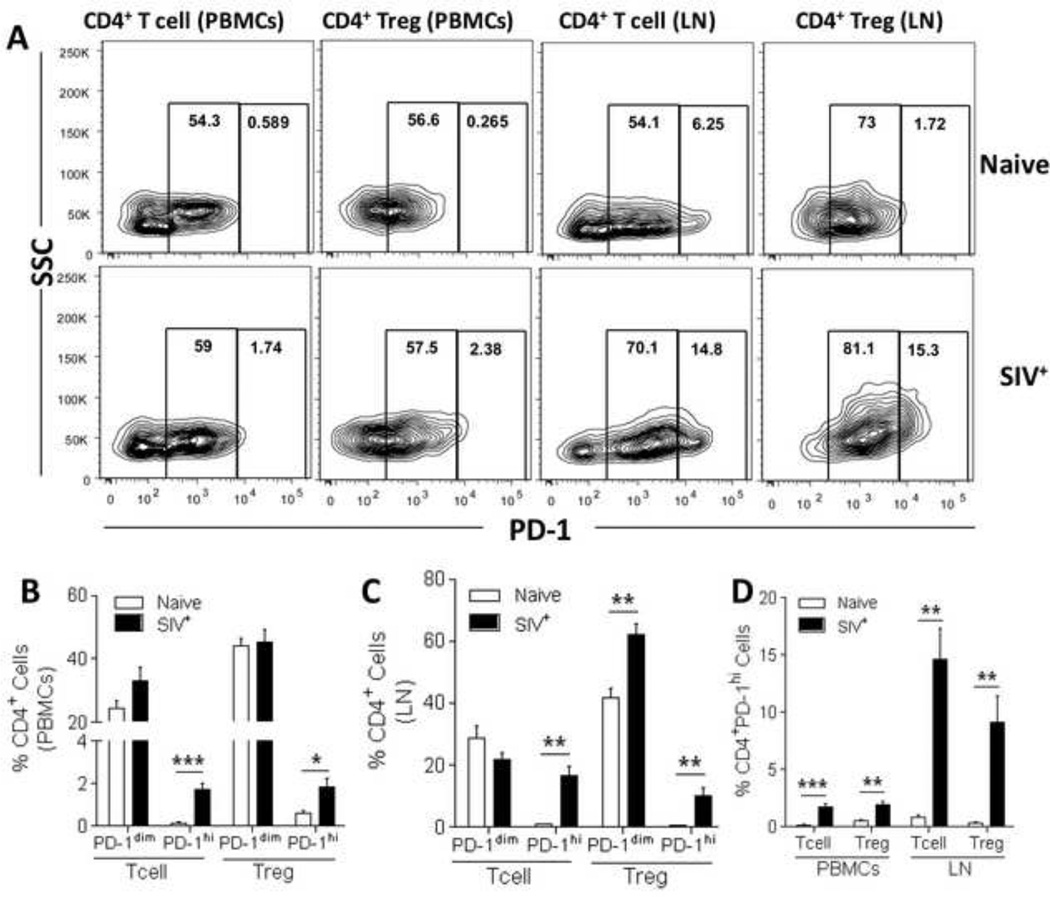
Figure 2. Proportional distribution of PD-1dim and PD-1hi T cells and Tregs in PBMCs and LN cells of naïve and SIV-infected macaques.
(A) PD-1 positive T cells and Tregs display a bi-modal expression pattern both in PBMCs as well as in lymph nodes. (B–D) Freshly isolated PBMC and LN single-cell suspensions from naïve and SIV-infected rhesus macaques were stained for analysis of PD-1 expression. Percentages of CD4+ PD-1dim and PD-1hi T cells and Tregs in PBMCs (B) and LN (C) cells. (D) Comparative representation of percentages of PD-1hi CD4+ T cells and Tregs in PBMCs and LN cells. Data are representative (A) or pooled (B–D) from at least 5 naïve and 12 SIV-infected macaques. Data are shown as means ± SEM. *, p<0.05, **, p<0.01, and ***, p<0.001 indicate statistically significant differences between the compared groups by Mann-Whitney Test.
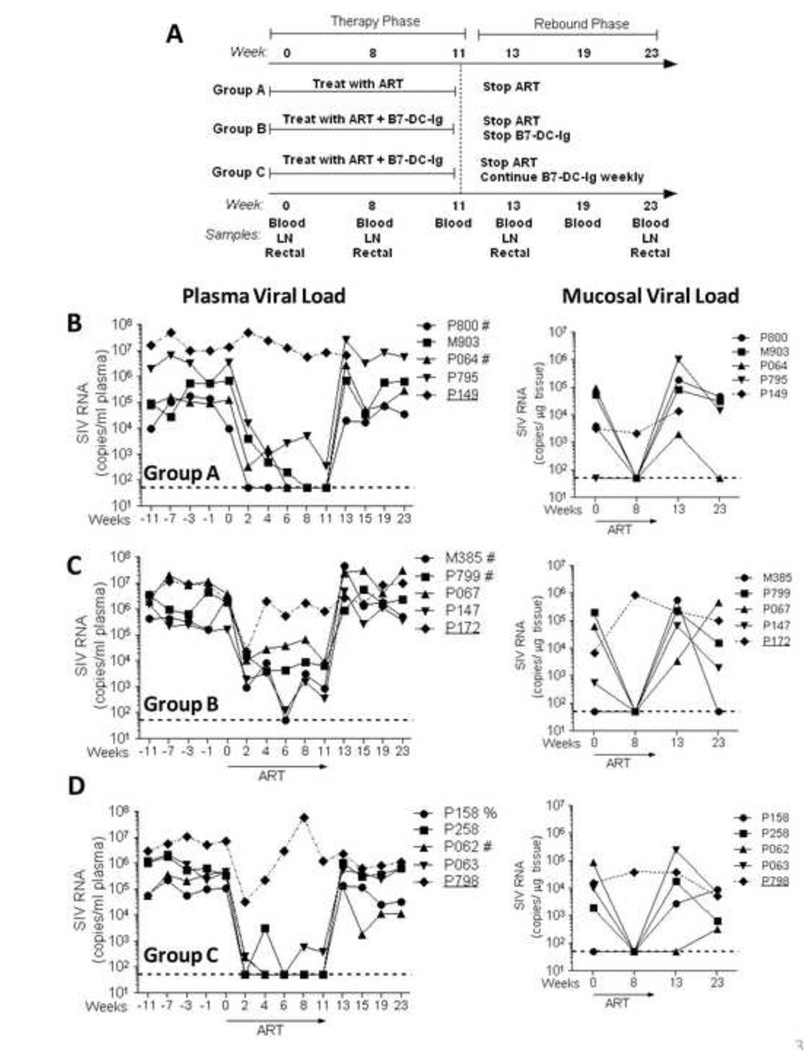
Figure 3. Therapeutic study design and plasma and rectal tissue viral loads for individual animals throughout the study.
(A) Schematic description of the study design indicating the experimental groups as well as the treatment administered in each group. Time points (in weeks) for sample collection as well as type of samples collected are indicated. (B–D) Plasma and rectal tissue viral loads were obtained at the indicated time points by NASBA. Dotted lines in B–D indicate the lower limit of viremia detection (50 copies) by NASBA. Viral loads in Group A (ART-only controls) were previously reported in (35). Animals P149 (Group A), P172 (Group B) and P798 (Group C) did not respond satisfactorily to ART and were not included in further analyses. #, Mamu A*01 positive; %, Mamu B*08 positive.
Blood samples were collected by venipuncture of anesthetized animals into EDTA-treated collection tubes. Peripheral blood mononuclear cells (PBMC) were obtained using Ficoll-Paque PLUS gradients (GE Healthcare; Piscataway, NJ). Cells were washed and resuspended at 1×106 cells/ml in R-10 medium (RPMI 1640 containing 10% FBS, 2 mM L-glutamine, 1% non-essential amino acids, 1% sodium pyruvate and antibiotics). Lymph node (LN) biopsies were minced, passed through a 40 µm cell strainer, and lysed to remove red blood cells. Rectal biopsies were digested for 60 min on an orbital shaker in R-10 medium containing 1 mg/mL of collagenase II (Sigma; St Louis, MO). VL in plasma and rectal tissue were determined by NASBA (36).
Flow Cytometry
Anti-human fluorochrome-conjugated monoclonal antibodies (mAbs) known to cross-react with rhesus macaques and used in the present study are described in Table 1. Yellow and aqua LIVE/DEAD viability dyes (Invitrogen; Grand Island, NY) were used to exclude dead cells. For multiparametric flow cytometry analysis, PBMCs and tissue mononuclear cells were stained for specific surface molecules, fixed and permeabilized with either the Transcription Factor Staining Buffer Set (eBioscience; San Diego, CA) or with the Cytofix/Cytoperm Kit (BD Biosciences; San Jose, CA), and then stained for specific intracellular molecules. At least 500,000 singlet events (PBMCs) or 30,000 CD3+ singlet events (tissue mononuclear cells) were acquired on a 4-laser LSR II SORP (BD Biosciences) and analyzed using FlowJo Software (TreeStar Inc; Ashland, OR). For all samples, gating was established using a combination of isotype and fluorescence-minus-one controls. Given that PD-1hi cells are enriched in the LN of SIV-infected macaques, we used LN cells to set up the flow cytometry gates that differentiate PD-1 negative, dim and high expression levels.
Table 1.
Fluorochrome-conjugated monoclonal antibodies used in the present study
| Antigen | Clone | Fluorochrome | Supplier |
|---|---|---|---|
| CD3 | SP34-2 | Alexa Fluor 700 | BD Biosciences |
| SP34-2 | PE-Cy7 | BD Biosciences | |
| CD4 | 19Thy5D7 | Qdot655 | NIH NHPRR |
| L200 | Qdot605 (custom) | BD Biosciences | |
| OKT4 | eFluor605NC | eBioscience | |
| CD8 | 3B5 | Qdot605 | Invitrogen |
| RPA-T8 | eFluor650NC | eBioscience | |
| RPA-T8 | APC-Cy7 | BD Biosciences | |
| CD25 | BC96 | PE-Cy7 | eBioscience |
| CD28 | CD28.2 | PE-Cy7 | eBioscience |
| CD28.2 | APC | BD Biosciences | |
| CD95 | DX2 | PE-Cy5 | BD Biosciences |
| DX2 | PE-Cy5 | BioLegend | |
| CD279 (PD-1) | eBioJ105 | PE | eBioscience |
| Ki67 | B56 | FITC | BD Biosciences |
| Foxp3 | PCH101 | PerCP-Cy5.5 | eBioscience |
| Alpha4Beta7 | Rhesus recombinant | APC | NIH NHPRR |
| IFN-g | B27 | PE | BD Biosciences |
| TNF-a | MAB11 | PerCP-Cy5.5 | BioLegend |
| IL-2 | MQ1-17H12 | APC-Cy7 | BD Biosciences |
T cell Recall Assays
T cell recall responses were assayed by stimulating 2 × 106 PBMCs with 1 µg/mL SIVmac239 Gag peptide pools (complete sets of 15-mer peptides, overlapping by 11 and spanning the entire protein; NIH AIDS Research & Reference Reagent Program) for 6 h. Stimulation was performed in the presence of 10 µg/mL Brefeldin A, 2 µg/mL anti-CD49d and 0.375 µg/mL PE-Cy7 anti-CD28 (all from BD Biosciences). CD3+ T cells were divided into CD4+ and CD8+ populations and for each, cells were further subdivided into CD28+ CD95+ central (CM) and CD28− CD95+ effector memory (EM) cells. Percent cytokine secreting cells among each memory subset was then determined. Non-stimulated and SEB-treated (5µg/mL; Sigma) tubes were used as negative and positive controls, respectively. Non-stimulated values were subtracted from Gag-stimulated samples to calculate Gag-specific cytokine secreting cells.
Statistical Analysis
Unless otherwise specified, all data reported were averaged from the number of macaques indicated in the figure legends. Results are shown as means ± standard errors of the mean. Data were analyzed using Prism (v5.03, GraphPad Software). A p value of ≤0.05 was considered statistically significant for each test.
Results
SIV-associated increased levels of PD-1 expression in T cells and Tregs
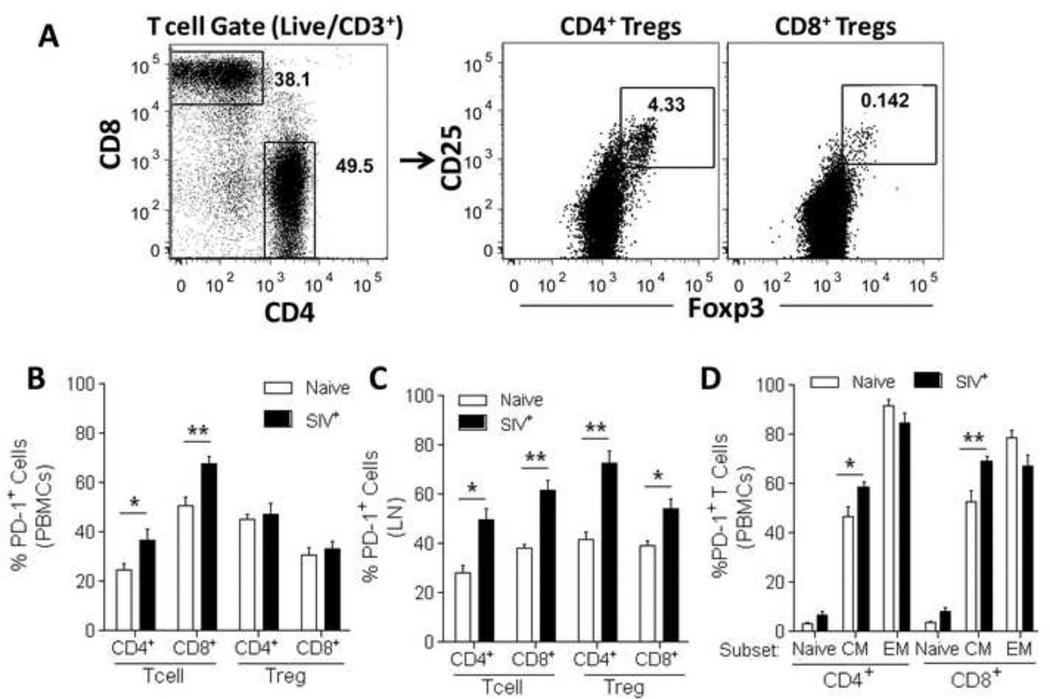
In order to evaluate the effect of in vivo targeting of the PD-1 pathway during and after ART, we first analyzed the distribution of PD-1 expressing T cells and Tregs (Fig. 1A) in peripheral blood mononuclear cells (PBMCs) and lymph nodes (LN). As previously reported (22), compared to naïve macaques, the frequency of total PD-1 expression in PBMCs of SIV-infected macaques was increased in both CD4+ and CD8+ T cells, without a significant increase in CD4+ or CD8+ Tregs (Fig. 1B). Interestingly, when evaluating LN of SIV-infected macaques, a significant increase in total PD-1 expression was observed in both T cells and Tregs when compared to naïve animals (Fig. 1C). Similar to previous reports (22), we confirmed that PD-1 is differentially expressed within CD4+ and CD8+ T cell subsets in naïve animals, and significantly increased in CD4+ and CD8+ central memory T cells of SIV-infected macaques (Fig. 1D). Given that PD-1 expression in T cells and Tregs follows a bimodal distribution (Fig. 2A), and that PD-1hi cells represent an anergic population of T cells that can be directly targeted by murine B7-DC-Ig in vivo treatment (32), we separated dimly (PD-1dim) and highly (PD-1hi) PD-1-expressing T cells and Tregs in SIV-infected macaques. We initially observed that PD-1 expression in CD4+ T cells and Tregs in SIV-infected macaques was significantly elevated in the PD-1hi subset compared to naïve macaques, both in PBMCs (Fig. 2B) and LN (Fig. 2C). Similarly, CD8+ PD-1hi T cells (PBMC and LN) and Tregs (LN only) were also significantly increased (p<0.05) in SIV-infected macaques when compared to naïve animals (data not shown). Notably, PD-1hi CD4+ T cells and Tregs in the LN of SIV-infected macaques represented approximately 10–15% of all CD4+ T cells and Tregs, a significant increase when compared to almost undetectable levels in naïve macaques (p<0.01, Fig. 2D).
Figure 1. Increased expression of PD-1 is observed in central memory T cells as well as in CD4+ and CD8+ Tregs in SIV-infected rhesus macaques.
Freshly isolated PBMC and LN single-cell suspensions from naïve and SIV-infected rhesus macaques were stained for analysis of PD-1 expression. (A) Gating strategy used to identify total CD4+ and CD8+ T cells as well as CD4+ and CD8+ Tregs. PD-1 expression was evaluated in CD4+ and CD8+ T cells and Tregs in PBMCs (B) and LN (C) cells. (D) PD-1 expression levels were analyzed in naïve (CD28+ CD95−), central memory (CM, CD28+CD95+), and effector memory (EM, CD28−CD95+) CD4+ and CD8+ T cells. PBMC data are pooled from 10 naïve and 12 SIV-infected macaques. LN data are pooled from 5 naïve and 12 SIV-infected macaques. Data are shown as means ± SEM. *, p<0.05 and **, p<0.01 indicate statistically significant differences between the compared groups by Mann-Whitney Test.
Impact of ART and ART plus B7-DC-Ig treatment regimens on plasma and mucosal viral loads
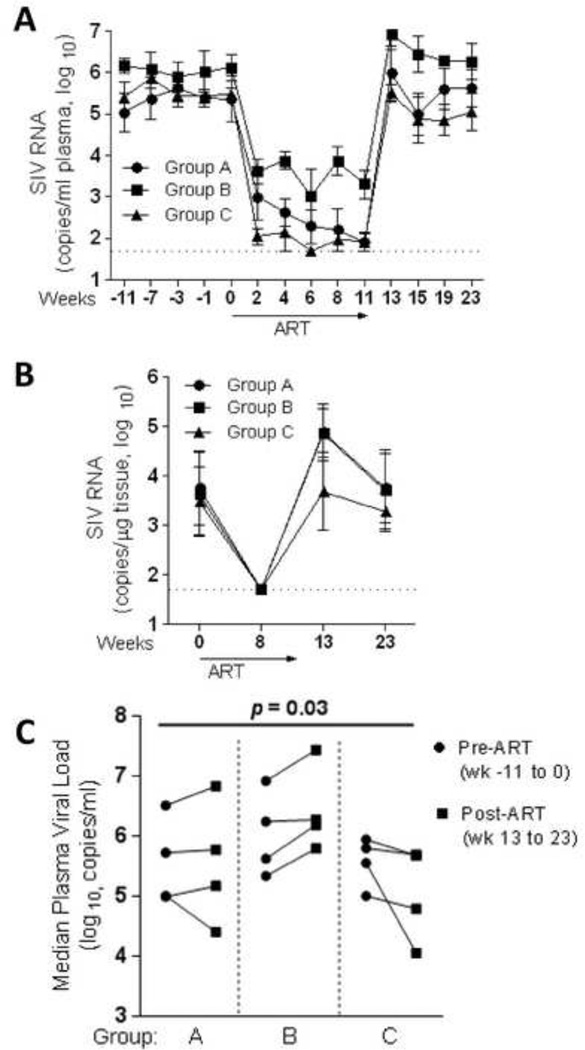
In preliminary pilot studies we determined that B7-DC-Ig given alone for 4 wks did not affect plasma viral loads in chronically SIV-infected macaques (data not shown). Additionally, administration of ART in order to first decrease viremia in chronically infected macaques followed by 4 wks of B7-DC-Ig treatment alone did not impact viral loads in comparison to controls (data not shown). Therefore, we decided to study the effect of B7-DC-Ig treatment given during 11 weeks of ART, and included one group in which B7-DC-Ig was administered for an extended period of time following ART cessation. For this purpose, we divided 15 chronically SIV-infected rhesus macaques into 3 treatment groups (Groups: A, B and C, Fig. 3A). The following treatments were administered to each group: 1) Group A: 11 wks of daily ART (ART control group); Group B: 11 wks of daily ART plus weekly doses of B7-DC-Ig (ART + PD-1 modulation group); group C: 11 wks of daily ART plus weekly doses of B7-DC-Ig, followed by an additional 12 wks of B7-DC-Ig alone (continued PD-1 modulation group). Figure 3B–D shows plasma and rectal tissue viral loads for each animal for the duration of the study. In each group, one animal failed to significantly respond to ART (Fig. 3B–D, underlined codes) and was therefore not included in further analyses. Geometric mean plasma viral loads for the remaining 4 animals per group are shown in Fig. 4A and display a significant response to ART (>90% reduction in plasma and tissue VL) over the 11 wk period of treatment in all treatment groups. Although plasma viral loads were reduced to undetectable levels in only some macaques (Fig. 3B–D), rectal tissue viral loads were undetectable after 8wk of ART in all assayed animals (Fig. 3B–D and Fig. 4B). As expected, upon ART cessation, plasma and rectal tissue viral loads rebounded in all animals (Fig. 4A–B). Interestingly, macaques in Group C which remained on B7-DC-Ig for an additional 12 wk were significantly different from macaques in Groups A and B (p=0.03 for the null hypothesis of equal changes in all 3 groups). Group C animals showed a decrease in median plasma viremia after ART release (wk 13 to 23) compared to median pre-ART levels (wk −11 to 0), whereas Groups A and B macaques did not (Fig. 4C).
Figure 4. Impact of ART and prolonged B7-DC-Ig treatment on viral load rebound.
Geometric mean plasma (A) and mucosal (B) viremia for each group was determined using the four animals per group that responded satisfactorily to ART. (C) Pre-ART vs. Post-ART median plasma viral load changes in each of the assayed groups. The changes in pre- vs post-ART median viral loads were compared across all three groups by the exact Kruskal-Wallis test.
Dynamics of T cell and Treg biodistribution during combined ART and in vivo PD-1 modulation
Given that during chronic SIV infection the proportional distribution and function of CD4+ and CD8+ T cells and Tregs are altered systemically (37, 38), and that ART treatment is capable of restoring their normal proportional distribution (39, 40), we monitored these cell populations throughout the duration of the study in PBMCs, LN and rectal tissue. During the first 8 wks of ART, we observed a significant increase in the proportion of CD4+ T cells (Fig. 5A) in all tissue compartments assayed. This increase was only transient, and CD4+ T cells started to decrease proportionally upon ART termination at wk 11. Conversely, CD8+ T cells transiently decreased in proportion (p<0.05, Fig. 5B) in PBMCs and LN during the first 8 wks of ART. Interestingly, while CD4+ Tregs increased proportionally during the first 8 wks of ART in LN and rectal tissue, they were proportionally decreased in PBMCs (p<0.05, Fig. 5C). Furthermore, CD8+ Tregs were significantly increased during the first 8 wks of ART only in LN (p<0.01, Fig. 5D). Overall, neither combined (during ART, group B) nor prolonged (during and after ART, group C) in vivo PD-1 modulation affected the bio-distribution of CD4+ and CD8+ T cells and Tregs (Fig. 5A–D).
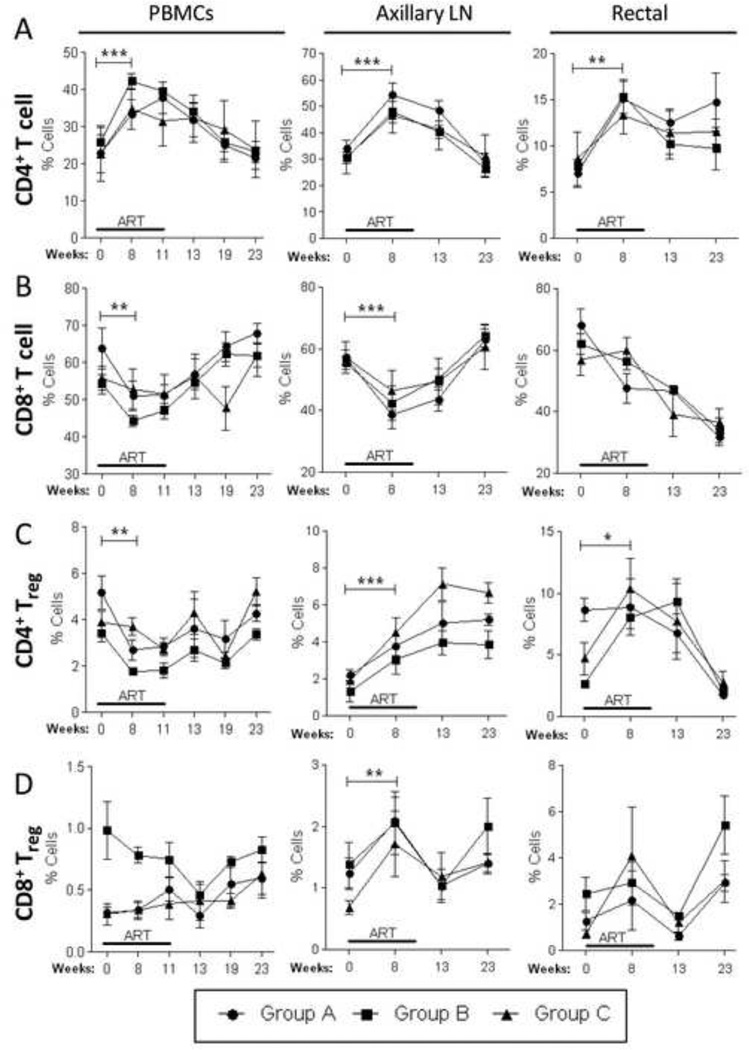
Figure 5. Proportional and compartmental distribution of CD4 and CD8 T cells and Tregs over the course of the study.
Blood, LN and rectal tissue samples (n=4 per group) were collected at different time points as indicated in Fig. 3A and stained for flow cytometric determination of T cell and Treg percentages. Proportional counts of CD4+ (A) and CD8+ (B) T cells, as well as, CD4+ (C) and CD8+ (D) Tregs were done at each indicated time point for each tissue. Data are shown as means ± SEM. *, p<0.05, **, p<0.01, and ***, p<0.001 indicate statistically significant differences between the indicated time points by the Wilcoxon signed rank test.
Effect of B7-DC-Ig on PD-1hi T cells and Tregs
Given that murine B7-DC-Ig affects only cells that express high amounts of surface PD-1 (32), and because high levels of PD-1 expression are observed during the chronic stage of HIV/SIV infection (2), we monitored the proportion of PD-1hi T cells and Tregs during the course of this study.
Although ART transiently decreased the percentage of PD-1hi expressing CD4+ (Fig. 6A) and CD8+ (Fig. 6B) T cells within PBMC, only animals treated with B7-DC-Ig decreased the percentage of PD-1hi T cells to levels comparable to those of naïve animals (Fig. 6A, B dotted line). Moreover, continuous B7-DC-Ig treatment (Group C) maintained PD-1hi T-cells at low proportions for up to 12 wks post- ART (Fig. 6A–B, wk 23). Similarly, PD-1hi Tregs within PBMC were reduced in Groups B and C that received B7-DC-Ig but not by ART alone (Fig. 6C–D). Although CD8+ PD-1hi Tregs were maintained at low levels after ART release by continuous B7-DC-Ig treatment (Fig. 6D wks 19 and 23), CD4+ PD-1hi Tregs were maintained at low levels by continuous B7-DC-Ig treatment for only 2 wks upon ART release (Fig. 6C). Similarly to results in PBMC, continuous B7-DC-Ig treatment also maintained significantly lower levels of LN PD-1hi CD4+ and PD-1hi CD8+ Iregs (Fig. 6G–H), but not T cells (Fig. 6E–F).
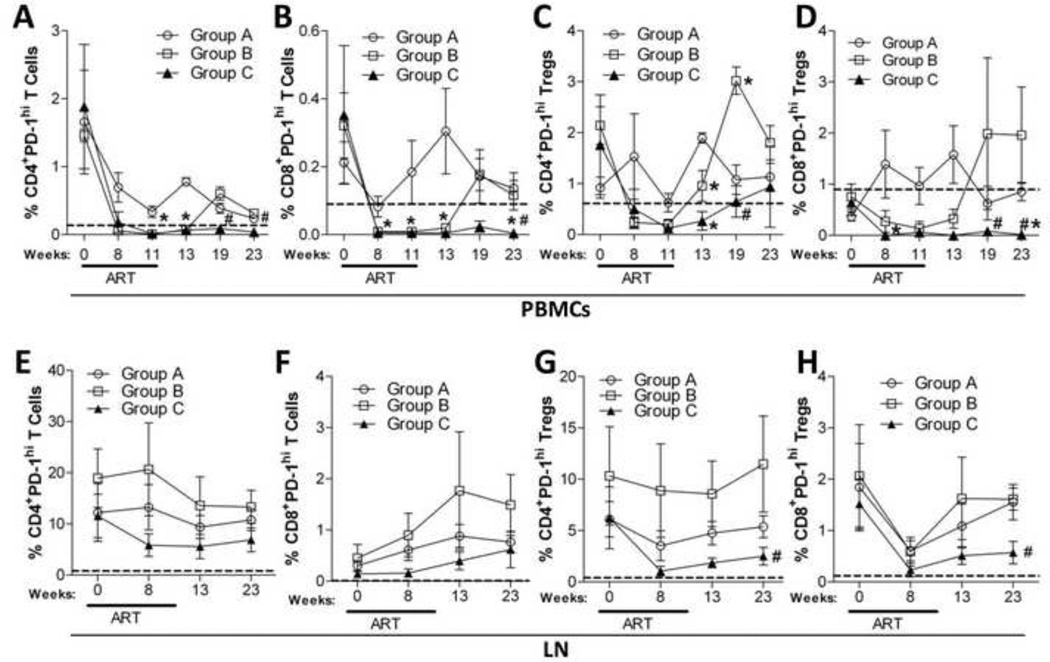
Figure 6. B7-DC-Ig treatment during and after ART significantly decreases the percentage of circulatory PD-1hi CD4+ and CD8+ T cells and Tregs.
PBMCs and LN cells were isolated at the indicated time points and stained for flow cytometric analysis of PD-1 expression. CD4+ (A and E) and CD8+ (B and F) PD-1hi T-cells, as well as, CD4+ (C and G) and CD8+ (D and H) PD-1hi Tregs were monitored for the duration of the study in PBMC (A–D) and LN (E–H). Dotted lines represent mean values of each cell population obtained from naïve macaques (10 for PBMCs and 5 for LN). Data are shown as means ± SEM. *, p<0.05 indicates statistically significant differences between Group A and Group B or C by the Mann-Whitney Test. #, p<0.05 indicates statistically significant differences between Group B and Group C by the Mann-Whitney Test.
Notably, the continuous B7-DC-Ig treatment did not elicit any toxicity. A previous GLP-compliant pharmacology and toxicology study in naïve healthy cynomolgus monkeys showed that a 10 mg/kg weekly repeated dose induced anti-drug antibodies (ADA) in only a small percentage of animals (data not shown). We did not assess ADA here, but the extracellular domain of B7-DC is highly conserved between humans, cynomolgus monkeys and rhesus macaques (≥96% homology) and we did not expect significant antibody induction that would alter treatment outcomes. This is supported by the continuous effects of B7-DC-Ig in Group C macaques on PD-1hi T cells and Tregs in PBMC and LN (Fig. 6A,B,D,G,H).
Prolonged B7-DC-Ig treatment transiently modulates Treg phenotype in SIV-infected macaques and controls the magnitude of Ag-specific T-cell responses
Increased PD-1 expression is a reliable marker of immune cell exhaustion (1, 41) and HIV/SIV viral replication and disease progression (16). Increased expression of α4β7 (42, 43) and Ki67 (44, 45) have also been associated with HIV/SIV pathogenesis and immune dysfunction. We monitored α4β7 and Ki67 co-expression in CD4+ and CD8+ T cells and Tregs during the course of the study. While no significant changes in the levels of α4β7 and Ki67 co-expression were observed in conventional T cells, α4β7 and Ki67 co-expression in CD4+ and CD8+ Tregs within PBMC and LN cells were impacted by continuous B7-DC-Ig treatment (Fig. 7A–D). Within PBMC, continuous B7-DC-Ig treatment significantly decreased CD4+ α4β7 Ki67+ Tregs 2 wks post-ART release (Fig. 7A, Group C vs B, wk 13), while a transient (albeit non-significant) decrease was observed at wk 13 and 19 in CD8+ α4β7 +Ki67+ Tregs (Fig. 7B). In the LN, ART significantly decreased α4β7 and Ki67 co-expression in CD4+ and CD8+ Tregs of all groups (Fig. 7C–D, wk 8). Continuous B7-DC-Ig treatment (Group C) maintained double-positive α4β7+ and Ki67+ CD4+ and CD8+ Tregs at significantly lower levels for 2 wks post-ART release (Fig. 7C– D, Group C vs B, wk 13). Group C macaques also exhibited a slower rebound in CD4+ and CD8+ α4 β 7+Ki67+ Tregs following ART release at week 11. During ART, both groups exhibited low levels of these cells (Fig. 7C–D, wk 8). However the subsequent increase between wks 8 and 13 in Group B was significantly greater than that of Group C (p = 0.029).
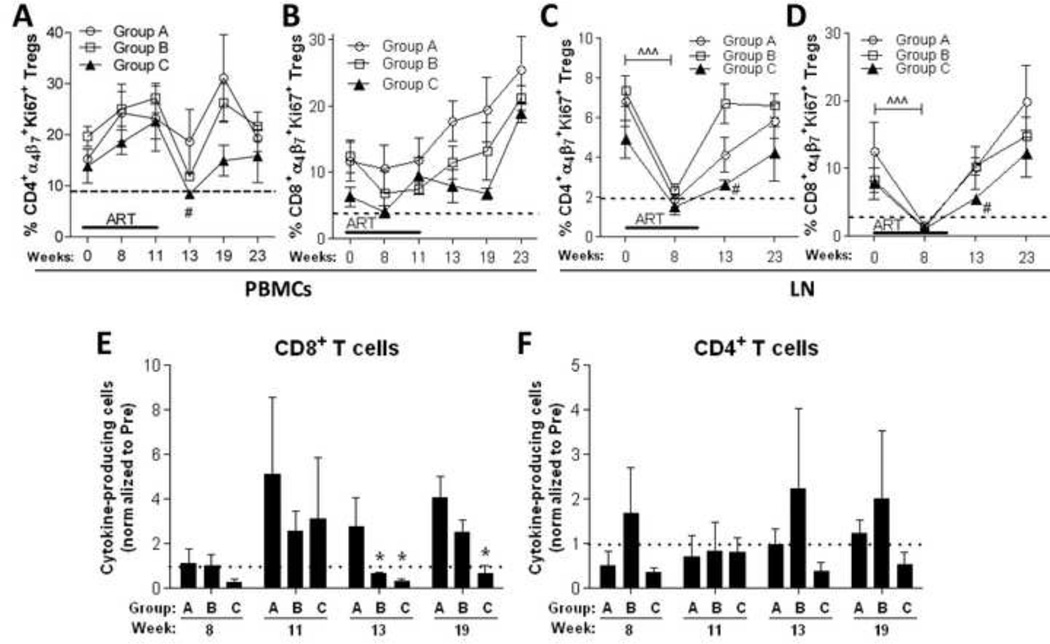
Figure 7. Prolonged B7-DC-Ig treatment transiently decreases Ki67 and α4β7 expression in Tregs and maintains Ag-specific CD8+ T-cell responses at levels lower than those observed before ART.
(A–D) PBMC and LN samples were isolated at the indicated time points and stained for flow cytometric analysis of Ki67 and α4β7 expression on CD4+ and CD8+ Treg cells. Co-expression of Ki67 and α4β7 on CD4+ (A) and CD8+ (B) Tregs within PBMCs. (C–D) Co-expression of Ki67 and α4β7 on CD4+ (C) and CD8+ (D) Tregs within LN cells. Dotted lines represent mean values of Ki67+α4β7+ CD4+ and CD8+ Tregs as calculated from naïve macaques (10 for PBMCs and 5 for LN). (E–F) Combined Gag-specific cytokine responses (IFN-γ/TNF-α/IL-2) in total memory (CD28+CD95−/+) CD8+ (E) and CD4+ (F) T-cells. Data are shown as means ± SEM. In C and D, ^^^, p<0.001 indicates statistically significant differences between the indicated time points by the Wilcoxon signed rank test. *, p<0.05 indicates statistically significant differences between Group A and Group B or C by the Mann-Whitney Test.#p<0.05 indicates statistically significant differences between Group B and Group C by the Mann-Whitney Test.
As increased PD-1 expression on T-cells during the chronic phase of HIV/SIV infection leads to T-cell exhaustion (1, 3, 41) and because anti-PD-1 therapy has successfully reversed T-cell exhaustion in cancer and viral infections (15, 46–48) we examined SIV-specific functional responses of CD4+ and CD8+ T cells over the course of this study. We evaluated the combined Gag-specific production of IFN-γ, IL-2 and TNF-α in total memory (CD95+CD28−/+) CD4+ and CD8+ T cells. B7-DC-Ig treatment (Groups B and C) maintained Gag-specific cytokine production by total memory CD8+ T cells at levels lower than those observed pre-ART for 2 weeks post-ART (Fig. 7E, wk 13). Moreover, continued B7-DC-Ig treatment (Group C) maintained lower Gag-specific cytokine responses in total memory CD8+ T cells for 8 weeks post-ART (Fig. 7E, wk 19). Similar albeit not statistically significant trends were observed in Gag-specific cytokine production by total memory CD4+ T cells under continuous B7-DC-Ig treatment (Fig. 7F, Group C).
Discussion
The potential use of immunomodulatory compounds during anti-HIV therapy is of great interest as a means to boost Ag-specific immune responses and to improve the anti-viral effects of ART and/or therapeutic vaccination (49–51). Although ART has been successful at increasing the life expectancy of HIV-infected individuals, the severity of ART-related side effects has prompted investigations of complementary and/or alternative therapeutic approaches that may lead to use of alternative anti-viral regimens. In this study, we investigated the immunomodulatory effects of B7-DC-Ig, during and after ART, in chronically SIV-infected rhesus macaques. We showed that continuous treatment with B7-DC-Ig caused a moderate overall decrease for up to 12 wks in the median rebound viremia after ART release when compared to median pre-ART levels. Furthermore, prolonged B7-DC-Ig treatment was associated with a decreased proportion of PD-1hi expressing CD8+ and CD4+ T cells and Tregs in PBMCs, and of PD-1hi Tregs in lymph nodes for up to 12 wks post-ART. Similarly, prolonged B7-DC-Ig treatment transiently decreased the expression of Ki67 and α4β7 in PBMC CD4+ and CD8+ Tregs for up to 8 wks post-ART and maintained Ag-specific T-cell responses at levels lower to those observed pre-ART. Our results suggest that continuous immune modulation through the PD-1 pathway using prolonged B7-DC-Ig treatment during and after ART helps maintain lower systemic VL, keeps a favorable Treg and T cell repertoire and modulates antigen-specific responses.
Despite the fact that in vivo PD-1 blockade has previously been reported to improve Ag-specific T cell responses during viral infection (15, 27, 46, 52–54), the immunomodulatory potential of a molecule that selectively targets PD-1hi -expressing cells had not been evaluated in the context of chronic SIV infection and antiretroviral therapy. Interestingly, although plasma viral loads decreased similarly during ART in animals treated with and without B7-DC-Ig, upon ART-release, macaques that continued to receive B7-DC-Ig (group C) displayed a lower rebound post-ART plasma viral load when compared to pre-ART values (Fig. 4A and C). Similar, albeit not statistically significant results were observed in rectal tissue viral loads 2 wks post-ART (Fig. 4B, wk 13). Further, although frequencies of CD4+ and CD8+ T cells and Tregs fluctuated similarly across all three treatment groups (Fig. 5), CD4+ and CD8+ PD-1hi T cells and Tregs within PBMCs maintained the lowest levels with continued B7-DC-Ig treatment post-ART release (Group C, Fig. 6). Our results are similar to observations in HBV-infected patients, where treatment with pegylated interferon led to long-lasting decreases in the percentage of circulating Tregs and levels of PD-1 expression in CD4+ and CD8+ T-cells in patients that responded to treatment (55). The mechanism(s) leading to the decreased frequency of PD-1hi T –cells and Tregs has not been determined, but may include inhibition of proliferation of PD-1hi CD4 T cells and Tregs as previously reported (32). Additionally, preliminary studies using murine B7-DC-Ig in vitro suggests the possibility of ADCC-mediated clearance of PD-1 over-expressing cells (Marshall, S.A. et al. manuscript in preparation).
The extent to which the absolute level of viral suppression during ART affected PD-1hi T cells and Tregs upon ART release requires further study. Although the magnitude of declines were similar, plasma viral loads in Groups A and C dropped to lower levels than that of Group B after 11 weeks of treatment, although tissue viral loads became undetectable in all 3 groups (Fig. 4A–B). In view of the quick viremia rebound in all groups upon ART release (Fig. 4A), the prolonged maintenance of low level CD4+ and CD8+ PD-1hi T cells and Tregs in Group C macaques appears unrelated to viral load rebound (Fig. 6). Similarly, the slower rebound of Group C macaques in α4 β 7+ Ki67+ CD4+ and CD8+ Tregs in LN over weeks 8 to 13 also appears unrelated to absolute viral suppression, as all groups exhibited similar low levels of these cells during ART (Fig. 7C–D, wk 8). Several factors have been shown to influence stronger control of HIV viremia following release of patients from ART, including not only a low plasma viral load during primary infection, but also a higher CD4 count and female gender (56). While CD4 counts here were similar among macaque groups at ART initiation (Fig. 5), two of the 4 macaques in Group C were females. Further studies using larger group sizes and extended ART to bring viral loads to near undetectable levels in all animals will be needed to confirm these initial results of B7-DC-Ig treatment. (Fig. 5)
Previous studies using anti-PD-1 antibodies have shown promising results in the treatment of cancerous and viral malignancies (5, 15, 27, 28). These approaches are based on the use of anti-PD-1 antibodies, which target cells that express PD-1 both at dim and high levels. Although PD-1 has long been considered a marker for cell exhaustion, recent evidence shows that in the context of chronic SIV infection, PD-1 expression by T cells is more likely to be related to a specific differentiation or trafficking stage rather than serving by itself as a reliable marker of immune exhaustion (57). Given that our B7-DC-Ig-mediated treatment uniquely targets PD-1hi expressing cells without blocking PD-1-mediated signaling (32), our therapy targets mostly exhausted PD-1hi -expressing cells, and does not eliminate all PD-1-expressing T cell subpopulations. Despite this selective targeting of exhausted PD-1hi-expressing T cells and Tregs, we did not see an improvement of antigen-specific responses by CD4+ and CD8+ T cells and thus further experiments are needed to directly compare both anti-PD-1 immunomodulation approaches (PD-1 blockade and B7-CD-Ig treatment) in anti-cancer and anti-viral models.
We speculate that the lower levels of exhausted cells in Group C led to improved functional activities, resulting in a slower rate of viral rebound. A decrease in dysfunctional PD-1hi Tregs could have favored better Treg functionality and better control of immune activation, therefore reducing the number of targets for SIV infection. Additionally, the decrease in PD-1hi T-cells in the PBMC compartment could have provided sustained immune control of viremia following the initial reductions attributable to ART. Moreover, although the ART/B7-DC-Ig-mediated decrease of CD4+ PD-1hi T-cells in the LN compartment was not significant (Fig. 4E), follicular helper CD4+PD-1hi T-cells (Tfh) have been recently shown to accumulate in LN and serve as SIV reservoirs during chronic infection (58–60). Thus, a B7-DC-Ig-mediated decrease in Tfh abundance may have contributed to the lower rebound in plasma VL observed. As the current study did not include phenotypic markers for Tfh cells, future studies need to further assess the impact of B7-DC-Ig on these cells. Furthermore, the SIV-mediated accumulation of PD-1hi T cells in the LN (Fig. 2D) may reflect the need of a higher B7-DC-Ig dose for significantly reducing PD-1hi T cells at this location.
Given that α4β7 blockade has previously been shown to reduce plasma and tissue viral loads in SIV-infected macaques (61), we hypothesize that the reductions in plasma viral loads observed in our study might also be due to the B7-DC-Ig-induced transient decrease of α4β7+ and Ki67+ CD4+ Tregs observed in PBMCs and LN (Fig 7A and C). As Foxp3 (62) and CD25 are markers of T cell activation, and α4β7hi memory CD4+ T cells are preferentially infected by SIV (42), the overall reduction of α4β7- and Ki67-expressing CD4+CD25+Foxp3 cells might also reflect an overall decrease in SIV target cell availability and therefore explain the reduction in plasma viral loads observed in group C.
We initially observed that in SIV-infected rhesus macaques, the highest level of PD-1 expression (PD-1hi cells) was exhibited by CD4+ Treg and T cells residing in the LN (Fig. 2D). Given that ART+B7-DC-Ig treatment significantly reduced the percentages of PD-1hi CD4+ and CD8+ T cells and Tregs within PBMCs (Fig. 6), we expected an overall increase in the responding capacity of CD4+ and CD8+ T cells in Ag-specific recall assays. However, as mentioned above, CD4+ and CD8+ T cell Ag-specific immune responses fluctuated during ART and ART+B7-DC-Ig treatment but were maintained at levels lower than those at the start of treatment by continuous administration of B7-DC-Ig (Fig. 7E–F). Although B7-DC-Ig treatment selectively depletes “exhausted” PD-1hi cells, the reduction in SIV antigenic load caused by ART presumably led to the decline observed in Ag-specific immune responses. In this regard, it has previously been reported that HIV-infected individuals receiving ART have a decrease in the magnitude and diversity of their virus-specific T cell receptor repertoire (63). Furthermore, initiation of ART has also been correlated with a decrease in the levels of HIV-specific cytotoxic responses of effector and memory CD8+ T cells (64, 65).
As summarized in recent reviews, the impact of Treg cells on HIV infection and disease progression is not clear (66, 67). Treg cells may be detrimental, as they suppress viral-specific immune responses. On the other hand, Treg cells can control immune activation, thereby decreasing overall pathology as well as the number of target cells available for HIV infection. The Treg dichotomy may be best exhibited during the different phases of disease progression. As hypothesized by Holmes et al., prior to or during acute HIV infection, Treg cells may decrease viral-specific immunity while increasing the number of available target cells (68). In contrast, during the chronic phase of infection, Tregs may reduce immune activation, thereby reducing viremia and slowing T cell loss. Here, in macaques studied during the chronic phase of infection, we observed no differences in Treg cells among groups over the course of the study. However, macaques treated with continuous B7-DC-Ig exhibited prolonged maintenance of a lower level of PD-1hi Tregs. Loss of these dysfunctional, exhausted cells could have provided enhanced function relative to the other groups of macaques, leading to better control of immune activation and better control of rebound viremia as we observed. Our results thus support the hypothesis of Holmes et al. (68). We expect that treatment with ART earlier in the disease course together with prolonged treatment with the B7-DC-Ig immune modulator might have more profound effects on viremia outcome and allow better discrimination of effects on immune response and disease progression.
Highlights.
B7-DC-Ig modulates PD-1hi cells in SIV-infected macaques during and post-ART Continued PD-1 modulation post-ART maintains PD-1hi cells at low levels Continued PD-1 modulation post-ART maintains a favorable T cell and Treg repertoire
Acknowledgments
We gratefully acknowledge the animal caretakers at Advanced BioScience Laboratories, Inc., for their expert care of our macaques and collection of serial samples. The following reagents were obtained through the NIH Nonhuman Primate Reagent Resource: Alpha-4/beta-7-APC and CD4-Qdot 655. The following reagent was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: SIVmac239 Gag peptides, Complete Set. This research was supported by the Intramural Research Program of the NIH, National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
L.L and S.L. are employees of Amplimmune, Inc., which provided B7-DC-Ig and input on study design.
References
- 1.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 2.Porichis F, Kaufmann DE. Role of PD-1 in HIV pathogenesis and as target for therapy. Curr HIV/AIDS Rep. 2012;9:81–90. doi: 10.1007/s11904-011-0106-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 4.Kim PS, Ahmed R. Features of responding T cells in cancer and chronic infection. Curr Opin Immunol. 2010;22:223–230. doi: 10.1016/j.coi.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norde WJ, Hobo W, van der Voort R, Dolstra H. Coinhibitory molecules in hematologic malignancies: targets for therapeutic intervention. Blood. 2012;120:728–736. doi: 10.1182/blood-2012-02-412510. [DOI] [PubMed] [Google Scholar]
- 6.Riley JL. PD-1 signaling in primary T cells. Immunol Rev. 2009;229:114–125. doi: 10.1111/j.1600-065X.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. Embo J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH, Sharpe AH. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh AK, Stock P, Akbari O. Role of PD-L1 and PD-L2 in allergic diseases and asthma. Allergy. 2011;66:155–162. doi: 10.1111/j.1398-9995.2010.02458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez-Garcia M, Porichis F, de Jong OG, Levi K, Diefenbach TJ, Lifson JD, Freeman GJ, Walker BD, Kaufmann DE, Kavanagh DG. Expression of PD-L1 and PD-L2 on human macrophages is up-regulated by HIV-1 and differentially modulated by IL-10. J Leukoc Biol. 2011;89:507–515. doi: 10.1189/jlb.0610327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Messal N, Serriari NE, Pastor S, Nunes JA, Olive D. PD-L2 is expressed on activated human T cells and regulates their function. Mol Immunol. 2011;48:2214–2219. doi: 10.1016/j.molimm.2011.06.436. [DOI] [PubMed] [Google Scholar]
- 12.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao ZQ, King E, Prayther D, Yin D, Moorman J. T cell dysfunction by hepatitis C virus core protein involves PD-1/PDL-1 signaling. Viral Immunol. 2007;20:276–287. doi: 10.1089/vim.2006.0096. [DOI] [PubMed] [Google Scholar]
- 14.Kasprowicz V, Schulze Zur Wiesch J, Kuntzen T, Nolan BE, Longworth S, Berical A, Blum J, McMahon C, Reyor LL, Elias N, Kwok WW, McGovern BG, Freeman G, Chung RT, Klenerman P, Lewis-Ximenez L, Walker BD, Allen TM, Kim AY, Lauer GM. High level of PD-1 expression on hepatitis C virus (HCV)-specific CD8+ and CD4+ T cells during acute HCV infection, irrespective of clinical outcome. J Virol. 2008;82:3154–3160. doi: 10.1128/JVI.02474-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, Vanderford TH, Chennareddi L, Silvestri G, Freeman GJ, Ahmed R, Amara RR. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrovas C, Price DA, Mattapallil J, Ambrozak DR, Geldmacher C, Cecchinato V, Vaccari M, Tryniszewska E, Gostick E, Roederer M, Douek DC, Morgan SH, Davis SJ, Franchini G, Koup RA. SIV-specific CD8+ T cells express high levels of PD1 and cytokines but have impaired proliferative capacity in acute and chronic SIVmac251 infection. Blood. 2007;110:928–936. doi: 10.1182/blood-2007-01-069112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moorman JP, Zhang CL, Ni L, Ma CJ, Zhang Y, Wu XY, Thayer P, Islam TM, Borthwick T, Yao ZQ. Impaired hepatitis B vaccine responses during chronic hepatitis C infection: involvement of the PD-1 pathway in regulating CD4(+) T cell responses. Vaccine. 2011;29:3169–3176. doi: 10.1016/j.vaccine.2011.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, Freeman GJ, Kuchroo VK, Ahmed R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A. 2010;107:14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franceschini D, Paroli M, Francavilla V, Videtta M, Morrone S, Labbadia G, Cerino A, Mondelli MU, Barnaba V. PD-L1 negatively regulates CD4+CD25+Foxp3+ Tregs by limiting STAT-5 phosphorylation in patients chronically infected with HCV. J Clin Invest. 2009;119:551–564. doi: 10.1172/JCI36604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen T, Zheng J, Liang H, Xu C, Chen X, Zhang T, Xu Q, Lu F. Characteristics and PD-1 expression of peripheral CD4+CD127loCD25hiFoxP3+ Treg cells in chronic HCV infected-patients. Virol J. 2011;8:279. doi: 10.1186/1743-422X-8-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grabmeier-Pfistershammer K, Steinberger P, Rieger A, Leitner J, Kohrgruber N. Identification of PD-1 as a unique marker for failing immune reconstitution in HIV-1-infected patients on treatment. J Acquir Immune Defic Syndr. 2011;56:118–124. doi: 10.1097/QAI.0b013e3181fbab9f. [DOI] [PubMed] [Google Scholar]
- 22.Salisch NC, Kaufmann DE, Awad AS, Reeves RK, Tighe DP, Li Y, Piatak M, Jr, Lifson JD, Evans DT, Pereyra F, Freeman GJ, Johnson RP. Inhibitory TCR coreceptor PD-1 is a sensitive indicator of low-level replication of SIV and HIV-1. J Immunol. 2010;184:476–487. doi: 10.4049/jimmunol.0902781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore RD, Keruly JC, Bartlett JG. Improvement in the health of HIV-infected persons in care: reducing disparities. Clin Infect Dis. 2012;55:1242–1251. doi: 10.1093/cid/cis654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Margolis DM. Eradication therapies for HIV Infection: time to begin again. AIDS Res Hum Retroviruses. 2011;27:347–353. doi: 10.1089/aid.2011.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pace MJ, Agosto L, Graf EH, O'Doherty U. HIV reservoirs and latency models. Virology. 2011;411:344–354. doi: 10.1016/j.virol.2010.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calza L. Renal toxicity associated with antiretroviral therapy. HIV Clin Trials. 2012;13:189–211. doi: 10.1310/hct1304-189. [DOI] [PubMed] [Google Scholar]
- 27.Nakamoto N, Kaplan DE, Coleclough J, Li Y, Valiga ME, Kaminski M, Shaked A, Olthoff K, Gostick E, Price DA, Freeman GJ, Wherry EJ, Chang KM. Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization. Gastroenterology. 2008;134:1927–1937. doi: 10.1053/j.gastro.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakthivel P, Gereke M, Bruder D. Therapeutic intervention in cancer and chronic viral infections: antibody mediated manipulation of PD-1/PD-L1 interaction. Rev Recent Clin Trials. 2012;7:10–23. doi: 10.2174/157488712799363262. [DOI] [PubMed] [Google Scholar]
- 29.Kaufmann DE, Walker BD. PD-1 and CTLA-4 inhibitory cosignaling pathways in HIV infection and the potential for therapeutic intervention. J Immunol. 2009;182:5891–5897. doi: 10.4049/jimmunol.0803771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, David O, Burow M, Gordon A, Dhurandhar N, Myers L, Berggren R, Hemminki A, Alvarez RD, Emilie D, Curiel DT, Chen L, Zou W. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 31.Mkrtichyan M, Najjar YG, Raulfs EC, Abdalla MY, Samara R, Rotem-Yehudar R, Cook L, Khleif SN. Anti-PD-1 synergizes with cyclophosphamide to induce potent anti-tumor vaccine effects through novel mechanisms. Eur J Immunol. 2011;41:2977–2986. doi: 10.1002/eji.201141639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mkrtichyan M, Najjar YG, Raulfs EC, Liu L, Langerman S, Guittard G, Ozbun L, Khleif SN. B7-DC-Ig Enhances Vaccine Effect by a Novel Mechanism Dependent on PD-1 Expression Level on T Cell Subsets. J Immunol. 2012;189:2338–2347. doi: 10.4049/jimmunol.1103085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pegu P, Vaccari M, Gordon S, Keele BF, Doster M, Guan Y, Ferrari G, Pal R, Ferrari MG, Whitney S, Hudacik L, Billings E, Rao M, Montefiori D, Tomaras G, Alam SM, Fenizia C, Lifson JD, Stablein D, Tartaglia J, Michael N, Kim J, Venzon D, Franchini G. Antibodies with high avidity to the gp120 envelope protein in protection from SIVmac251 acquisition in an immunization regimen that mimics the RV-144 Thai Trial. J Virol. 2012;87:1708–1719. doi: 10.1128/JVI.02544-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao P, Patterson LJ, Kuate S, Brocca-Cofano E, Thomas MA, Venzon D, Zhao J, DiPasquale J, Fenizia C, Lee EM, Kalisz I, Kalyanaraman VS, Pal R, Montefiori D, Keele BF, Robert-Guroff M. Replicating adenovirus-simian immunodeficiency virus (SIV) recombinant priming and envelope protein boosting elicits localized, mucosal IgA immunity in rhesus macaques correlated with delayed acquisition following a repeated low-dose rectal SIV(mac251) challenge. J Virol. 2012;86:4644–4657. doi: 10.1128/JVI.06812-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demberg T, Brocca-Cofano E, Xiao P, Venzon D, Vargas-Inchaustegui D, Lee EM, Kalisz I, Kalyanaraman VS, Dipasquale J, McKinnon K, Robert-Guroff M. Dynamics of memory B-cell populations in blood, lymph nodes, and bone marrow during antiretroviral therapy and envelope boosting in simian immunodeficiency virus SIVmac251-infected rhesus macaques. J Virol. 2012;86:12591–12604. doi: 10.1128/JVI.00298-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee EM, Chung HK, Livesay J, Suschak J, Finke L, Hudacik L, Galmin L, Bowen B, Markham P, Cristillo A, Pal R. Molecular methods for evaluation of virological status of nonhuman primates challenged with simian immunodeficiency or simian-human immunodeficiency viruses. J Virol Methods. 2010;163:287–294. doi: 10.1016/j.jviromet.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 37.Pereira LE, Villinger F, Onlamoon N, Bryan P, Cardona A, Pattanapanysat K, Mori K, Hagen S, Picker L, Ansari AA. Simian immunodeficiency virus (SIV) infection influences the level and function of regulatory T cells in SIV-infected rhesus macaques but not SIV-infected sooty mangabeys. J Virol. 2007;81:4445–4456. doi: 10.1128/JVI.00026-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fauci AS, Desrosiers RC. Pathogenesis of HIV and SIV. In: Coffin JM, Hughes SH, Varmus HE, editors. Retroviruses. Cold Spring Harbor (NY); 1997. [PubMed] [Google Scholar]
- 39.Sellier P, Mannioui A, Bourry O, Dereuddre-Bosquet N, Delache B, Brochard P, Calvo J, Prevot S, Roques P. Antiretroviral treatment start-time during primary SIV(mac) infection in macaques exerts a different impact on early viral replication and dissemination. PLoS One. 2010;5:10570. doi: 10.1371/journal.pone.0010570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benlhassan-Chahour K, Penit C, Dioszeghy V, Vasseur F, Janvier G, Riviere Y, Dereuddre-Bosquet N, Dormont D, Le Grand R, Vaslin B. Kinetics of lymphocyte proliferation during primary immune response in macaques infected with pathogenic simian immunodeficiency virus SIVmac251: preliminary report of the effect of early antiviral therapy. J Virol. 2003;77:12479–12493. doi: 10.1128/JVI.77.23.12479-12493.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr Opin Immunol. 2007;19:408–415. doi: 10.1016/j.coi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 42.Kader M, Wang X, Piatak M, Lifson J, Roederer M, Veazey R, Mattapallil JJ. Alpha4(+)beta7(hi)CD4(+) memory T cells harbor most Th-17 cells and are preferentially infected during acute SIV infection. Mucosal Immunol. 2009;2:439–449. doi: 10.1038/mi.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kader M, Bixler S, Roederer M, Veazey R, Mattapallil JJ. CD4 T cell subsets in the mucosa are CD28+Ki-67-HLA-DR-CD69+ but show differential infection based on alpha4beta7 receptor expression during acute SIV infection. J Med Primatol. 2009;1(38 Suppl):24–31. doi: 10.1111/j.1600-0684.2009.00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chattopadhyay PK, Roederer M. Good cell, bad cell: flow cytometry reveals T-cell subsets important in HIV disease. Cytometry A. 2010;77:614–622. doi: 10.1002/cyto.a.20905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okoye A, Meier-Schellersheim M, Brenchley JM, Hagen SI, Walker JM, Rohankhedkar M, Lum R, Edgar JB, Planer SL, Legasse A, Sylwester AW, Piatak M, Jr, Lifson JD, Maino VC, Sodora DL, Douek DC, Axthelm MK, Grossman Z, Picker LJ. Progressive CD4+ central memory T cell decline results in CD4+ effector memory insufficiency and overt disease in chronic SIV infection. J Exp Med. 2007;204:2171–2185. doi: 10.1084/jem.20070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Macatangay BJ, Rinaldo CR. PD-1 blockade: A promising immunotherapy for HIV? Cellscience. 2009;5:61–65. [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenblatt J, Glotzbecker B, Mills H, Vasir B, Tzachanis D, Levine JD, Joyce RM, Wellenstein K, Keefe W, Schickler M, Rotem-Yehudar R, Kufe D, Avigan D. PD-1 blockade by CT-011, anti-PD-1 antibody, enhances ex vivo T-cell responses to autologous dendritic cell/myeloma fusion vaccine. J Immunother. 2011;34:409–418. doi: 10.1097/CJI.0b013e31821ca6ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vezys V, Penaloza-MacMaster P, Barber DL, Ha SJ, Konieczny B, Freeman GJ, Mittler RS, Ahmed R. 4-1BB signaling synergizes with programmed death ligand 1 blockade to augment CD8 T cell responses during chronic viral infection. J Immunol. 2011;187:1634–1642. doi: 10.4049/jimmunol.1100077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zajac AJ, Murali-Krishna K, Blattman JN, Ahmed R. Therapeutic vaccination against chronic viral infection: the importance of cooperation between CD4+ and CD8+ T cells. Curr Opin Immunol. 1998;10:444–449. doi: 10.1016/s0952-7915(98)80119-2. [DOI] [PubMed] [Google Scholar]
- 50.Ha SJ, Mueller SN, Wherry EJ, Barber DL, Aubert RD, Sharpe AH, Freeman GJ, Ahmed R. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J Exp Med. 2008;205:543–555. doi: 10.1084/jem.20071949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Finnefrock AC, Tang A, Li F, Freed DC, Feng M, Cox KS, Sykes KJ, Guare JP, Miller MD, Olsen DB, Hazuda DJ, Shiver JW, Casimiro DR, Fu TM. PD-1 blockade in rhesus macaques: impact on chronic infection and prophylactic vaccination. J Immunol. 2009;182:980–987. doi: 10.4049/jimmunol.182.2.980. [DOI] [PubMed] [Google Scholar]
- 52.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 53.Tzeng HT, Tsai HF, Liao HJ, Lin YJ, Chen L, Chen PJ, Hsu PN. PD-1 blockage reverses immune dysfunction and hepatitis B viral persistence in a mouse animal model. PLoS One. 2012;7:e39179. doi: 10.1371/journal.pone.0039179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Porichis F, Kwon DS, Zupkosky J, Tighe DP, McMullen A, Brockman MA, Pavlik DF, Rodriguez-Garcia M, Pereyra F, Freeman GJ, Kavanagh DG, Kaufmann DE. Responsiveness of HIV-specific CD4 T cells to PD-1 blockade. Blood. 2011;118:965–974. doi: 10.1182/blood-2010-12-328070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma H, Zhang HH, Wei L. Frequency of T-cell FoxP3(+) Treg and CD4(+)/CD8(+) PD-1 expression is related to HBeAg seroconversion in hepatitis B patients on pegylated interferon. Chin Med J (Engl) 2013;126:267–273. [PubMed] [Google Scholar]
- 56.Goujard C, Girault I, Rouzioux C, Lécuroux C, Deveau C, Chaix ML, Jacomet C, Talamali A, Delfraissy JF, Venet A, Meyer L, Sinet M. and the ANRS CO6 PRIMO Study Group. 2012. HIV-1 control after transient antiretroviral treatment initiated in primary infection: role of patient characteristics and effect of therapy. Antivir Ther. 17:1001–9. doi: 10.3851/IMP2273. [DOI] [PubMed] [Google Scholar]
- 57.Hong JJ, Amancha PK, Rogers K, Ansari AA, Villinger F. Re-evaluation of PD-1 expression by T cells as a marker for immune exhaustion during SIV infection. PLoS One. 2013;8:e60186. doi: 10.1371/journal.pone.0060186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klatt NR, Vinton CL, Lynch RM, Canary LA, Ho J, Darrah PA, Estes JD, Seder RA, Moir SL, Brenchley JM. SIV infection of rhesus macaques results in dysfunctional T- and B-cell responses to neo and recall Leishmania major vaccination. Blood. 2011;118:5803–5812. doi: 10.1182/blood-2011-07-365874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perreau M, Savoye AL, De Crignis E, Corpataux JM, Cubas R, Haddad EK, De Leval L, Graziosi C, Pantaleo G. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med. 2013;210:143–156. doi: 10.1084/jem.20121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hong JJ, Amancha PK, Rogers K, Ansari AA, Villinger F. Spatial alterations between CD4(+) T follicular helper, B, and CD8(+) T cells during simian immunodeficiency virus infection: T/B cell homeostasis, activation, and potential mechanism for viral escape. J Immunol. 2012;188:3247–3256. doi: 10.4049/jimmunol.1103138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ansari AA, Reimann KA, Mayne AE, Takahashi Y, Stephenson ST, Wang R, Wang X, Li J, Price AA, Little DM, Zaidi M, Lyles R, Villinger F. Blocking of alpha4beta7 gut-homing integrin during acute infection leads to decreased plasma and gastrointestinal tissue viral loads in simian immunodeficiency virus-infected rhesus macaques. J Immunol. 2011;186:1044–1059. doi: 10.4049/jimmunol.1003052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suchard MS, Mayne E, Green VA, Shalekoff S, Donninger SL, Stevens WS, Gray CM, Tiemessen CT. FOXP3 expression is upregulated in CD4T cells in progressive HIV-1 infection and is a marker of disease severity. PLoS One. 2010;5:e11762. doi: 10.1371/journal.pone.0011762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Conrad JA, Ramalingam RK, Duncan CB, Smith RM, Wei J, Barnett L, Simons BC, Lorey SL, Kalams SA. Antiretroviral therapy reduces the magnitude and T cell receptor repertoire diversity of HIV-specific T cell responses without changing T cell clonotype dominance. J Virol. 2012;86:4213–4221. doi: 10.1128/JVI.06000-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Casazza JP, Betts MR, Picker LJ, Koup RA. Decay kinetics of human immunodeficiency virus-specific CD8+ T cells in peripheral blood after initiation of highly active antiretroviral therapy. J Virol. 2001;75:6508–6516. doi: 10.1128/JVI.75.14.6508-6516.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kalams SA, Goulder PJ, Shea AK, Jones NG, Trocha AK, Ogg GS, Walker BD. Levels of human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte effector and memory responses decline after suppression of viremia with highly active antiretroviral therapy. J Virol. 1999;73:6721–6728. doi: 10.1128/jvi.73.8.6721-6728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moreno-Fernandez ME, Presicce P, Chougnet CA. Homeostasis and function of regulatory T cells in HIV/SIV infection. J Virol. 2012;86:10262–10269. doi: 10.1128/JVI.00993-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chevalier MF, Weiss L. The split personality of regulatory T cells in HIV infection. Blood. 2013;121:29–37. doi: 10.1182/blood-2012-07-409755. [DOI] [PubMed] [Google Scholar]
- 68.Holmes D, Jiang Q, Zhang L, Su L. Foxp3 and Treg cells in HIV-1 infection and immuno-pathogenesis. Immunol Res. 2008;41:248–266. doi: 10.1007/s12026-008-8037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]