Abstract
Significance: Vitamin C, or ascorbic acid, has long been known to participate in several important functions in the vascular bed in support of endothelial cells. These functions include increasing the synthesis and deposition of type IV collagen in the basement membrane, stimulating endothelial proliferation, inhibiting apoptosis, scavenging radical species, and sparing endothelial cell-derived nitric oxide to help modulate blood flow. Although ascorbate may not be able to reverse inflammatory vascular diseases such as atherosclerosis, it may well play a role in preventing the endothelial dysfunction that is the earliest sign of many such diseases. Recent Advances: Beyond simply preventing scurvy, evidence is mounting that ascorbate is required for optimal function of many dioxygenase enzymes in addition to those involved in collagen synthesis. Several of these enzymes regulate the transcription of proteins involved in endothelial function, proliferation, and survival, including hypoxia-inducible factor-1α and histone and DNA demethylases. More recently, ascorbate has been found to acutely tighten the endothelial permeability barrier and, thus, may modulate access of ascorbate and other molecules into tissues and organs. Critical Issues: The issue of the optimal cellular content of ascorbate remains unresolved, but it appears that low millimolar ascorbate concentrations are normal in most animal tissues, in human leukocytes, and probably in the endothelium. Although there may be little benefit of increasing near maximal cellular ascorbate concentrations in normal people, many diseases and conditions have either systemic or localized cellular ascorbate deficiency as a cause for endothelial dysfunction, including early atherosclerosis, sepsis, smoking, and diabetes. Future Directions: A key focus for future studies of ascorbate and the vascular endothelium will likely be to determine the mechanisms and clinical relevance of ascorbate effects on endothelial function, permeability, and survival in diseases that cause endothelial dysfunction. Antioxid. Redox Signal. 19, 2068–2083.
Introduction
Vitamin C, or ascorbic acid, is required to prevent scurvy, but debate continues as to whether any single function of the vitamin is really necessary and the extent to which ascorbate contributes to optimal function of an organ or even a cell. One of the organs most affected by ascorbate is the endothelium, which regulates the distribution of ascorbate throughout the body and where ascorbate has many functions. Ascorbate has long been known to enhance endothelial synthesis and deposition of Type IV collagen to form the basement membrane of blood vessels. More recent studies reveal other potential functions of the vitamin in the endothelium, especially as related to control of endothelial cell proliferation and apoptosis, smooth muscle-mediated vasodilation, and endothelial permeability barrier function. Accordingly, this review will consider the extent to which ascorbate helps maintain the health of the endothelium, the mechanisms by which it does so, and how ascorbate might aid in the normal functions of the endothelium.
Ascorbate Chemistry and Biochemical Functions
Ascorbate chemistry
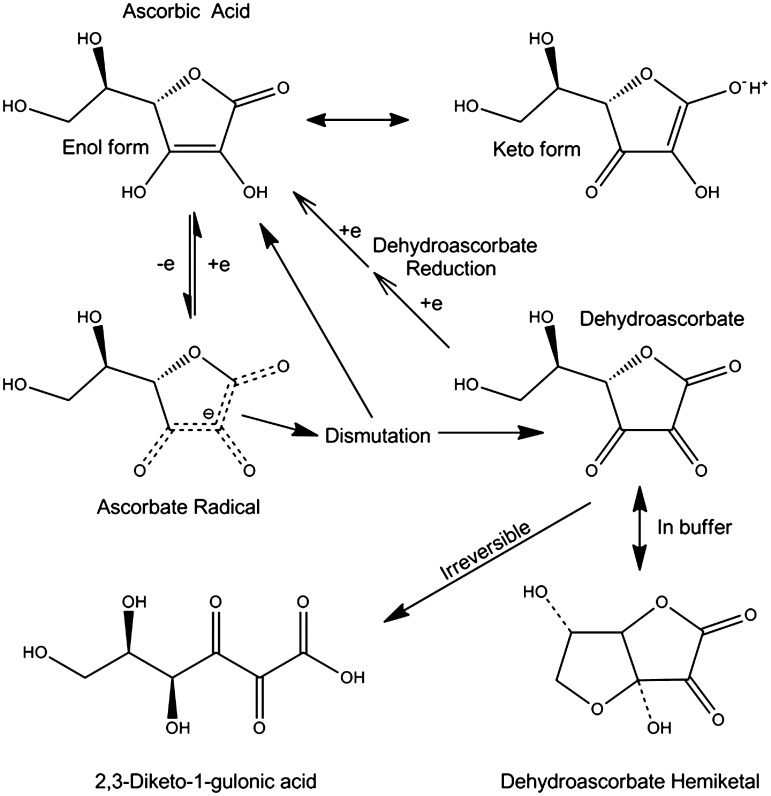
As shown in Figure 1, four of the six ascorbic acid carbons form a cyclic 5-membered lactone ring that is strained because of carbon bond angle preferences. Although aliphatic alcohols are usually not acidic, the presence of a double bond between carbons 2 and 3 allows for keto-enol tautomerism, lowering the pKa of ascorbic acid to 4.1 (Fig. 1). Thus, it is effectively a monoanion at physiologic pH. Ascorbate donates a single electron in all its redox reactions, generating the ascorbate radical. This radical is not very reactive with anything but itself (17). Dismutation of two ascorbate radicals forms a molecule each of ascorbate and dehydroascorbate (Fig. 1). Dehydroascorbate, a tri-ketone lactone ring structure, is very unstable, with a half life in physiologic buffer of about 6 min (47, 178). Hydrolysis of the lactone ring irreversibly converts it to 2,3-diketo-1-gulonic acid (Fig. 1) (19, 30). In buffer, dehydroascorbate preferentially forms a hemiketal (43, 126) (Fig. 1) that resembles glucose in its molecular configuration and has affinity for the GLUT-type glucose transporter (165).
FIG. 1.
Ascorbic acid metabolism. Ascorbate donates a single electron to become the ascorbate radical, which reacts with another ascorbate radical to form a molecule each of ascorbate and dehydroascorbate (DHA). The latter is unstable at physiologic pH and if not reduced back to ascorbate via GSH-dependent mechanisms, it will undergo irreversible ring opening and loss. In buffers, DHA forms a hemiketal that has a molecular structure resembling that of glucose.
Ascorbate uptake
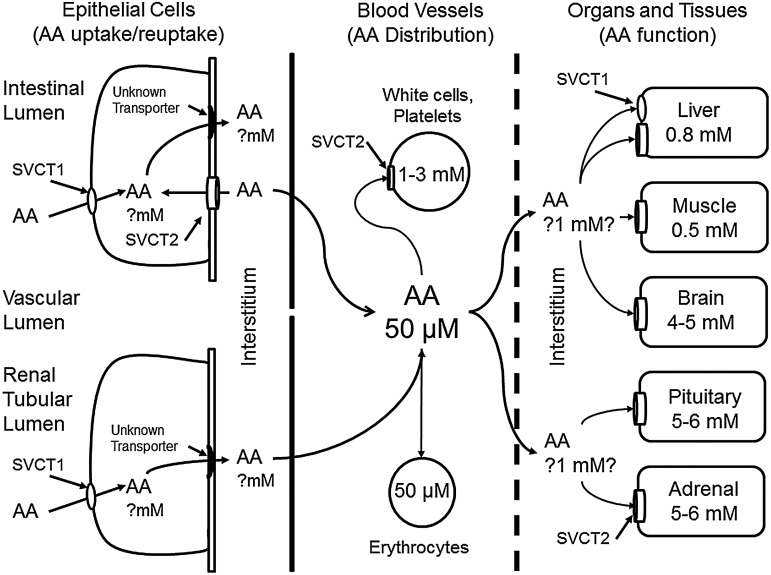
Since humans cannot synthesize their own vitamin C, it should be absorbed in the intestine and carried through the circulation to the various organs (Fig. 2). The vitamin is taken up as ascorbate into intestinal cells on a dedicated sodium- and energy-dependent transporter, termed the Sodium-dependent Vitamin C Transporter 1 (SVCT1). Dehydroascorbate uptake on the intestinal Sodium-dependent Glucose Transporter-1 (SGLT1) may also contribute to absorbed ascorbate (16). Ascorbate probably exits the enterocytes via an unknown transporter (Fig. 2, left side) and somehow enters the circulation where it typically circulates at concentrations of 40–60 μM, depending on dietary intake and rates of disposition. Due to its low molecular weight, vitamin C is freely filtered by the kidney, but reabsorbed in the renal proximal tubule, again by the SVCT1 (Fig. 2, left side). This mechanism helps conserve ascorbate in the blood for uptake into the tissues. Cellular uptake of ascorbate occurs largely on the Sodium-dependent Vitamin C Transporter 2 (SVCT2), which is closely related to the SVCT1 in structure and function (161), but is located mostly in non-epithelial cells (Fig. 2). It is the only known ascorbate transporter in endothelial cells (141). This transporter generates a sharp gradient of ascorbate from the blood to human white blood cells, resulting in intracellular concentrations of the vitamin as high as 3 mM in monocytes (14) and 2 mM in neutrophils (173) and platelets (53). Mature erythrocytes, which do not contain either SVCT (109), have the same intracellular ascorbate concentrations as in the surrounding blood plasma (Fig. 2, center). Ascorbate exits from the bloodstream, as discussed in greater detail next, then enters various cells and organs, again largely on the SVCT2 (Fig. 2, right side).
FIG. 2.
Uptake and distribution of ascorbate across the vascular bed. Ascorbate (AA) is taken up from the intestine either on the SVCT1 or as DHA on glucose transporters (not shown). Once inside the intestinal epithelium, it exits by an unknown mechanism on the basolateral membrane into the interstitium and then into nearby capillaries. Ascorbate in the bloodstream is taken up by erythrocytes (either as DHA or as slow diffusion) and by leukocytes and endothelial cells on the SVCT2. Plasma ascorbate is distributed by the vascular tree to organ beds. Interstitial ascorbate is then taken up by the SVCT2 on nucleated cells in the organs. In the central nervous system, ascorbate enters the cerebrospinal fluid largely by secretion from the choroid plexus (not shown). SVCT1, sodium-dependent vitamin C transporter 1; SVCT2, sodium-dependent vitamin C transporter 2.
The intracellular ascorbate concentration in endothelial cells has not been directly measured, but based on levels that can be achieved by adding ascorbate to cultured cells, it is likely in the range of 2–4 mM (95, 103). Perhaps, of significance, such low millimolar concentrations are required for optimal synthesis of type IV collagen (103). As expected, the SVCT2 is expressed in primary culture endothelial cells derived from both pigs (15) and mice (131). However, not all endothelial cells express the SVCT proteins. Endothelial cells forming the blood–brain barrier appear to lack the SVCT2 in vivo (57) and at least initially ex vivo (131). Perhaps in line with this, ascorbate does not cross the blood–brain barrier in vivo at appreciable rates (2). Although lack of the SVCT2 might at first glance be considered the cause of blood–brain barrier impermeability to ascorbate, as discussed next, ascorbate in other vascular beds likely crosses the endothelial barrier by diffusion around the cells, which is severely limited by the tight junctions of the blood–brain barrier.
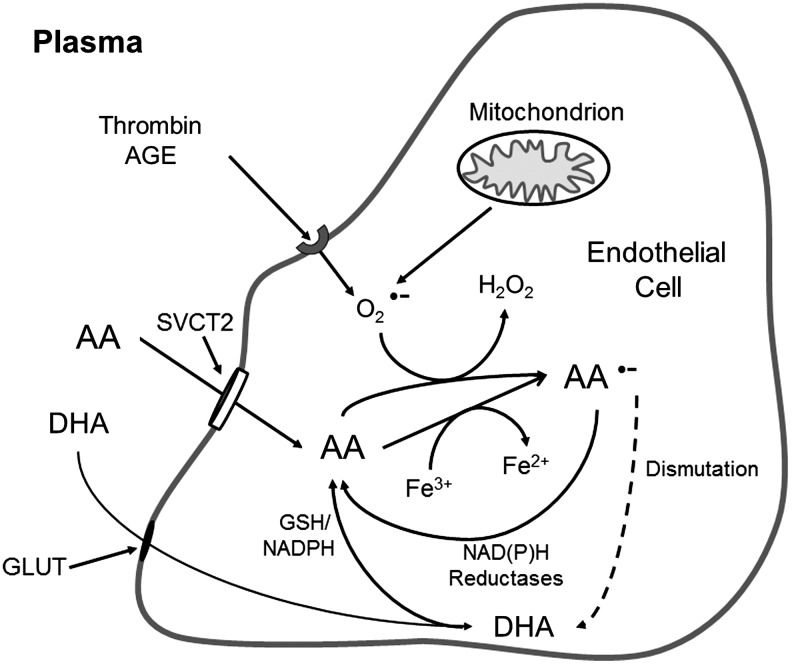
Ascorbate can also enter cells via its two-electron-oxidized form, dehydroascorbate (Fig. 3). As noted earlier, the hemiketal of dehydroascorbate (Fig. 1) is transported into cells by facilitated diffusion on the ubiquitous GLUT-type glucose transporters. Once inside the cell, dehydroascorbate is rapidly reduced back to ascorbate, However, given that plasma dehydroascorbate concentrations are usually 2 μM or less (42), and that the normal circulating level of 5 mM glucose will compete for uptake with dehydroascorbate, it is unlikely that ascorbate concentrations in cells are bolstered much by dehydroascorbate, except perhaps in erythrocytes or in conditions of high oxidative stress in blood, such as during the respiratory burst of phagocytic cells (124, 172). Additional evidence suggesting that the SVCT2 may be the only route of ascorbate entry in most cells is that brains of embryonic mice lacking both copies of the SVCT2 have undetectable ascorbate levels (146), as do primary culture macrophages lacking the SVCT2 (8).
FIG. 3.
Endothelial cell ascorbate uptake and recycling. Ascorbate (AA) enters endothelial cells largely on the SVCT2, although a small amount may come in as DHA on glucose transporters (GLUT), to be rapidly reduced to ascorbate in the cell. Once in the cell, ascorbate can donate an electron ferric iron, superoxide (O2•−), and other radical species generated in mitochondria or via activation of cell surface receptors, such as those for thrombin or advanced glycation end-products (AGE). The resulting ascorbate radical (AA•−) is mostly reduced directly back to ascorbate by NADH- and NADPH-dependent reductases. However, if the oxidative stress is overwhelming, the ascorbate radical may dismutate to form ascorbate and DHA, with subsequent reduction of the latter back to ascorbate.
Ascorbate recycling
Once inside cells, ascorbate is essentially trapped due to its hydrophilic nature and negative charge. Intracellular ascorbate can then scavenge a variety of radicals, with superoxide being one of the most important. Superoxide might be generated within the cell as a result of receptor activation, such as by advanced glycation end products or by thrombin (Fig. 3). Superoxide can also originate as a byproduct of mitochondrial metabolism, especially in response to excessive glucose metabolism in diabetes. The major scavenger of superoxide in cells is likely to be superoxide dismutase, which has been noted to react in vitro with superoxide 105 times faster than ascorbate (78) (see Table 1 for rate constants). In the endothelial cell, there will also be plentiful nitric oxide, which will react at diffusion-limited rates with superoxide [rate constant 0.7–1.9×1010 M·s−1 (76)], generating the strong oxidant peroxynitrite (13). Nonetheless, ascorbate at concentrations of 1–10 mM does scavenges superoxide and enhances arterial relaxation to acetylcholine in rabbit thoracic aorta (78), suggesting that the presumed low millimolar ascorbate concentrations in endothelial cells may allow ascorbate to aid in scavenging both superoxide (78) and peroxynitrite (78, 83). Ascorbate can also reduce enzyme-bound ferric to ferrous iron in enzymes (Fig. 3), as discussed in detail next.
Table 1.
Rate Constants for Reaction of Antioxidants with Superoxide and Peroxynitrite
In all these reactions, the resulting ascorbate radical is rapidly reduced back to ascorbate by one or more poorly characterized NADH-dependent dehydrogenases (67, 77, 91), as well as by thioredoxin reductase using NADPH as the reducing agent (98) (Fig. 3). As noted earlier in Figure 1, two ascorbate radicals can also dismutate to form a molecule each of ascorbate and dehydroascorbate. The latter is reduced directly via a two-step mechanism by reduced glutathione, or enzymatically by GSH-dependent thiol transferases (174, 179) or by NADPH-dependent reductases (41), the latter again including thioredoxin reductase (100) (Fig. 3).
Ascorbate transfer across the endothelial barrier
The barrier to diffusion of substances out of blood vessels varies with vessel type (artery, vein, or capillary) and tissue bed. Indeed, the tightness of the endothelial permeability barrier changes along the vascular tree, from well-organized and numerous connections between endothelial cells in large arteries and veins (where there is strict control of permeability), to near absence in some postcapillary venules, where exchange of plasma and interstitial constituents is efficient (40). Endothelial cells in some organs, such as those in liver sinusoids and the renal glomerulus, have fenestrations that clearly facilitate such exchange. In larger vessels or vascular beds where the endothelium presents a substantial barrier to the exchange of both large and small molecules, ascorbate could enter the interstitium by transiting either through endothelial cells or going between them.
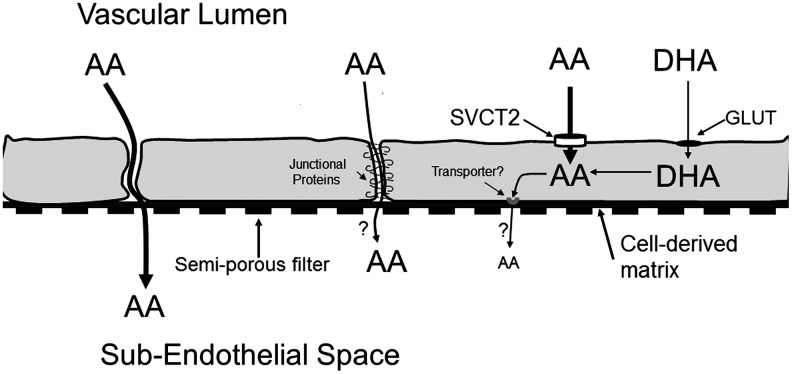
Ascorbate readily enters most endothelial cells on the luminal SVCT2. However, ascorbate is also known to efflux from cultured endothelial cells (104, 162), an effect enhanced by increases in intracellular calcium (37). It follows that this efflux could reflect vectorial ascorbate efflux from the abluminal or basolateral side of the cells (104). This model would mirror that which exists for epithelial cell barriers (Fig. 2), which are much tighter than those generated by the endothelium. For example, the colon carcinoma-derived cell line Caco-2, which has been used to model the intestinal epithelial barrier, expresses the SVCT1 in the apical cell membrane (20, 96). The SVCT2 isoform is expressed on the basolateral membrane (20). Both transporters are thought to mediate unidirectional ascorbate transport (161); so, their orientation in this manner would serve only to bring ascorbate into the cells. Unless the functional orientation of the SVCT2 can reverse, this would require a yet-to-be-described transporter or channel to facilitate ascorbate efflux from the basolateral cell border.
At least in endothelial cells that form a permeability barrier, it appears that ascorbate crosses this barrier by going around the cells in a paracellular manner (108) (Fig. 4). The evidence for this is that intracellular ascorbate did not appreciably efflux below endothelial cells cultured on semi-porous filters for more than 90 min. Indeed, there was an efflux of intracellular ascorbate into the luminal, not the abluminal compartment. Further, ascorbate accumulation below the cells and filters was decreased rather than increased as the intracellular ascorbate concentration was increased (108). It was concluded that short-term ascorbate transfer occurs on gaps between the cells or even across one or more cellular junctional connections (Fig. 4). Endothelial cells in culture have 0.5–2 μm gaps between as many as 10% of cells, which decrease in size with time in culture (3, 108). Ascorbate could, thus, diffuse across any gaps between the cells with little sieving effect. Ascorbate could also diffuse across the cellular connections that form rings of attachment between endothelial cells (4) (Fig. 4). Cell-to-cell endothelial connections are made up of tight junctions, gap junctions, adherence junctions, and syndesmos (11, 40), although they are not as tight as those found in epithelial layers (3). These junctions have been proposed as a barrier that contains pores and transporters which might regulate the passage of small charged molecules such as ions (11, 155). Our previous finding that ascorbate transfer across an endothelial cell barrier was decreased by several anion channel inhibitors, including sulfinpyrazone, 5-nitro-2-(3-phenylpropylamino)benzoic acid, niflumic acid, and probenecid (108), suggests that such a mechanism might apply to ascorbate.
FIG. 4.
Routes of transfer of ascorbate out of the vascular bed as represented by endothelial cells in culture on semi-porous filters. Ascorbate (AA) or DHA added on the luminal side of endothelial cells cultured on semi-porous membranes enter the cells on the SVCT2 or GLUT-type transporter, respectively. The resulting ascorbate is trapped with little exit on the basolateral side of the cells over a 90 min time-frame. Rather, most ascorbate passes between the cells by a paracellular route, which intracellular ascorbate tightens. There may also be some transit of ascorbate between the cells as sieving across tight junctional proteins.
If ascorbate leaves the vascular space by diffusion between endothelial cells as suggested by experiments using cells cultured on semi-permeable membranes, then the interstitial ascorbate concentration will be no greater than the plasma concentration and will decrease near cells taking ascorbate up on the SVCT2. However, direct measurement of subcutaneous dermal ascorbate concentrations by microdialysis coupled with gas chromatography-mass spectroscopy showed an interstitial ascorbate concentration of 1 mM (87). This ascorbate concentration is similar to that generated by the SVCT2 in many cell types and is 15–20-fold higher than present in blood (Fig. 2). If correct, this measurement suggests that the simple diffusion of ascorbate which we observed between dermal microcapillary endothelial cells in primary culture (108) does not reflect the situation in vivo. Rather, it suggests that the normal dermal capillary endothelial barrier is very tight to ascorbate passage and that there is an as-yet-undiscovered secretory component from the basolateral endothelium (or from cells in the interstitial space) which is not detected in short-term cell culture experiments.
Ascorbate Function in Endothelial Cells
Antioxidant function: scavenging of endogenous and exogenous radicals
Ascorbate has long been considered a key cellular antioxidant, serving as a primary antioxidant by detoxifying exogenous radical species that have entered cells or which have arisen within cells due to excess superoxide generation by mitochondrial metabolism, by NADPH oxidase, xanthine oxidase, or by uncoupled nitric oxide synthase (NOS). As previously noted, at the low millimolar concentrations of ascorbate likely to exist in endothelial cells, ascorbic acid will aid superoxide dismutase in scavenging superoxide and its more toxic breakdown products (Fig. 3). The question arises as to just how much intracellular ascorbate contributes to the antioxidant capacity of cells. GSH is often thought of as the major low-molecular-weight antioxidant in cells, both scavenging radicals and serving as the electron donor for antioxidant enzymes such as glutathione peroxidase. It is certainly a stronger reducing agent than ascorbate (24) and even recycles ascorbate, as noted earlier. However, GSH is more than 100-fold less reactive with superoxide than is ascorbate (Table 1) and although GSH is converted to GSSG, the overall reaction does not reduce superoxide to H2O2 (180). The reaction rate of peroxynitrite with GSH is twice that of peroxynitrite with ascorbate (Table 1), so it should effectively scavenge the oxidant. Thus, although GSH might be a more effective scavenger of peroxynitrite than is ascorbate, only ascorbate will effectively remove superoxide.
It is likely that endothelial cells treated with ascorbate concentrations similar to those found in plasma will have similar intracellular ascorbate and GSH concentrations, both of which are in the low millimolar range (102, 107). Further, ascorbate concentrations decrease at lower levels of oxidative stress than do GSH concentrations when these are generated by several oxidants, including menadione (110), ferricyanide (101), and oxidized low-density lipoprotein (LDL) (99). Although these results might suggest that ascorbate “takes the first hit” in the defense against reactive oxygen or nitrogen species, ascorbate could also appear to be more sensitive to oxidative stress than GSH, because the latter has a more robust recycling mechanism and as GSH can be synthesized by the cell.
The reaction rates of superoxide and peroxynitrite with three other cellular antioxidants are shown in Table 1. With regard to superoxide, ascorbate reacts at a similar rate compared with tetrahydrobiopterin (BH4), but reacts 100-fold faster than estimated rates for α-tocopherol in micelles. Uric acid itself it not reactive with superoxide, although its mono-radical species are quite reactive. Both BH4 and α-tocopherol react much faster than ascorbate with peroxynitrite, whereas rates with uric acid are similar.
Although ascorbate is widely considered an antioxidant, its reduction of transition metals such as Fe3+ and Cu2+ is well known to generate reactive oxygen species, including the hydroxyl radical (25). These reactions are potentially important for interpretation of in vitro, cell culture, or tissue incubation experiments, where free metal ions might exist. On the other hand, an extensive review of in vivo studies by Carr and Frei (28) found little evidence for a pro-oxidant effect of ascorbate on markers of DNA, protein, and lipid oxidation.
Recycling of intracellular radicals of cellular constituents and enzyme co-factors
Ascorbate also recycles and, thus, preserves several readily oxidized molecules in the cell. It repairs both protein (45) and lipid radicals (55). The latter occurs indirectly as ascorbate recycling of α-tocopherol in cell membranes. When α-tocopherol reacts with lipid peroxyl radicals in the lipid phase, it is oxidized to the α-tocopheroxyl radical, which ascorbate subsequently reduces to α-tocopherol (24, 122). Ascorbate recycling of the α-tocopheroxyl radical has been observed in lipid micelles (18, 122) and likely accounts for sparing of α-tocopherol in erythrocyte ghosts (34), in intact cells (111), and in human smokers (23). This sparing of α-tocopherol by ascorbate has also been observed in endothelial cells cultured in the presence of oxidized LDL (135).
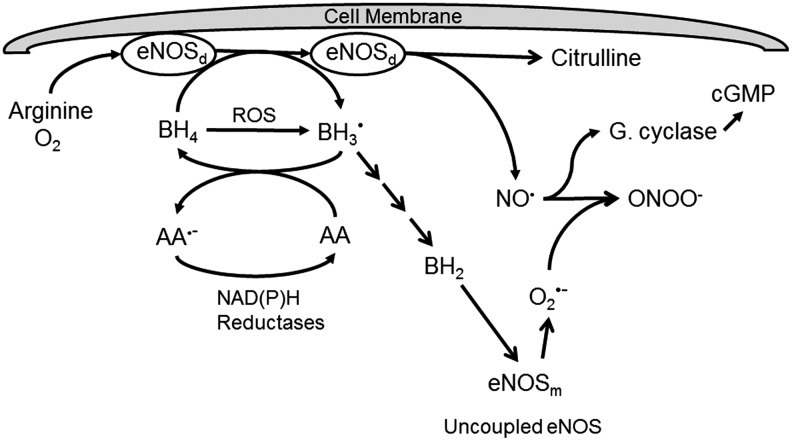
An important enzyme co-factor recycled by ascorbate is BH4. BH4 maintains the catalytic iron of several dioxygenase enzymes in the active ferrous form. These enzymes mediate the hydroxylation of key neurotransmitter precursors, triglyceride precursors, and the synthesis of nitric oxide (Table 2). Regarding the latter, ascorbate recycling of BH4 is especially important for the proper function of endothelial nitric oxide synthase (eNOS) (Fig. 5) (72, 83). BH4 is synthesized de novo through a pathway controlled by GTP-cyclohydrolase-1 and by recycling of dihydrobiopterin (BH2) by dihydrofolate reductase. BH4 (but not ascorbate) is a required co-factor for eNOS, along with arginine and molecular oxygen. During the enzyme cycle, BH4 donates an electron to the active dimeric form of eNOS (eNOSd) and becomes the trihydrobiopterin radical (BH3•), which is efficiently reduced back to BH4 by ascorbate, with a rate constant of ∼1.7×105 M·s−1 (127) (Fig. 5). This reaction is very specific for ascorbate, as thiols are ineffective (83). Failure to reduce the BH3• results in its decomposition to inactive BH2, which then competes with a similar affinity as BH4 for binding on eNOS, but does not support NO• production (36). Lack of BH4, combined with increased BH2, uncouples eNOS (152), causing the enzyme to generate superoxide instead of NO•. What NO• is generated then may rapidly react with this superoxide to form peroxynitrite (13), which will both consume NO• and oxidize more BH4 (Table 1) (83). In this regard, any scavenging of superoxide by ascorbate will also prevent its reaction with NO• to form peroxynitrite (148). Another consequence of uncoupled eNOS noted in Figure 5 is that it dissociates from the plasma membrane as well as from itself to form unbound monomers (eNOSm) that generate superoxide (83, 89).
Table 2.
Tetrahydrobiopterin-Dependent Enzymes
| Enzyme | Substrate | Product(s) | Relevance |
|---|---|---|---|
| Phenylalanine hydroxylase |
L-phenylalanine |
L-tyrosine |
Phenylketonuria when deficient |
| Tyrosine hydroxylase |
L-tyrosine |
L-DOPA |
First and rate-limiting step in catecholamine synthesis |
| Tryptophan hydroxylase |
L-tryptophan |
5-hydroxy-tryptophan |
First and rate-limiting step in serotonin synthesis |
| Nitric oxide synthase |
L-arginine |
L-citrulline, nitric oxide |
Nitric oxide generation |
| Alkylglycerol monooxygenase | 1-alkyl-sn-glycerol | 1-hydroxyalkyl-sn-glycerol | Glycerol and lipid metabolism |
FIG. 5.
Ascorbate recycling of BH4 and preservation of nitric oxide. Dimeric eNOS (eNOSd) attached to the endothelial cell plasma membrane utilizes arginine, molecular oxygen, and BH4 to generate nitric oxide (NO•) that subsequently activates endothelial and smooth muscle guanylate cyclase (G. cyclase). In the enzyme cycle, the trihydrobiopterin radical (BH3•) is generated, which is recycled by ascorbate (AA). The resulting ascorbate radical (AA•−) is recycled by various NAD(P)H-dependent reductases. Failure to recycle BH3•, or its formation due to BH4 oxidation by reactive oxygen species (ROS), results in the formation of dihydrobiopterin (BH2), which competes with BH4 for the enzyme. This, and loss of BH4 uncouples eNOS, which then dissociates from the membrane into monomers (eNOSm) that generate superoxide (O2•−) rather than NO•. Reaction of O2•− with any available NO• forms peroxynitrite, a strong nitrating oxidant. By initially recycling BH4, ascorbate prevents loss of BH4 and sustains eNOS activity. BH4, tetrahydrobiopterin
It is evident that the ability of eNOS to generate NO• depends both on synthesizing BH4 and on recycling it from BH2 through the pathways controlled by GTP-cyclohydrolase-1 and dihydrofolate reductase, respectively. Nonetheless, ascorbate recycling of the BH3• at a site proximal in the pathway of oxidation of BH4 to BH2 (Fig. 5) would also be expected to maintain effective BH4/BH2 ratios, thus lessening the need for recycling from BH2 by dihydrofolate reductase.
Regulation of enzyme phosphorylation
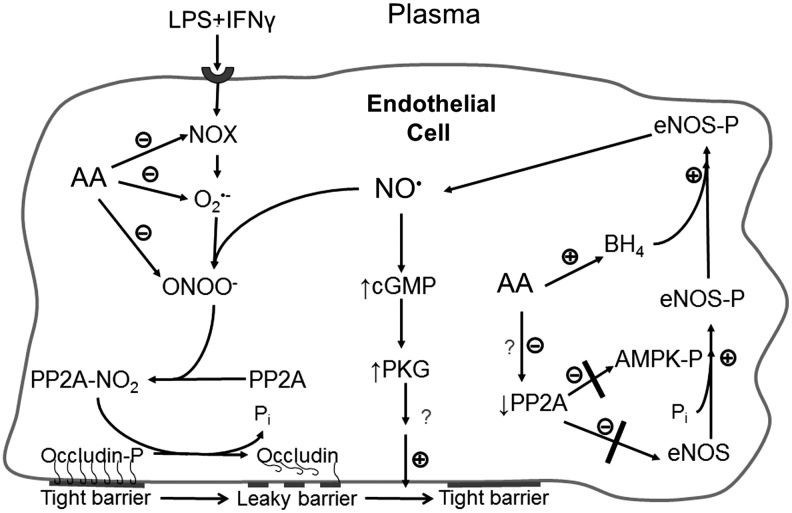
Ascorbate was found by Wilson's group to inhibit the increased activity of protein phosphatase type 2A (PP2A) in cultured primary endothelial cells exposed to a septic insult (bacterial lipopolysaccharide [LPS] + interferon-γ [IFNγ]) (66). The proposed mechanism of this effect is depicted in the left-hand cascade in Figure 6. Stimulation of NADPH oxidase by LPS + IFNγ generates superoxide, which in endothelial cells rapidly reacts with available nitric oxide, generating peroxynitrite. The latter can then nitrate the catalytic subunit of PP2A, leading to its activation (183), which, in turn, was shown to dephosphorylate occludin and disrupt the endothelial permeability barrier (66). Ascorbate inhibits this process by scavenging both superoxide and peroxynitrite (Fig. 6). Wu, et al. (182) hypothesized that such scavenging by ascorbate would decrease positive feedback of reactive oxygen/nitrogen species on NADPH oxidase subunit assembly and, thus, indirectly inhibit enzyme function.
FIG. 6.
Multiple mechanisms by which ascorbate preserves nitric oxide and tightens the endothelial permeability barrier. In endothelial cells in which NADPH oxidase (NOX) is activated by septic insult (or other mechanisms), the resulting superoxide (O2•−) reacts with available nitric oxide (NO•) to form peroxynitrite (ONOO−), which nitrates and activates PP2A. The phosphatase then dephosphorylates occludin, causing it to pull away from the membrane and weaken tight junctional structures. Ascorbate prevents the activation of PP2A in this pathway by inhibiting NOX function and scavenging O2•− and ONOO−. In unstimulated cells (with presumably low levels of ONOO−, ascorbate also enhances nitric oxide generation by inhibiting PP2A by an unknown mechanism. This prevents PP2A from dephosphorylating and thus deactivating eNOS itself, as well as the AMP-dependent kinase (AMPK). The resulting increase in eNOS phosphorylation is mediated at least in part by phosphorylation-dependent activation of AMPK, which activates eNOS to generate nitric oxide. This, along with the preservation of BH4 by ascorbate, increases intracellular nitric oxide, which then generates cyclic GMP through the canonical pathway to eventually tighten the endothelial permeability barrier. PP2A, protein phosphatase type 2A.
More recently, ascorbate inhibition of PP2A activity has been implicated in the regulation of eNOS in non-stimulated primary and immortalized human umbilical vein endothelial cells (85) (Fig. 6). PP2A is a key phosphatase involved in dephosphorylation of multiple protein kinases (116) and other enzymes, including eNOS (117). In the study by Ladurner, et al. (85), ascorbate was shown to increase phosphorylation of eNOS-Ser1177 and decrease phosphorylation of eNOS-Thr495. This pattern of phosphorylation changes is known to substantially activate the enzyme (117). These phosphorylation effects were rapid and complete within 30 min, although the comparable increase in eNOS activity required 16 h of incubation with ascorbate. An ascorbate-dependent increase in BH4 required 24 h, suggesting that the observed enzyme activation was not due to preservation of BH4 by ascorbate. These investigators went on to implicate inhibition of PP2A as the mechanism of ascorbate-stimulated eNOS-Ser1177 phosphorylation. It is known that PP2A dephosphorylates eNOS-Ser1177 (117), so that its inhibition could allow kinases to increase eNOS-Ser1177 phosphorylation (Fig. 6). Ladurner, et al. (85) found that the ascorbate-dependent increase in phosphorylation of eNOS-Ser1177 was blocked by overexpression of the catalytic subunit of PP2A and that the ascorbate-induced phosphorylation patterns of both eNOS-Ser1177 and eNOS-Thr495 were mimicked by specific inhibition of PP2A with okadaic acid. They also determined by inhibitor and knockdown experiments that the ascorbate-induced phosphorylation of eNOS-Ser1177 was likely due to the activation of AMP kinase (AMPK), which showed rapid and sustained increased phosphorylation at AMPK-Thr172 in response to ascorbate. PP2A is known to dephosphorylate AMPK on Thr172 (181), so that its inhibition by ascorbate could result in an increase in its phosphorylation and activation (Fig. 6). Although inhibition of PP2A could nicely explain the stimulation of eNOS activity by ascorbate, there remains the caveat that Han, et al. (66) did not find ascorbate to inhibit PP2A activity in unstimulated endothelial cells. This, as well as the mechanism of PP2A inhibition by ascorbate in unstimulated cells, will require further studies.
Enzyme co-factor function
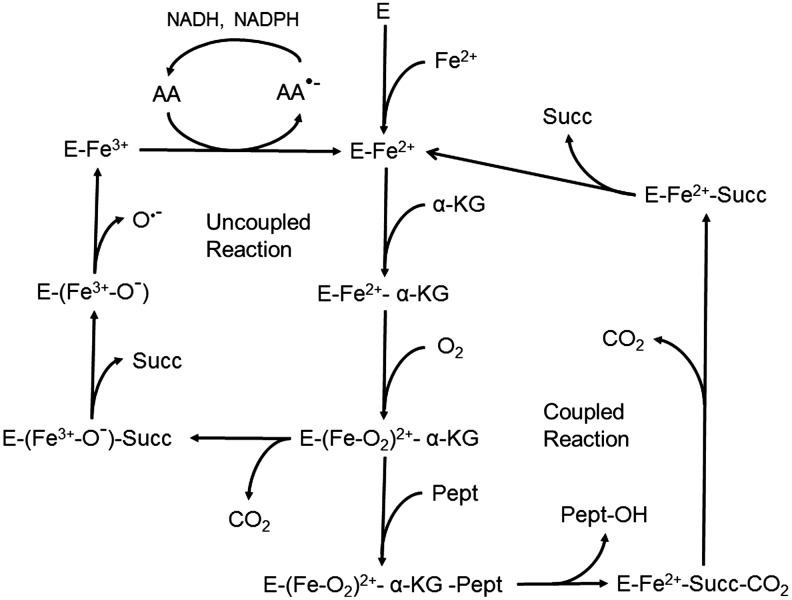
Another well-established function of ascorbate is to sustain the activity of mono- and dioxygenase enzymes (5). These enzymes vary in their substrates, tissue localizations, and mechanisms (Table 3). The two monooxygenases that are dependent on ascorbate are important in catecholamine and neuropeptide synthesis. Of the more numerous dioxygenases, only 4-hydroxyphenylpyruvate hydroxylase mediates the incorporation of both atoms of dioxygen into the same molecule, whereas all the other enzymes incorporate one atom of dioxygen into α-ketoglutarate and the other into the product of the reaction (Fig. 7). The dioxygenase enzymes that hydroxylate collagen and other substrates are perhaps the best known of the group. They serve to hydroxylate proline and lysine in procollagen, thus allowing proper folding of the procollagen chain and release from the cell as mature collagen (75, 118). As with the other dioxygenases, these hydroxylases have a non-heme iron in the active site that is kept in its active, reduced form by ascorbate (39, 119, 120). Ascorbate, however, is not involved in the coupled reaction that hydroxylates the protein and converts α-ketoglutarate to succinate and CO2. Rather, ascorbate prevents deactivation of the enzyme during an occasional uncoupled reaction cycle in which the peptide substrate fails to bind or α-ketoglutarate is cleaved directly (Fig. 7). The ferryl ion bound to the enzyme decomposes, leaving ferric iron bound to the enzyme, which is then directly reduced by ascorbate to ferrous iron and thus able to enter a new reaction cycle. The resulting ascorbate radical is then recycled by mechanisms described earlier.
Table 3.
Molecular Functions of Ascorbate
| Function | Substrate | Product(s) | Relevance |
|---|---|---|---|
| Primary antioxidant |
−OH, O2•−, RO• |
H2O, H2O2, ROH |
Scavenges damaging radicals (24) |
| Pro-oxidant |
Fe3+, O2 |
Fe2+, H2O2 |
Redox regulation (140), DNA, or protein damage (25, 65) |
| Recycling | |||
| Tetrahydrobiopterin |
BH3• |
BH4 |
Numerous, see Table 2 |
| Lipid peroxidation |
α-Tocopheroxyl radical |
α-Tocopherol |
Chain breaking of lipid peroxidation (79, 122) |
| Monoxygenases | |||
| Dopamine β-hydroxylase |
Dopamine |
Norepinephrine |
Catecholamine biosynthesis (113, 149) |
| Peptidylglycine α-amidating monoxygenase |
Neuroendocrine peptides |
α-Amidated peptides |
Neuropeptide and neurotransmitter synthesis (50) |
| Dioxygenases | |||
| 4-Hydroxyphenylpyruvate hydroxylase |
4-Hydroxyphenyl-pyruvate |
Homogentisate |
Breakdown of L-tyrosine (88) |
| Prolyl/lysyl hydroxylase (collagen, elastin) |
α-KG, peptide |
Peptide-OH |
Collagen, elastin synthesis (10, 118) |
| Prolyl/lysyl hydroxylase (HIF-1α) |
α-KG, HIF-1α |
Hydroxylated HIF-1α |
Proteosomal degradation of HIF-1α (21, 63) |
| Thymine/pyrimidine hydroxylases |
α-KG, thymine, deoxyuridine |
Hydroxylated nucleobases |
Salvage pathways (51) |
| 6-N-Trimethyl-L-lysine hydroxylase |
α-KG, 6-N-trimethyl-L-lysine |
3-hydroxy-Nɛ-trimethyllysine |
1st step in carnitine biosynthesis (121, 132) |
| γ-Butyrobetaine hydroxylase |
α-KG, γ-butyrobetaine |
L-carnitine |
Last step in carnitine biosynthesis (121, 132) |
| Histone demethylase Jhdm1a/1b |
α-KG, trimethylated histone H3 |
Mono-methylated histone H3 |
Epigenomic regulation, somatic stem cell reprogramming (74, 171) |
| Nucleic acid demethylase | α-KG, 3-methyl-thymine in DNA | Thymine in DNA | Reprogramming of fibroblasts to induced pluripotent stem cells (32) |
HIF-1α, hypoxia inducible factor-1α; Jhdm1a/1b, Jumonji histone demethylase 1a/1b; α-KG, α-ketoglutarate; R, protein or lipid carbon.
FIG. 7.
Role of ascorbate in maintaining the activity of α-ketoglutarate-dependent hydroxylation reactions. The proposed mechanism of α-ketoglutarate-dependent hydroxylases is first a sequential binding of ferrous iron, α-ketoglutarate, and molecular oxygen. At this point, there is the formation of a key ferryl-oxygen intermediate that not only usually binds the substrate protein (Pept), but can also generate an uncoupled cycle by cleaving α-ketoglutarate to succinate (Succ) and releasing CO2. In the functional enzyme cycle, a proline or lysine is hydroxylated with subsequent sequential release of the modified protein, CO2, and succinate leaving enzyme-bound ferrous iron that can restart the cycle. In an uncoupled cycle, the enzyme-bound iron is oxidized to ferric iron by oxygen, and the latter is released, perhaps as the hydroxyl radical (O•−). The function of ascorbate is to reduce the enzyme-bound ferric to ferrous iron, thus allowing the enzyme to enter a potentially functional cycle again.
Although the mechanism by which ascorbate stimulates collagen synthesis is often considered to involve procollagen hydroxylation, many studies suggest that the major effect of ascorbate is to stimulate de novo collagen synthesis. For example, scorbutic guinea pigs had no differences in urinary hydroxyproline excretion (9) and only about a 10% decrease in skin collagen synthesis (10) compared with non-scorbutic guinea pigs. These findings are in agreement with more recent studies of mice lacking the SVCT2 transporter. Despite very low tissue levels of ascorbate in these mice, skin hydroxyproline content was normal (146). On the other hand, moderate vitamin C deficiency in guinea pigs caused substantial decreases in type IV collagen mRNA in vessel walls (94). Addition of ascorbate increases procollagen mRNA in cultured fibroblasts (58, 118) and stimulates type IV collagen maturation and release in a concentration-dependent manner in cultured endothelial cells (96, 103, 131, 184). As reviewed by Englard and Seifter (51), although many studies report contradictory evidence, both pre- and post-translational mechanisms are likely to be involved in ascorbate-stimulated deposition of mature collagen in the vessel wall and integument.
A second Fe2+-dependent prolyl hydroxylase isoform that requires ascorbate is the enzyme that hydroxylates the hypoxia-inducible transcription factor subunit HIF-1α. Hydroxylation of the oxygen-sensitive HIF-1α subunit on specific proline or lysine residues targets it for ubiquitination and proteosomal destruction (29). Lack of ascorbate prevents this hydroxylation and allows HIF-1α to accumulate (21, 63). The surviving HIF-1α then forms a nuclear transcription complex that activates diverse pathways, including those for glucose transport and metabolism, angiogenesis, inflammation, and cell survival (22, 68, 168). In both primary culture (167) and immortalized (130) human umbilical vein endothelial cells, provision of ascorbate abolished basal HIF-1α expression and decreased the HIF-1α expression stimulated by culture of cells under hypoxic conditions or in the presence of cobalt.
Recent studies have also suggested that ascorbate can affect gene expression by serving as a co-factor for demethylases of both DNA and histones. Similar to the prolyl/lysyl hydroxylases, these demethylases are Fe2+-α-ketoglutarate dioxygenases, having a non-heme active site iron that is maintained in the ferrous state by ascorbate. The DNA demethylases remove methyl groups from thymine residues (59), which may, in turn, activate a variety of genes (32). Activation of these demethylases by ascorbate could account for the widespread DNA demethylation observed in response to ascorbate treatment of human embryonic stem cells (32) and could promote their differentiation (33). The histone demethylases having Jumonji C catalytic domains remove methyl groups from trimethylated lysines on DNA-bound histones, thus opening up the DNA for potential transcription (160). These enzymes may also promote widespread histone demethylation in response to ascorbate, as well as enhance the ability of the vitamin to remove the senescence block on mouse and human induced pluripotent stem cells (52, 171) and facilitate T-cell maturation (Manning, et al., this Forum).
Role of Ascorbate in Endothelial Function
Ascorbate effects on endothelial cell proliferation and apoptosis
Ascorbate has several effects on endothelial function and survival. It causes endothelial cells to proliferate and to form capillary-like structures in cell in culture (136, 139). It is likely that the effect of ascorbate to stimulate endothelial cell proliferation is due to its ability to increase the synthesis of type IV collagen (143, 157), as in the absence of ascorbate there is little generation of mature type IV collagen by cultured endothelial cells (184) or of type IV collagen mRNA relative to that of elastin in blood vessels (94). Further, it has been shown that type IV collagen is required for both basement membrane formation (114) and endothelial cell adhesion (133), processes that are not facilitated by collagen types I and III (133).
Ascorbate also prevents apoptosis of endothelial cells in culture induced by high glucose conditions (73, 154), tumor necrosis factor-α (136), and LPS (64). In humans with congestive heart failure, an intravenous bolus of 2.5 g of ascorbate followed by 3 days of 2 g vitamin C a day decreased plasma levels of circulating endothelial apoptotic microparticles to 32% of baseline levels, an effect also significant compared with a placebo-treated control group of the same clinical characteristics (134).
Although the foregoing evidence favors a positive effect of ascorbate on endothelial growth and survival, ascorbate has also been noted as an angiostatic factor, inhibiting the formation of capillary-like structures in cultured endothelial cells and decreasing in vivo angiogenesis in chick chorioallantoic membranes (6). It is likely, however, that such effects are related to the use of very high ascorbate concentrations, which have been shown to inhibit angiogenesis in excised aortic rings and in subcutaneous Matrigel plugs in vivo (115). In addition, ascorbate deficiency in mice unable to synthesize vitamin C markedly impaired angiogenesis in a transplanted tumor model (156). Although ascorbate deficiency clearly impairs endothelial proliferation and angiogenesis, the effects of ascorbate may differ with the amount of ascorbate available.
Ascorbate modulation of vascular tone
Deficiency of nitric oxide causes endothelial dysfunction that manifests as failure of arterioles to dilate in response to stimuli such as shear stress or acetylcholine. As previously noted, ascorbate spares endothelial cell-derived nitric oxide by recycling BH4 (Fig. 5) and by inhibiting the function of PP2A (Fig. 6). It may also preserve nitric oxide by several other mechanisms, including direct reduction of nitrite to nitric oxide, release of nitric oxide from nitrosothiols, and, as noted earlier, by scavenging superoxide that would otherwise react with nitric oxide to form peroxynitrite [reviewed in (97)]. These mechanisms may combine to generate more nitric oxide, which then diffuses out of endothelial cells and binds to an iron coordinate site on the active site heme of guanylate cyclase in adjacent smooth muscle cells (26). This activates the enzyme and increases production of cyclic GMP, causing the smooth muscle cells to relax and the vessel to dilate (Fig. 5). Nitric oxide also inhibits the toxicity of pro-inflammatory cytokines and adhesion molecules that are responsible for endothelial dysfunction and ultimately, atherosclerosis (38, 81, 82).
The effects of ascorbate to promote endothelial- and nitric oxide-dependent vasodilation have also been confirmed in clinical studies of endothelium-dependent vasodilatation in patients with endothelial dysfunction due to atherosclerosis (60, 71, 159), hypertension (48, 49, 153), smoking (70, 80, 145), and diabetes (158). Most of these studies used high doses of intravenous ascorbate, which is often infused directly into coronary arteries. However, it has been shown in a randomized, double-blind, placebo-controlled study that long-term moderate oral ascorbate supplements improved endothelial-dependent flow-mediated brachial artery dilation in people with atherosclerosis (60). In this study, 46 subjects with coronary artery disease were administered 500 mg/day of ascorbate orally for 30 days. The results showed that the already normal plasma ascorbate concentrations were doubled by the supplement and that flow-mediated brachial artery dilation increased by 50%. Although it has not been possible to show that ascorbate or related antioxidants decrease event frequency in established atherosclerosis (69, 142), these results suggest that ascorbate might prevent or delay the early endothelial dysfunction that is associated with atherosclerosis.
Although ascorbate facilitates nitric oxide-dependent vasodilation, it impairs tonic vasodilation due to endothelial-derived hyperpolarizing factor (EDHF) (112, 150). Despite considerable effort, the identity of EDHF has not been ascertained (151). Ascorbate inhibits both flow- and agonist-mediated vasodilatation due to EDHF, the latter probably due to an antioxidant effect, as it is inhibited by thiols and reducing agents (112).
Ascorbate-stimulated tightening of the endothelial permeability barrier
Ascorbate has been shown to tighten the permeability barrier of endothelial cells in long-term culture on porous filter supports (163). In those studies, bovine arterial endothelial cells were cultured for 5–7 days, and daily addition of (10–100 μM) ascorbate progressively decreased transfer of fluorescein dextran across the cells and filter barriers. Inhibitors of collagen synthesis prevented ascorbate-dependent barrier tightening, leading the authors to conclude that the effect required collagen synthesis. Subsequent studies carried out at much shorter times of culture with ascorbate (90 min or less) found that intracellular ascorbate acutely tightened the endothelial barrier to its own transit in both EA.hy926 cells (an immortalized line derived from human umbilical vein endothelial cells) and primary culture dermal microcapillary endothelial cells (108). The tightening in EA.hy926 cells was significant after 15 min of treatment with dehydroascorbate (used to rapidly load them with ascorbate) and paralleled the intracellular ascorbate concentration. The effect of ascorbate persisted with time in culture, as a single addition of 30–300 μM ascorbate caused progressive 30%–70% decreases in ascorbate transfer when performed after 18 h in culture. In these experiments, removal of the cells from the filters and matrix had no effect on ascorbate permeability, indicating that the effect was not due to collagen deposition (108). Although there were no major changes in F-actin configuration in the EA.hy926 cells, inhibition of microtubule function with colchicine prevented the effect of ascorbate to inhibit its own transfer (108).
Intracellular ascorbate also decreased transfer of radiolabeled inulin (5000–5500 kDa) and mannitol across the EA.hy926-generated endothelial barrier, showing that the effect was not limited to ascorbate transfer alone (108). Loading endothelial cells with ascorbate also prevented the increases in barrier permeability caused by thrombin and high glucose concentrations (J.M. May, unpublished observations). Finally, ascorbate both prevented and reversed the effect of oxidized LDL to increase endothelial barrier permeability, an effect that was mimicked by several other antioxidants (105). Although this might suggest a simple antioxidant effect, a subsequent study showed that the ascorbate-induced decrease in radiolabeled inulin permeabilty in EA.hy926 cells was blocked either by inhibiting the function of eNOS with Nω-nitro-L-arginine methyl ester (L-NAME) or by inhibiting the effect of nitric oxide to activate guanylate cyclase (106). Nitric oxide derived from eNOS has been shown to decrease both basal (128) and stimulated (46) endothelial permeability, an effect that is dependent on the generation of cyclic GMP (46, 175). Although details of the mechanism by which ascorbate might tighten endothelial permeabilty by the nitric oxide-cyclic GMP pathway remain to be determined, the mechanisms depicted in Figure 6 support a coordinated function for ascorbate-induced increases in nitric oxide in decreasing both basal and agonist-stimulated permeability.
Conclusion and Clinical Relevance
The foregoing section generally describes a beneficial role for ascorbate in endothelial growth, survival, and function to maintain vascular responsiveness and integrity. Due to the kinetics of the SVCT2, ascorbate concentrations in human white blood cells and platelets saturate at high normal plasma ascorbate concentrations. The observation that cultured endothelial cells expressing the SVCT2 saturate over a similar concentration range of ascorbate suggests that they have similar low millimolar ascorbate concentrations in vivo, which also cannot be increased even by substantial increases in dietary intake. This, and the fact that most intracellular functions of ascorbate would be saturated as well, suggests that megadoses of vitamin C supplements will not be beneficial when normal intakes are much more than 200 mg a day. As reviewed and commented on (1, 125), this likely is a contributing factor to the fact that supplements of ascorbate and other antioxidants to participants in large clinical trials failed to show benefit on atherosclerosis progression (35, 69, 142). Indeed, in most clinical trials, plasma ascorbate levels were not measured, making it possible to say that the trials could have failed because ascorbate levels were not significantly increased. Other confounding factors could be combined use of several antioxidants (69) or a high rate of statin use in the trials (166). Although there is strong preclinical and clinical evidence to support an effect of ascorbate on endothelial dysfunction in early atherosclerosis, clinical trials thus far have studied subjects either with established atherosclerosis or with a high risk of disease. Unfortunately, given the size and duration that would be required for a primary prevention trial, it seems unlikely that one will ever be carried out.
More revealing may be to study the numerous conditions and diseases in which ascorbate is depleted, some of which are clearly known to have endothelial dysfunction as a prelude to vascular dysregulation, leakage, or atherosclerosis. These conditions/diseases include metabolic syndrome (54), diabetes with poor glycemic control (129), atherosclerosis (86), smoking (92, 93, 138), poor dietary intake (7, 27), inflammatory neurodegenerative diseases (129), sepsis (31, 44, 169), and hemodialysis (170, 185). Indeed, it may even require megadoses of the vitamin to replete the vitamin and improve endothelial function in some of these conditions. For example, taking 800 mg a day of oral vitamin C for 4 weeks doubled low vitamin C plasma levels in subjects with insulin-resistant type 2 diabetes, but not to levels expected from this dose of the vitamin. Moreover, this treatment did not improve forearm blood flow in response to acetylcholine or insulin resistance. A similar difficulty in repleting low vitamin C levels was found in critically injured or infected patients, even with intravenous infusions of approximtaely 1000 mg of vitamin C a day (90).
Supplementation to upper normal plasma ascorbate levels is clearly indicated in most diseases and conditions in which ascorbate is depleted. However, it is seldom a priority, because patients, physicians, and health authorities are unaware of the increasing evidence for multiple potentially important functions of ascorbate. With regard to the endothelium, it is worth emphasizing observations made more than 50 years ago that early scurvy generates endothelial disruption in guinea pigs, which resembles atherosclerosis (176, 177) and is fully and rapidly reversible with ascorbate repletion (177).
Abbreviations Used
- AMPK
AMP kinase
- BH2
dihydrobiopterin
- BH3•
trihydrobiopterin radical
- BH4
tetrahydrobiopterin
- DHA
dehydroascorbate
- EDHF
endothelial-derived hyperpolarizing factor
- eNOS
endothelial nitric oxide synthase
- HIF-1α
hypoxia-inducible factor-1α
- IFNγ
interferon-γ
- LDL
low density lipoprotein
- LPS
lipopolysaccharide
- PP2A
protein phosphatase type 2A
- SVCT1
sodium-dependent vitamin C transporter 1
- SVCT2
sodium-dependent vitamin C transporter 2
Acknowledgment
This work was supported by NIH grant DK50435.
References
- 1.Aguirre R. and May JM. Inflammation in the vascular bed: importance of vitamin C. Pharmacol Ther 119: 96–103, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agus DB, Gambhir SS, Pardridge WM, Speilholz C, Baselga J, and Vera JC. Vitamin C crosses the blood-brain barrier in the oxidized form through the glucose transporters. J Clin Invest 100: 2842–2848, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albelda SM, Sampson PM, Haselton FR, McNiff JM, Mueller SN, Williams SK, Fishman AP, and Levine EM. Permeability characteristics of cultured endothelial cell monolayers. J Appl Physiol 64: 308–322, 1988 [DOI] [PubMed] [Google Scholar]
- 4.Antonov AS, Lukashev ME, Romanov YA, Tkachuk VA, Repin VS, and Smirnov VN. Morphological alterations in endothelial cells from human aorta and umbilical vein induced by forskolin and phorbol 12-myristate 13-acetate: a synergistic action of adenylate cyclase and protein kinase C activators. Proc Natl Acad Sci U S A 83: 9704–9708, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arrigoni O. and De Tullio MC. Ascorbic acid: much more than just an antioxidant. Biochim Biophys Acta Gen Subj 1569: 1–9, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Ashino H, Shimamura M, Nakajima H, Dombou M, Kawanaka S, Oikawa T, Iwaguchi T, and Kawashima S. Novel function of ascorbic acid as an angiostatic factor. Angiogenesis 6: 259–269, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Assefa F, Jabarkhil MZ, Salama P, and Spiegel P. Malnutrition and mortality in Kohistan District, Afghanistan, April 2001. JAMA 286: 2723–2728, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Babaev VR, Whitesell RR, Li L, Linton MF, Fazio S, and May JM. Selective macrophage ascorbate deficiency suppresses early atherosclerosis. Free Radic Biol Med 50: 27–36, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes MJ, Constable BJ, and Kodicek E. Excretion of hydroxyproline and other amino acids in scorbutic guinea-pigs. Biochim Biophys Acta 184: 358–365, 1969 [DOI] [PubMed] [Google Scholar]
- 10.Barnes MJ, Constable BJ, and Kodicek E. Studies in vivo on the biosynthesis of collagen and elastin in ascorbic acid-deficient guinea pigs. Biochem J 113: 387–397, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bazzoni G. Endothelial tight junctions: permeable barriers of the vessel wall. Thromb Haemost 95: 36–42, 2006 [PubMed] [Google Scholar]
- 12.Becker BF, Reinholz N, Ozcelik T, Leipert B, and Gerlach E. Uric acid as radical scavenger and antioxidant in the heart. Pflugers Arch 415: 127–135, 1989 [DOI] [PubMed] [Google Scholar]
- 13.Beckman JS. and Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and the ugly. Am J Physiol Cell Physiol 271: C1424–C1437, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Bergsten P, Amitai G, Kehrl J, Dhariwal KR, Klein HG, and Levine M. Millimolar concentrations of ascorbic acid in purified human mononuclear leukocytes. Depletion and reaccumulation. J Biol Chem 265: 2584–2587, 1990 [PubMed] [Google Scholar]
- 15.Best KA, Holmes ME, Samson SE, Mwanjewe J, Wilson JX, Dixon SJ, and Grover AK. Ascorbate uptake in pig coronary artery endothelial cells. Mol Cell Biochem 271: 43–49, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Bianchi J, Wilson FA, and Rose RC. Dehydroascorbic acid and ascorbic acid transport systems in the quinea pig ileum. Am J Physiol 250: G461–G468, 1986 [DOI] [PubMed] [Google Scholar]
- 17.Bielski BH, Richter HW, and Chan PC. Some properties of the ascorbate free radical. Ann N Y Acad Sci 258: 231–237, 1975 [DOI] [PubMed] [Google Scholar]
- 18.Bisby RH. and Parker AW. Reaction of ascorbate with the α-tocopheroxyl radical in micellar and bilayer membrane systems. Arch Biochem Biophys 317: 170–178, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Bode AM, Cunningham L, and Rose RC. Spontaneous decay of oxidized ascorbic acid (dehydro-L-ascorbic acid) evaluated by high-pressure liquid chromatography. Clin Chem 36: 1807–1809, 1990 [PubMed] [Google Scholar]
- 20.Boyer JC, Campbell CE, Sigurdson WJ, and Kuo SM. Polarized localization of vitamin C transporters, SVCT1 and SVCT2, in epithelial cells. Biochem Biophys Res Commun 334: 150–156, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Bruick RK. and McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294: 1337–1340, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Bruick RK. and McKnight SL. Transcription. Oxygen sensing gets a second wind. Science 295: 807–808, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Bruno RS, Leonard SW, Atkinson J, Montine TJ, Ramakrishnan R, Bray TM, and Traber MG. Faster plasma vitamin E disappearance in smokers is normalized by vitamin C supplementation. Free Radic Biol Med 40: 689–697, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Buettner GR. The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys 300: 535–543, 1993 [DOI] [PubMed] [Google Scholar]
- 25.Buettner GR. and Jurkiewicz BA. Catalytic metals, ascorbate and free radicals: Combinations to avoid. Radiat Res 145: 532–541, 1996 [PubMed] [Google Scholar]
- 26.Burstyn JN, Yu AE, Dierks EA, Hawkins BK, and Dawson JH. Studies of the heme coordination and ligand binding properties of soluble guanylyl cyclase (sGC): characterization of Fe(II)sGC and Fe(II)sGC(CO) by electronic absorption and magnetic circular dichroism spectroscopies and failure of CO to activate the enzyme. Biochemistry 34: 5896–5903, 1995 [DOI] [PubMed] [Google Scholar]
- 27.Cahill L, Corey PN, and El Sohemy A. Vitamin C deficiency in a population of young Canadian adults. Am J Epidemiol 170: 464–471, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Carr A. and Frei B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J 13: 1007–1024, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Cash TP, Pan Y, and Simon MC. Reactive oxygen species and cellular oxygen sensing. Free Radic Biol Med 43: 1219–1225, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chatterjee IB. Biosynthesis of L-ascorbate in animals. Methods Enzymol 18: 28–34, 1970 [Google Scholar]
- 31.Cherian S, Jameson S, Rajarajeswari C, Helena V, Latha L, Anu Rekha MR, Nagamma T, Subba R V, Kini PG, and Rao A. Oxidative stress in sepsis in children. Indian J Med Res 125: 143–148, 2007 [PubMed] [Google Scholar]
- 32.Chung TL, Brena RM, Kolle G, Grimmond SM, Berman BP, Laird PW, Pera MF, and Wolvetang EJ. Vitamin C Promotes Widespread Yet Specific DNA Demethylation of the Epigenome in Human Embryonic Stem Cells. Stem Cells 28: 1848–1855, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Chung TL, Turner JP, Thaker NY, Kolle G, Cooper-White JJ, Grimmond SM, Pera MF, and Wolvetang EJ. Ascorbate promotes epigenetic activation of CD30 in human embryonic stem cells. Stem Cells 28: 1782–1793, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Constantinescu A, Han D, and Packer L. Vitamin E recycling in human erythrocyte membranes. J Biol Chem 268: 10906–10913, 1993 [PubMed] [Google Scholar]
- 35.Cook NR, Albert CM, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, Buring JE, and Manson JE. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women's Antioxidant Cardiovascular Study. Arch Intern Med 167: 1610–1618, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crabtree MJ, Smith CL, Lam G, Goligorsky MS, and Gross SS. Ratio of 5,6,7,8-tetrahydrobiopterin to 7,8-dihydrobiopterin in endothelial cells determines glucose-elicited changes in NO vs. superoxide production by eNOS. Am J Physiol Heart Circ Physiol 294: H1530–H1540, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davis KA, Samson SE, Best K, Mallhi KK, Szewczyk M, Wilson JX, Kwan CY, and Grover AK. Ca(2+)-mediated ascorbate release from coronary artery endothelial cells. Br J Pharmacol 147: 131–139, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA, Jr., Shin WS, and Liao JK. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest 96: 60–68, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Jong L, Albracht SP, and Kemp A. Prolyl 4-hydroxylase activity in relation to the oxidation state of enzyme-bound iron. The role of ascorbate in peptidyl proline hydroxylation. Biochim Biophys Acta 704: 326–332, 1982 [DOI] [PubMed] [Google Scholar]
- 40.Dejana E, Corada M, and Lampugnani MG. Endothelial cell-to-cell junctions. FASEB J 9: 910–918, 1995 [PubMed] [Google Scholar]
- 41.Del Bello B, Maellaro E, Sugherini L, Santucci A, Comporti M, and Casini AF. Purification of NADPH-dependent dehydroascorbate reductase from rat liver and its identification with 3α-hydroxysteroid dehydrogenase. Biochem J 304: 385–390, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dhariwal KR, Hartzell WO, and Levine M. Ascorbic acid and dehydroascorbic acid measurements in human plasma and serum. Am J Clin Nutr 54: 712–716, 1991 [DOI] [PubMed] [Google Scholar]
- 43.DiLabio GA. and Wright JS. Hemiketal formation of dehydroascorbic acid drives ascorbyl radical anion disproportionation. Free Radic Biol Med 29: 480–485, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Doise JM, Aho LS, Quenot JP, Guilland JC, Zeller M, Vergely C, Aube H, Blettery B, and Rochette L. Plasma antioxidant status in septic critically ill patients: a decrease over time. Fundam Clin Pharmacol 22: 203–209, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Domazou AS, Koppenol WH, and Gebicki JM. Efficient repair of protein radicals by ascorbate. Free Radic Biol Med 46: 1049–1057, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Draijer R, Atsma DE, van der LA, and van Hinsbergh VW. cGMP and nitric oxide modulate thrombin-induced endothelial permeability. Regulation via different pathways in human aortic and umbilical vein endothelial cells. Circ Res 76: 199–208, 1995 [DOI] [PubMed] [Google Scholar]
- 47.Drake BB, Smythe CV, and King CG. Complexes of dehydroascorbic acid with three sulfhydryl compounds. J Biol Chem 143: 89–98, 1942 [Google Scholar]
- 48.Duffy SJ, Gokce N, Holbrook M, Huang A, Frei B, Keaney JF, Jr., and Vita JA. Treatment of hypertension with ascorbic acid. Lancet 354: 2048–2049, 1999 [DOI] [PubMed] [Google Scholar]
- 49.Duffy SJ, Gokce N, Holbrook M, Hunter LM, Biegelsen ES, Huang AO, Keaney JF, Jr., and Vita JA. Effect of ascorbic acid treatment on conduit vessel endothelial dysfunction in patients with hypertension. Am J Physiol Heart Circ Physiol 280: H528–H534, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Eipper BA. and Mains RE. The role of ascorbate in the biosynthesis of neuroendocrine peptides. Am J Clin Nutr 54: 1153S–1156S, 1991 [DOI] [PubMed] [Google Scholar]
- 51.Englard S. and Seifter S. The biochemical functions of ascorbic acid. Annu Rev Nutr 6: 365–406, 1986 [DOI] [PubMed] [Google Scholar]
- 52.Esteban MA, Wang T, Qin B, Yang J, Qin D, Cai J, Li W, Weng Z, Chen J, Ni S, et al. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell 6: 71–79, 2010 [DOI] [PubMed] [Google Scholar]
- 53.Evans RM, Currie L, and Campbell A. The distribution of ascorbic acid between various cellular components of blood, in normal individuals, and its relation to the plasma concentration. Br J Nutr 47: 473–482, 1982 [DOI] [PubMed] [Google Scholar]
- 54.Ford ES, Mokdad AH, Giles WH, and Brown DW. The metabolic syndrome and antioxidant concentrations: findings from the Third National Health and Nutrition Examination Survey. Diabetes 52: 2346–2352, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Frei B. Molecular and biological mechanisms of antioxidant action. FASEB J 13: 963–964, 1999 [DOI] [PubMed] [Google Scholar]
- 56.Fridovich I. Superoxide radical: an endogenous toxicant. Annu Rev Pharmacol Toxicol 23: 239–257, 1983 [DOI] [PubMed] [Google Scholar]
- 57.García ML, Salazar K, Millán C, Rodríguez F, Montecinos H, Caprile T, Silva C, Cortes C, Reinicke K, Vera JC, et al. Sodium vitamin C cotransporter SVCT2 is expressed in hypothalamic glial cells. Glia 50: 32–47, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Geesin JC, Darr D, Kaufman R, Murad S, and Pinnell SR. Ascorbic acid specifically increases type I and type III procollagen messenger RNA levels in human skin fibroblast. J Invest Dermatol 90: 420–424, 1988 [DOI] [PubMed] [Google Scholar]
- 59.Gerken T, Girard CA, Tung YC, Webby CJ, Saudek V, Hewitson KS, Yeo GS, McDonough MA, Cunliffe S, McNeill LA, et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science 318: 1469–1472, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gokce N, Keaney JF, Jr., Frei B, Holbrook M, Olesiak M, Zachariah BJ, Leeuwenburgh C, Heinecke JW, and Vita JA. Long-term ascorbic acid administration reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation 99: 3234–3240, 1999 [DOI] [PubMed] [Google Scholar]
- 61.Goss SPA, Hogg N, and Kalyanaraman B. The effect of α-tocopherol on the nitration of gamma-tocopherol by peroxynitrite. Arch Biochem Biophys 363: 333–340, 1999 [DOI] [PubMed] [Google Scholar]
- 62.Gotoh N. and Niki E. Rates of interactions of superoxide with vitamin E, vitamin C and related compounds as measured by chemiluminescence. Biochim Biophys Acta 1115: 201–207, 1992 [DOI] [PubMed] [Google Scholar]
- 63.Grano A. and De Tullio MC. Ascorbic acid as a sensor of oxidative stress and a regulator of gene expression: The Yin and Yang of vitamin C. Med Hypotheses 69: 953–954, 2007 [DOI] [PubMed] [Google Scholar]
- 64.Haendeler J, Zeiher AM, and Dimmeler S. Vitamin C and E prevent lipopolysaccharide-induced apoptosis in human endothelial cells by modulation of Bcl-2 and Bax. Eur J Pharmacol 317: 407–411, 1996 [DOI] [PubMed] [Google Scholar]
- 65.Halliwell B. Vitamin C: poison, prophylactic or panacea? Trends Biochem Sci 24: 255–259, 1999 [DOI] [PubMed] [Google Scholar]
- 66.Han M, Pendem S, Teh SL, Sukumaran DK, Wu F, and Wilson JX. Ascorbate protects endothelial barrier function during septic insult: Role of protein phosphatase type 2A. Free Radic Biol Med 48: 128–135, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hara T. and Minakami S. On functional role of cytochrome b5. II. NADH-linked ascorbate radical reductase activity in microsomes. J Biochem (Tokyo) 69: 325–330, 1971 [DOI] [PubMed] [Google Scholar]
- 68.Harris AL. Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer 2: 38–47, 2002 [DOI] [PubMed] [Google Scholar]
- 69.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 360: 23–33, 2002. 12114037 [Google Scholar]
- 70.Heitzer T, Just H, and Münzel T. Antioxidant vitamin C improves endothelial dysfunction in chronic smokers. Circulation 94: 6–9, 1996 [DOI] [PubMed] [Google Scholar]
- 71.Heitzer T, Schlinzig T, Krohn K, Meinertz T, and Münzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 104: 2673–2678, 2001 [DOI] [PubMed] [Google Scholar]
- 72.Heller R, Unbehaun A, Schellenberg B, Mayer B, Werner-Felmayer G, and Werner ER. L-ascorbic acid potentiates endothelial nitric oxide synthesis via a chemical stabilization of tetrahydrobiopterin. J Biol Chem 276: 40–47, 2001 [DOI] [PubMed] [Google Scholar]
- 73.Ho FM, Liu SH, Lin WW, and Liau CS. Opposite effects of high glucose on MMP-2 and TIMP-2 in human endothelial cells. J Cell Biochem 101: 442–450, 2007 [DOI] [PubMed] [Google Scholar]
- 74.Horton JR, Upadhyay AK, Qi HH, Zhang X, Shi Y, and Cheng X. Enzymatic and structural insights for substrate specificity of a family of jumonji histone lysine demethylases. Nat Struct Mol Biol 17: 38–43, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hudson BG, Reeders ST, and Tryggvason K. Type IV collagen: structure, gene organization, and role in human diseases. Molecular basis of Goodpasture and Alport syndromes and diffuse leiomyomatosis. J Biol Chem 268: 26033–26036, 1993 [PubMed] [Google Scholar]
- 76.Huie RE. and Padmaja S. The reaction of no with superoxide. Free Radic Res Commun 18: 195–199, 1993 [DOI] [PubMed] [Google Scholar]
- 77.Iyanagi T. and Yamazaki I. One-electron-transfer reactions in biochemical systems. 3. One- electron reduction of quinones by microsomal flavin enzymes. Biochim Biophys Acta 172: 370–381, 1969 [DOI] [PubMed] [Google Scholar]
- 78.Jackson TS, Xu AM, Vita JA, and Keaney JF, Jr. Ascorbate prevents the interaction of superoxide and nitric oxide only at very high physiological concentrations. Circ Res 83: 916–922, 1998 [DOI] [PubMed] [Google Scholar]
- 79.Kagan VE, Serbinova EA, Forte T, Scita G, and Packer L. Recycling of vitamin E in human low density lipoproteins. J Lipid Res 33: 385–397, 1992 [PubMed] [Google Scholar]
- 80.Kaufmann PA, Gnecchi-Ruscone T, Di Terlizzi M, Schäfers KP, Lüscher TF, and Camici PG. Coronary heart disease in smokers—Vitamin C restores coronary microcirculatory function. Circulation 102: 1233–1238, 2000 [DOI] [PubMed] [Google Scholar]
- 81.Khan BV, Harrison DG, Olbrych MT, Alexander RW, and Medford RM. Nitric oxide regulates vascular cell adhesion molecule 1 gene expression and redox-sensitive transcriptional events in human vascular endothelial cells. Proc Natl Acad Sci U S A 93: 9114–9119, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kubes P, Suzuki M, and Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A 88: 4651–4655, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kuzkaya N, Weissmann N, Harrison DG, and Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols—Implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem 278: 22546–22554, 2003 [DOI] [PubMed] [Google Scholar]
- 84.Kuzkaya N, Weissmann N, Harrison DG, and Dikalov S. Interactions of peroxynitrite with uric acid in the presence of ascorbate and thiols: implications for uncoupling endothelial nitric oxide synthase. Biochem Pharmacol 70: 343–354, 2005 [DOI] [PubMed] [Google Scholar]
- 85.Ladurner A, Schmitt CA, Schachner D, Atanasov AG, Werner ER, Dirsch VM, and Heiss EH. Ascorbate stimulates endothelial nitric oxide synthase enzyme activity by rapid modulation of its phosphorylation status. Free Radic Biol Med 52: 2082–2090, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Langlois M, Duprez D, Delanghe J, De Buyzere M, and Clement DL. Serum vitamin C concentration is low in peripheral arterial disease and is associated with inflammation and severity of atherosclerosis. Circulation 103: 1863–1868, 2001 [DOI] [PubMed] [Google Scholar]
- 87.Leveque N, Robin S, Muret P, Mac-Mary S, Makki S, and Humbert P. High iron and low ascorbic acid concentrations in the dermis of atopic dermatitis patients. Dermatology 207: 261–2642003 [DOI] [PubMed] [Google Scholar]
- 88.Levine SZ, Marples E, and Gordon HH. A defect in the metabolism of aromatic amino acids in premature infants: The role of vitamin C. Science 90: 620–621, 1939 [DOI] [PubMed] [Google Scholar]
- 89.Li L, Chen W, Rezvan A, Jo H, and Harrison DG. Tetrahydrobiopterin deficiency and nitric oxide synthase uncoupling contribute to atherosclerosis induced by disturbed flow. Arterioscler Thromb Vasc Biol 31: 1547–1554, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Long CL, Maull KI, Krishnan RS, Laws HL, Geiger JW, Borghesi L, Franks W, Lawson TC, and Sauberlich HE. Ascorbic acid dynamics in the seriously ill and injured. J Surg Res 109: 144–148, 2003 [DOI] [PubMed] [Google Scholar]
- 91.Lumper L, Schneider W, and Staudinger H. Untersuchungen zur Kinetik der mikrosomalen NADH:Semidehydroascorbat-Oxydoreduktase. Hoppe Seylers Z Physiol Chem 348: 323–328, 1967 [PubMed] [Google Scholar]
- 92.Lykkesfeldt J, Christen S, Wallock LM, Chang HH, Jacob RA, and Ames BN. Ascorbate is depleted by smoking and repleted by moderate supplementation: a study in male smokers and nonsmokers with matched dietary antioxidant intakes. Am J Clin Nutr 71: 530–536, 2000 [DOI] [PubMed] [Google Scholar]
- 93.Madruga d.O., Rondo PH, and Barros SB. Concentrations of ascorbic acid in the plasma of pregnant smokers and nonsmokers and their newborns. Int J Vitam Nutr Res 74: 193–198, 2004 [DOI] [PubMed] [Google Scholar]
- 94.Mahmoodian F. and Peterkofsky B. Vitamin C deficiency in guinea pigs differentially affects the expression of type IV collagen, laminin, and elastin in blood vessels. J Nutr 129: 83–91, 1999 [DOI] [PubMed] [Google Scholar]
- 95.Martin A. and Frei B. Both intracellular and extracellular vitamin C inhibit atherogenic modification of LDL by human vascular endothelial cells. Arterioscler Thromb Vasc Biol 17: 1583–1590, 1997 [DOI] [PubMed] [Google Scholar]
- 96.Maulen NP, Henriquez EA, Kempe S, Carcamo JG, Smid-Kotsas A, Bachem M, Grunen A, Bustamante ME, Nualart F, and Vera JC. Upregulation and polarized expression of the sodium-ascorbic acid transporter SVCT1 in post-confluent differentiated CaCo-2 cells. J Biol Chem 278: 9035–9041, 2003 [DOI] [PubMed] [Google Scholar]
- 97.May JM. How does ascorbic acid prevent endothelial dysfunction? Free Radic Biol Med 28: 1421–1429, 2000 [DOI] [PubMed] [Google Scholar]
- 98.May JM, Cobb CE, Mendiratta S, Hill KE, and Burk RF. Reduction of the ascorbyl free radical to ascorbate by thioredoxin reductase. J Biol Chem 273: 23039–23045, 1998 [DOI] [PubMed] [Google Scholar]
- 99.May JM, Li L, and Qu ZC. Oxidized LDL up-regulates the ascorbic acid transporter SVCT2 in endothelial cells. Mol Cell Biochem 343: 217–222, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.May JM, Mendiratta S, Hill KE, and Burk RF. Reduction of dehydroascorbate to ascorbate by the selenoenzyme thioredoxin reductase. J Biol Chem 272: 22607–22610, 1997 [DOI] [PubMed] [Google Scholar]
- 101.May JM, Qu Z, and Morrow JD. Mechanisms of ascorbic acid recycling in human erythrocytes. Biochim Biophys Acta 1528: 159–166, 2001 [DOI] [PubMed] [Google Scholar]
- 102.May JM. and Qu ZC. Redox regulation of ascorbic acid transport: Role of transporter and intracellular sulfhydryls. Biofactors 20: 199–211, 2004. 15706057 [Google Scholar]
- 103.May JM. and Qu ZC. Transport and intracellular accumulation of vitamin C in endothelial cells: relevance to collagen synthesis. Arch Biochem Biophys 434: 178–186, 2005 [DOI] [PubMed] [Google Scholar]
- 104.May JM. and Qu ZC. Ascorbic acid efflux and re-uptake in endothelial cells: maintenance of intracellular ascorbate. Mol Cell Biochem 325: 79–88, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.May JM. and Qu ZC. Ascorbic acid prevents increased endothelial permeability caused by oxidized low density lipoprotein. Free Radic Res 44: 1359–1368, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.May JM. and Qu ZC. Nitric oxide mediates tightening of the endothelial barrier by ascorbic acid. Biochem Biophys Res Commun 404: 701–705, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.May JM, Qu ZC, and Li X. Requirement for GSH in recycling of ascorbic acid in endothelial cells. Biochem Pharmacol 62: 873–881, 2001 [DOI] [PubMed] [Google Scholar]
- 108.May JM, Qu ZC, and Qiao H. Transfer of ascorbic acid across the vascular endothelium: mechanism and self-regulation. Am J Physiol Cell Physiol 297: C169–C178, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.May JM, Qu ZC, Qiao H, and Koury MJ. Maturational loss of the vitamin C transporter in erythrocytes. Biochem Biophys Res Commun 360: 295–298, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.May JM, Qu Z-C, and Li X. Ascorbic acid blunts oxidant stress due to menadione in endothelial cells. Arch Biochem Biophys 411: 136–144, 2003 [DOI] [PubMed] [Google Scholar]
- 111.May JM, Qu Z-C, and Mendiratta S. Protection and recycling of α-tocopherol in human erythrocytes by intracellular ascorbic acid. Arch Biochem Biophys 349: 281–289, 1998 [DOI] [PubMed] [Google Scholar]
- 112.McNeish AJ, Wilson WS, and Martin W. Ascorbate blocks endothelium-derived hyperpolarizing factor (EDHF)-mediated vasodilatation in the bovine ciliary vascular bed and rat mesentery. Br J Pharmacol 135: 1801–1809, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Menniti FS, Knoth J, and Diliberto EJ., Jr.Role of ascorbic acid in dopamine beta-hydroxylation. The endogenous enzyme cofactor and putative electron donor for cofactor regeneration. J Biol Chem 261: 16901–16908, 1986 [PubMed] [Google Scholar]
- 114.Mercier P. and Ekindjian OG. Collagen type IV: major component of basement membranes. Current knowledge. Ann Biol Clin (Paris) 48: 695–711, 1990 [PubMed] [Google Scholar]
- 115.Mikirova NA, Casciari JJ, and Riordan NH. Ascorbate inhibition of angiogenesis in aortic rings ex vivo and subcutaneous Matrigel plugs in vivo. J Angiogenes Res 2: 2, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Millward TA, Zolnierowicz S, and Hemmings BA. Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem Sci 24: 186–191, 1999 [DOI] [PubMed] [Google Scholar]
- 117.Mount PF, Kemp BE, and Power DA. Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol 42: 271–279, 2007 [DOI] [PubMed] [Google Scholar]
- 118.Murad S, Grove D, Lindberg KA, Reynolds G, Sivarajah A, and Pinnell SR. Regulation of collagen synthesis by ascorbic acid. Proc Natl Acad Sci U S A 78: 2879–2882, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Myllylä R, Kuutti-Savolainen ER, and Kivirikko KI. The role of ascorbate in the prolyl hydroxylase reaction. Biochem Biophys Res Commun 83: 441–448, 1978 [DOI] [PubMed] [Google Scholar]
- 120.Myllylä R, Tuderman L, and Kivirikko KI. Mechanism of the prolyl hydroxylase reaction. 2. Kinetic analysis of the reaction sequence. Eur J Biochem 80: 349–357, 1977 [DOI] [PubMed] [Google Scholar]
- 121.Nelson PJ, Pruitt RE, Henderson LL, Jenness R, and Henderson LM. Effect of ascorbic acid deficiency on the in vivo synthesis of carnitine. Biochim Biophys Acta 672: 123–127, 1981 [DOI] [PubMed] [Google Scholar]
- 122.Niki E, Noguchi N, Tsuchihashi H, and Gotoh N. Interaction among vitamin C, vitamin E, and β-carotene. Am J Clin Nutr 62Suppl: 1322S–1326S, 1995 [DOI] [PubMed] [Google Scholar]
- 123.Nishikimi M. Oxidation of ascorbic acid with superoxide anion generated by the xanthine-xanthine oxidase system. Biochem Biophys Res Commun 63: 463–468, 1975 [DOI] [PubMed] [Google Scholar]
- 124.Nualart FJ, Rivas CI, Montecinos VP, Godoy AS, Guaiquil VH, Golde DW, and Vera JC. Recycling of vitamin C by a bystander effect. J Biol Chem 278: 10128–10133, 2003 [DOI] [PubMed] [Google Scholar]
- 125.Padayatty SJ. and Levine M. Antioxidant supplements and cardiovascular disease in men. JAMA 301: 1336–1337, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pastore P, Rizzetto T, Curcuruto O, Cin MD, Zaramella A, and Marton D. Characterization of dehydroascorbic acid solutions by liquid chromatography/mass spectrometry. Rapid Commun Mass Spectrom 15: 2051–2057, 2001 [DOI] [PubMed] [Google Scholar]
- 127.Patel KB, Stratford MRL, Wardman P, and Everett SA. Oxidation of tetrahydrobiopterin by biological radicals and scavenging of the trihydrobiopterin radical by ascorbate. Free Radic Biol Med 32: 203–211, 2002 [DOI] [PubMed] [Google Scholar]
- 128.Predescu D, Predescu S, Shimizu J, Miyawaki-Shimizu K, and Malik AB. Constitutive eNOS-derived nitric oxide is a determinant of endothelial junctional integrity. Am J Physiol Lung Cell Mol Physiol 289: L371–L381, 2005 [DOI] [PubMed] [Google Scholar]
- 129.Price KD, Price CS, and Reynolds RD. Hyperglycemia-induced ascorbic acid deficiency promotes endothelial dysfunction and the development of atherosclerosis. Atherosclerosis 158: 1–12, 2001 [DOI] [PubMed] [Google Scholar]
- 130.Qiao H, Li L, Qu ZC, and May JM. Cobalt-induced oxidant stress in cultured endothelial cells: Prevention by ascorbate in relation to HIF-1alpha. Biofactors 35: 306–313, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Qiao H. and May JM. Development of ascorbate transport in brain capillary endothelial cells in culture. Brain Res 1208: 79–86, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rebouche CJ. Ascorbic acid and carnitine biosynthesis. Am J Clin Nutr 54: 1147S–1152S, 1991 [DOI] [PubMed] [Google Scholar]
- 133.Rixen H, Kirkpatrick CJ, Schmitz U, Ruchatz D, and Mittermayer C. Interaction between endothelial cells and basement membrane components. In vitro studies on endothelial cell adhesion to collagen types I, III, IV and high molecular weight fragments of IV. Exp Cell Biol 57: 315–323, 1989 [PubMed] [Google Scholar]
- 134.Rössig L, Hoffmann J, Hugel B, Mallat Z, Haase A, Freyssinet JM, Tedgui A, Aicher A, Zeiher AM, and Dimmeler S. Vitamin C inhibits endothelial cell apoptosis in congestive heart failure. Circulation 104: 2182–2187, 2001 [DOI] [PubMed] [Google Scholar]
- 135.Sabharwal AK. and May JM. alpha-Lipoic acid and ascorbate prevent LDL oxidation and oxidant stress in endothelial cells. Mol Cell Biochem 309: 125–132, 2008 [DOI] [PubMed] [Google Scholar]
- 136.Saeed RW, Peng T, and Metz CN. Ascorbic acid blocks the growth inhibitory effect of tumor necrosis factor-alpha on endothelial cells. Exp Biol Med (Maywood) 228: 855–865, 2003 [DOI] [PubMed] [Google Scholar]
- 137.Santus R, Patterson LK, Filipe P, Morliere P, Hug GL, Fernandes A, and Maziere JC. Redox reactions of the urate radical/urate couple with the superoxide radical anion, the tryptophan neutral radical and selected flavonoids in neutral aqueous solutions. Free Radic Res 35: 129–136, 2001 [DOI] [PubMed] [Google Scholar]
- 138.Schectman G, Byrd JC, and Gruchow HW. The influence of smoking on vitamin C status in adults. Am J Public Health 79: 158–162, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Schor AM, Schor SL, and Allen TD. Effects of culture conditions on the proliferation, morphology and migration of bovine aortic endothelial cells. J Cell Sci 62: 267–285, 1983 [DOI] [PubMed] [Google Scholar]
- 140.Sen CK. and Packer L. Antioxidant and redox regulation of gene transcription. FASEB J 10: 709–720, 1996 [DOI] [PubMed] [Google Scholar]
- 141.Seno T, Inoue N, Matsui K, Ejiri J, Hirata K, Kawashima S, and Yokoyama M. Functional expression of sodium-dependent vitamin C transporter 2 in human endothelial cells. J Vasc Res 41: 345–351, 2004 [DOI] [PubMed] [Google Scholar]
- 142.Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Glynn RJ, and Gaziano JM. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial. JAMA 300: 2123–2133, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shekhonin BV, Domogatsky SP, Idelson GL, Koteliansky VE, and Rukosuev VS. Relative distribution of fibronectin and type I, III, IV, V collagens in normal and atherosclerotic intima of human arteries. Atherosclerosis 67: 9–16, 1987 [DOI] [PubMed] [Google Scholar]
- 144.Sies H. and Arteel GE. Interaction of peroxynitrite with selenoproteins and glutathione peroxidase mimics. Free Radic Biol Med 28: 1451–1455, 2000 [DOI] [PubMed] [Google Scholar]
- 145.Solzbach U, Hornig B, Jeserich M, and Just H. Vitamin C improves endothelial dysfunction of epicardial coronary arteries in hypertensive patients. Circulation 96: 1513–1519, 1997 [DOI] [PubMed] [Google Scholar]
- 146.Sotiriou S, Gispert S, Cheng J, Wang YH, Chen A, Hoogstraten-Miller S, Miller GF, Kwon O, Levine M, Guttentag SH, et al. Ascorbic-acid transporter Slc23a1 is essential for vitamin C transport into the brain and for perinatal survival. Nat Med 8: 514–517, 2002 [DOI] [PubMed] [Google Scholar]
- 147.Squadrito GL, Cueto R, Splenser AE, Valavanidis A, Zhang H, Uppu RM, and Pryor WA. Reaction of uric acid with peroxynitrite and implications for the mechanism of neuroprotection by uric acid. Arch Biochem Biophys 376: 333–337, 2000 [DOI] [PubMed] [Google Scholar]
- 148.Squadrito GL, Jin X, and Pryor WA. Stopped-flow kinetic study of the reaction of ascorbic acid with peroxynitrite. Arch Biochem Biophys 322: 53–59, 1995 [DOI] [PubMed] [Google Scholar]
- 149.Stewart LC. and Klinman JP. Cooperativity in the dopamine beta-monooxygenase reaction. Evidence for ascorbate regulation of enzyme activity. J Biol Chem 266: 11537–11543, 1991 [PubMed] [Google Scholar]
- 150.Stirrat A, Nelli S, Dowell FJ, and Martin W. Flow-induced enhancement of vasoconstriction and blockade of endothelium-derived hyperpolarizing factor (EDHF) by ascorbate in the rat mesentery. Br J Pharmacol 153: 1162–1168, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Stoner L, Erickson ML, Young JM, Fryer S, Sabatier MJ, Faulkner J, Lambrick DM, and McCully KK. There's more to flow-mediated dilation than nitric oxide. J Atheroscler Thromb 19: 589–600, 2012 [DOI] [PubMed] [Google Scholar]
- 152.Sugiyama T, Levy BD, and Michel T. Tetrahydrobiopterin recycling, a key determinant of endothelial nitric-oxide synthase-dependent signaling pathways in cultured vascular endothelial cells. J Biol Chem 284: 12691–12700, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Taddei S, Virdis A, Ghiadoni L, Magagna A, and Salvetti A. Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation 97: 2222–2229, 1998 [DOI] [PubMed] [Google Scholar]
- 154.Tamareille S, Mignen O, Capiod T, Rucker-Martin C, and Feuvray D. High glucose-induced apoptosis through store-operated calcium entry and calcineurin in human umbilical vein endothelial cells. Cell Calcium 39: 47–55, 2006 [DOI] [PubMed] [Google Scholar]
- 155.Tang VW. and Goodenough DA. Paracellular ion channel at the tight junction. Biophys J 84: 1660–1673, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Telang S, Clem AL, Eaton JW, and Chesney J. Depletion of ascorbic acid restricts angiogenesis and retards tumor growth in a mouse model. Neoplasia 9: 47–56, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Thiele J, Rompcik V, Wagner S, and Fischer R. Vascular architecture and collagen type IV in primary myelofibrosis and polycythaemia vera: an immunomorphometric study on trephine biopsies of the bone marrow. Br J Haematol 80: 227–234, 1992 [DOI] [PubMed] [Google Scholar]
- 158.Tousoulis D, Antoniades C, Vasiliadou C, Kourtellaris P, Koniari K, Marinou K, Charakida M, Ntarladimas I, Siasos G, and Stefanadis C. Effects of atorvastatin and vitamin C on forearm hyperaemic blood flow, asymmentrical dimethylarginine levels and the inflammatory process in patients with type 2 diabetes mellitus. Heart 93: 244–246, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tousoulis D, Xenakis C, Tentolouris C, Davies G, Antoniades C, Crake T, and Stefanadis C. Effects of vitamin C on intracoronary L-arginine dependent coronary vasodilatation in patients with stable angina. Heart 91: 1319–1323, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tsukada Y, Fang J, Erdjument-Bromage H, Warren ME, Borchers CH, Tempst P, and Zhang Y. Histone demethylation by a family of JmjC domain-containing proteins. Nature 439: 811–816, 2006 [DOI] [PubMed] [Google Scholar]
- 161.Tsukaguchi H, Tokui T, Mackenzie B, Berger UV, Chen X.-Z., Wang YX, Brubaker RF, and Hediger MA. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature 399: 70–75, 1999 [DOI] [PubMed] [Google Scholar]
- 162.Upston JM, Karjalainen A, Bygrave FL, and Stocker R. Efflux of hepatic ascorbate: a potential contributor to the maintenance of plasma vitamin C. Biochem J 342: 49–56, 1999 [PMC free article] [PubMed] [Google Scholar]
- 163.Utoguchi N, Ikeda K, Saeki K, Oka N, Mizuguchi H, Kubo K, Nakagawa S, and Mayumi T. Ascorbic acid stimulates barrier function of cultured endothelial cell monolayer. J Cell Physiol 163: 393–399, 1995 [DOI] [PubMed] [Google Scholar]
- 164.Vasquez-Vivar J, Whitsett J, Martasek P, Hogg N, and Kalyanaraman B. Reaction of tetrahydrobiopterin with superoxide: EPR-kinetic analysis and characterization of the pteridine radical. Free Radic Biol Med 31: 975–985, 2001 [DOI] [PubMed] [Google Scholar]
- 165.Vera JC, Rivas CI, Fischbarg J, and Golde DW. Mammalian facilitative hexose transporters mediate the transport of dehydroascorbic acid. Nature 364: 79–82, 1993 [DOI] [PubMed] [Google Scholar]
- 166.Violi F. and Cangemi R. Antioxidant supplements and cardiovascular disease in men. JAMA 301: 1335–1337, 2009 [DOI] [PubMed] [Google Scholar]
- 167.Vissers MC, Gunningham SP, Morrison MJ, Dachs GU, and Currie MJ. Modulation of hypoxia-inducible factor-1 alpha in cultured primary cells by intracellular ascorbate. Free Radic Biol Med 42: 765–772, 2007 [DOI] [PubMed] [Google Scholar]
- 168.Vissers MC. and Wilkie RP. Ascorbate deficiency results in impaired neutrophil apoptosis and clearance and is associated with up-regulation of hypoxia-inducible factor 1{alpha}. J Leukoc Biol 81: 1236–1244, 2007 [DOI] [PubMed] [Google Scholar]
- 169.Voigt K, Kontush A, Stuerenburg HJ, Muench-Harrach D, Hansen HC, and Kunze K. Decreased plasma and cerebrospinal fluid ascorbate levels in patients with septic encephalopathy. Free Radic Res 36: 735–739, 2002 [DOI] [PubMed] [Google Scholar]
- 170.Wang S, Eide TC, Sogn EM, Berg KJ, and Sund RB. Plasma ascorbic acid in patients undergoing chronic haemodialysis. Eur J Clin Pharmacol 55: 527–532, 1999 [DOI] [PubMed] [Google Scholar]
- 171.Wang T, Chen K, Zeng X, Yang J, Wu Y, Shi X, Qin B, Zeng L, Esteban MA, Pan G, et al. The histone demethylases Jhdm1a/1b enhance somatic cell reprogramming in a vitamin-C-dependent manner. Cell Stem Cell 9: 575–587, 2011 [DOI] [PubMed] [Google Scholar]
- 172.Washko P. and Levine M. Inhibition of ascorbic acid transport in human neutrophils by glucose. J Biol Chem 267: 23568–23574, 1992 [PubMed] [Google Scholar]
- 173.Washko PW, Wang Y, and Levine M. Ascorbic acid recycling in human neutrophils. J Biol Chem 268: 15531–15535, 1993 [PubMed] [Google Scholar]
- 174.Wells WW, Xu DP, Yang YF, and Rocque PA. Mammalian thioltransferase (glutaredoxin) and protein disulfide isomerase have dehydroascorbate reductase activity. J Biol Chem 265: 15361–15364, 1990 [PubMed] [Google Scholar]
- 175.Westendorp RG, Draijer R, Meinders AE, and van Hinsbergh VW. Cyclic-GMP-mediated decrease in permeability of human umbilical and pulmonary artery endothelial cell monolayers. J Vasc Res 31: 42–51, 1994 [DOI] [PubMed] [Google Scholar]
- 176.Willis GC. An experimental study of the intimal ground substance in atherosclerosis. Can Med Assoc J 69: 17–22, 1953 [PMC free article] [PubMed] [Google Scholar]
- 177.Willis GC. The reversibility of atherosclerosis. Can Med Assoc J 77: 106–108, 1957 [PMC free article] [PubMed] [Google Scholar]
- 178.Winkler BS. In vitro oxidation of ascorbic acid and its prevention by GSH. Biochim Biophys Acta 925: 258–264, 1987 [DOI] [PubMed] [Google Scholar]
- 179.Winkler BS, Orselli SM, and Rex TS. The redox couple between glutathione and ascorbic acid: A chemical and physiological perspective. Free Radic Biol Med 17: 333–349, 1994 [DOI] [PubMed] [Google Scholar]
- 180.Winterbourn CC. and Metodiewa D. The reaction of superoxide with reduced glutathione. Arch Biochem Biophys 314: 284–290, 1994 [DOI] [PubMed] [Google Scholar]
- 181.Witczak CA, Sharoff CG, and Goodyear LJ. AMP-activated protein kinase in skeletal muscle: from structure and localization to its role as a master regulator of cellular metabolism. Cell Mol Life Sci 65: 3737–3755, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wu F, Schuster DP, Tyml K, and Wilson JX. Ascorbate inhibits NADPH oxidase subunit p47phox expression in microvascular endothelial cells. Free Radic Biol Med 42: 124–131, 2007 [DOI] [PubMed] [Google Scholar]
- 183.Wu F. and Wilson JX. Peroxynitrite-dependent activation of protein phosphatase type 2A mediates microvascular endothelial barrier dysfunction. Cardiovasc Res 81: 38–45, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yoshikawa K, Takahashi S, Imamura Y, Sado Y, and Hayashi T. Secretion of non-helical collagenous polypeptides of alpha1(IV) and alpha2(IV) chains upon depletion of ascorbate by cultured human cells. J Biochem (Tokyo) 129: 929–936, 2001 [DOI] [PubMed] [Google Scholar]
- 185.Zhang K, Dong J, Cheng X, Bai W, Guo W, Wu L, and Zuo L. Association between Vitamin C deficiency and dialysis modalities. Nephrology (Carlton) 17: 452–457, 2012 [DOI] [PubMed] [Google Scholar]