Abstract
Aim: l-ascorbic acid (vitamin C) insufficiency is considered one of the major risk factors for the development of liver disease. However, its specific effects and related mechanisms in vivo are largely unknown. The objective of this study was to investigate the in vivo protective role of vitamin C and its related mechanisms in liver injury with Gulo(−/−) mice that cannot synthesize vitamin C like humans due to the lack of l-gulonolactone-γ-oxidase (Gulo), an essential enzyme for vitamin C synthesis. Results: When liver injury was induced in Gulo(−/−) mice by injection of concanavalin A (Con A), there was greater extensive liver damage accompanied by an increased number of apoptotic hepatocytes in vitamin C-insufficient Gulo(−/−) mice. Additionally, the plasma and hepatic levels of the proinflammatory cytokines, such as TNF-α and IFN-γ, were much higher in the vitamin C-insufficient Gulo(−/−) mice than in the control mice. Moreover, increased numbers of liver-infiltrating T-cells in the vitamin C-insufficient Gulo(−/−) mice were related to the increased hepatic levels of IFN-inducible factor (IP-10). Although the vitamin C-insufficient Gulo(−/−) mice had higher amounts of interleukin-22 (IL-22), a hepatoprotective cytokine, a defect in IL-22Rα expression and its downstream STAT3 activation in hepatocytes were found. Innovation: We first demonstrate the novel in vivo action mechanisms of vitamin C on the prevention of disease development in the liver, through the regulation of excessive immune activation and maintenance of the IL-22Rα signaling pathways. Conclusion: These results suggest that severe liver damage induced by inflammation could be prevented by sufficient supplementation with vitamin C. Antioxid. Redox Signal. 19, 2040–2053.
Introduction
L-ascorbic acid (vitamin C) is a well-known antioxidant that maintains the intracellular antioxidant network, which mainly consists of glutathione and vitamin E (29, 31). Therefore, vitamin C could play a role as an anti-inflammatory molecule through the elimination of reactive oxygen species (ROS) that induce proinflammatory cytokines in several inflammatory diseases (4, 9, 45). It suggests that vitamin C insufficiency might be closely related with the development or facilitation of inflammatory diseases. In fact, vitamin C effectively suppresses inflammatory responses in UVB-irradiated skin keratinocytes through the elimination of ROS (19). In addition, a positive correlation of lower vitamin C concentrations in gastric juice with the incidence of chronic gastritis has been reported (1). Moreover, vitamin C supplementation with vitamin E attenuates the proinflammatory states in patients with gastric inflammation and improves hepatic fibrosis in patients with nonalcoholic steatohepatitis (13, 47). In an animal model of sepsis, vitamin C administration suppresses inflammation in the lung and decreases oxidative damages and lipid peroxidation in the liver (2, 10). The significant decrease in the plasma level of vitamin C in chronic active hepatitis patients has also been reported (60). Therefore, it seems that vitamin C insufficiency is also closely related with the development or progression of inflammatory liver disease. However, the mechanism regarding the specific role of vitamin C in vivo remains to be elucidated.
Innovation.
It is impossible to investigate the effects of l-ascorbic acid (vitamin C) not only in humans due to the fatal effects caused by vitamin C insufficiency, but also in animals, since they can synthesize vitamin C. Therefore, our experiment was done with Gulo(−/−) mice while controlling vitamin C intake. Since we could examine the direct effects on primary hepatocytes, even the systemic effects of vitamin C, our research is highly innovative, since it examined vitamin C effects in vivo on inflammatory diseases in the liver and other organs. Therefore, our data provide the new insights on preventive measures against liver diseases through sufficient vitamin C intake.
l-gulonolactone-γ-oxidase (Gulo) is an essential enzyme for the synthesis of vitamin C from glucose (6). It is impossible for humans, guinea pigs, and some of the primates to synthesize vitamin C, since the Gulo gene is mutated (5, 39). Therefore, humans should take sufficient amounts of vitamin C through their diet or with supplements. Insufficiency of dietary vitamin C is closely related with the development of acute or chronic diseases, including scurvy, diabetes mellitus, myocardial infarction, acute pancreatitis, and atrophic gastritis (3, 41, 46, 50). Gulo(−/−) mice without supplementation of vitamin C show levels of plasma vitamin C concentrations relevant to those of scurvy in humans (23, 32). In addition, reduced red blood cell (RBC) counts, hematocrit, and hemorrhages in the knee joints have been observed (32). Moreover, alteration of the aortic wall, including rupture of the elastic lamina, smooth muscle cell proliferation, and injury of the luminal surface, has been found (32). These results suggests that Gulo(−/−) mice are a suitable animal model that provides numerous opportunities for systematic studies regarding the in vivo effects of vitamin C reflecting what is happening during the development of diseases in humans.
Hepatitis is localized inflammation of the liver characterized by massive infiltration of inflammatory cells. Especially, activated T-cells are frequently responsible for mediating the damages in hepatocytes through IFN-γ production (36, 52). Concanavalin A (Con A)-induced hepatitis is a well-known model of viral hepatitis and autoimmune hepatitis (17, 54). In this model, CD4+ T-cells play a major role in the induction of liver injury through the release of a variety of proinflammatory cytokines, such as IFN-γ and TNF-α (26, 27, 52). Conversely, CD4+ T-cells also protect hepatocytes from damages by the production of interleukin-22 (IL-22) (42, 43, 61). IL-22 production is restricted to immune cells, but its receptor is restricted to nonhematopoietic cells in the skin, pancreas, lung, intestine, and liver (57, 58). IL-22 shows its protective effect through the activation of signal transducers and activators of transcription 3 (STAT3) downstream of the IL-22 receptor, which is composed of two subunits, IL-22Rα and IL-10Rβ (22, 25, 43, 59). Therefore, it is necessary to increase IL-22 and its receptor expression in the liver for protection against liver injury during inflammatory responses. However, it is, as of yet, unclear which factors regulate IL-22 production and its receptor expression under in vivo inflammatory conditions in the liver.
In the present study, we investigated the effects of vitamin C insufficiency on liver injury, followed by inflammation and its related mechanisms in Gulo(−/−) mice with Con A-mediated hepatitis. Especially, we focused on the regulation of the production of TNF-α, IFN-γ, and IL-22 from T-cells and the protective signaling through IL-22Rα on hepatocytes.
Results
Characteristics of Gulo(−/−) mice upon vitamin C withdrawal
Upon vitamin C withdrawal, by week 4, the Gulo(−/−) mice began to lose weight rapidly, whereas the body weights of the wild-type (WT) and vitamin C-supplemented Gulo(−/−) mice steadily increased (23). Finally, withdrawal of vitamin C for longer than 5 weeks caused death (23, 32). These results resemble the development of scurvy in humans (14). Additionally, we confirmed that there were no differences in the liver weight and body weight until 3 weeks after vitamin C withdrawal, but remarkable, decrease in vitamin C in the plasma and liver of Gulo(−/−) mice was observed (Supplementary Fig. S1A, B; Supplementary Data are available online at www.liebertpub.com/ars). To examine that decrease in vivo vitamin C concentration has increased oxidative stress in the liver of vitamin C-insufficient Gulo(−/−) mice, the intracellular ROS level was measured by using of the cell-permeable fluorogenic probe 2′, 7′-dichlorodihydrofluorescin diacetate. Coinciding with the result of decreases in the vitamin C concentration in the liver, the ROS levels in the liver of vitamin C-insufficient Gulo(−/−) mice at 4 h after Con A injection were higher than those in the mice with vitamin C supplementation (Supplementary Fig. S2). It suggests that vitamin C plays an important role as an antioxidant in our experimental model. So, all of the following experiments were done in the Gulo(−/−) mice under a vitamin C-insufficient status, not deficiency, through vitamin C withdrawal for 3 weeks, and these mice were referred to as vitamin C-insufficient Gulo(−/−) mice [Gulo(−/−)VC-insufficient]. The vitamin C concentration in the liver between the WT and vitamin C-supplemented Gulo(−/−) mice was almost the same (Supplementary Fig. S1B). Therefore, vitamin C-supplemented Gulo(−/−) mice were referred as vitamin C-sufficient Gulo−/− mice [Gulo(−/−)VC-sufficient].
Vitamin C has a protective role against Con A-induced liver injury
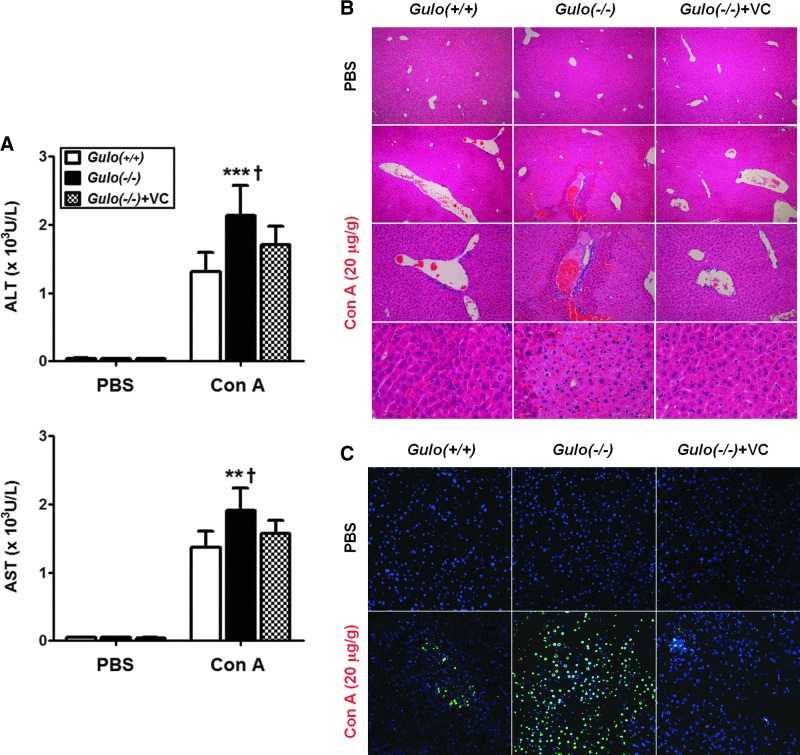
To examine the role of vitamin C in liver injury, we injected WT, Gulo(−/−)VC-insufficient, and Gulo(−/−)VC-sufficient mice with Con A (20 μg/g). To examine liver damage, we measured the amounts of alanine transaminase (ALT) and aspartate transaminase (AST) in the plasma. Eighteen hours after Con A injection, higher plasma levels of ALT and AST, more extensive damage, a larger number of lesions, and more extensive apoptosis of hepatocytes were observed in the liver of Gulo(−/−)VC-insufficient (Fig. 1). We observed no change among all groups in the ALT and AST levels or histological changes in the liver of the phosphate buffered saline (PBS)-injected Gulo(−/−)VC-insufficient mice, indicating that vitamin C insufficiency itself could not induce liver damage.
FIG. 1.
The extensive liver damage in vitamin C-insufficient Gulo(−/−) mice in response to Con A. WT [Gulo(+/+)], vitamin C-insufficient [Gulo(−/−)], and vitamin C-sufficient [Gulo(−/−)+VC] mice were intravenously injected with 20 μg/g of Con A or PBS. Mice were sacrificed at 18 h after injection. (A) The increase of the plasma ALT and AST levels by Con A injection [n=8–9, **p<0.01 or ***p<0.001 vs. Gulo(+/+), †p<0.05 vs. Gulo(−/−)+VC]. Liver injury was quantified by measuring the plasma levels of ALT and AST using a kit according to the manufacturer's instructions. (B) The extensive liver injury by Con A injection. Liver tissues were harvested, fixed, sectioned, and stained with hematoxylin and eosin as described in the Materials and Methods section. The lesions were examined surrounding the hepatic veins, and the results are the representative of more than three experiments (n=9). (C) The extensive apoptosis in the liver by Con A injection. Apoptotic cells in the liver were also detected by the TUNEL method as described in the Materials and Methods section. The nuclei were counterstained with DAPI, and the results are the representative of more than three experiments (n=9). ALT, alanine transaminase; AST, aspartate transaminase; Con A, concanavalin A; Gulo, l-gulonolactone-γ-oxidase; TUNEL, terminal deoxynucleotidyl transferase dUTP nick-end labeling; Vitamin C, l-ascorbic acid; WT, wild-type. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Vitamin C suppresses proinflammatory cytokine production by Con A injection
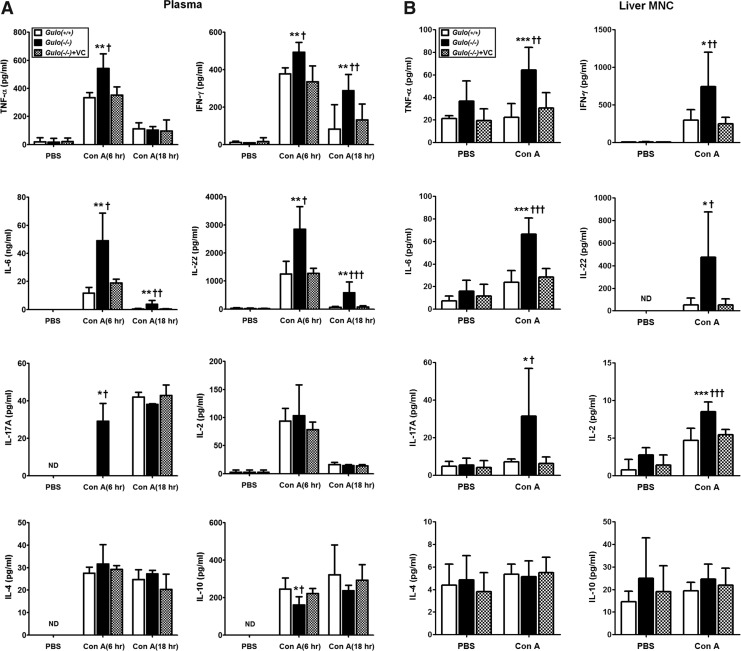
It has already been reported that proinflammatory cytokines, such as IFN-γ and TNF-α, have a key role in Con A-induced hepatic injury (26, 27, 52). Thus, the plasma levels of cytokines, including the proinflammatory cytokines, were analyzed. At 6 or 18 h, after Con A injection, the production of IL-6, IL-17, IL-22, IFN-γ, and TNF-α was significantly increased in the Gulo(−/−)VC-insufficient mice compared with the levels in the WT and Gulo(−/−)VC-sufficient mice (Fig. 2A). On the contrary, IL-10 production in the Gulo(−/−)VC-insufficient mice was suppressed. To analyze the localized production of such cytokines in the liver, isolated liver mononuclear cells (MNCs) were cultured, and then cytokine production was examined 4 h after Con A injection. Just as the results from the experiment using plasma, cytokine production increased in the liver MNCs of Gulo(−/−)VC-insufficient mice by Con A injection (Fig. 2B). There was no difference in IL-2 production in the plasma; however, it increased in the liver MNCs of Gulo(−/−)VC-insufficient mice. An increase in IL-4 and IL-10 in response to Con A was not detected in the liver MNCs. In addition, the frequency of CD4+ Foxp3+ regulatory T-cells was not affected after the injection of Con A (Supplementary Fig. S3).
FIG. 2.
The excessive production of proinflammatory cytokines in vitamin C-insufficient Gulo(−/−) mice in response to Con A. WT [Gulo(+/+)], vitamin C-insufficient [Gulo(−/−)], and vitamin C-sufficient [Gulo(−/−)+VC] mice were intravenously injected with 20 μg/g of Con A or PBS. (A) Increased cytokine levels in plasma. Blood was collected from the intraorbital plexus of each mouse in all experimental groups at 6 and 18 h after Con A injection, and then plasma was prepared as described in the Materials and Methods section. The cytokine levels were measured by cytometric bead array (CBA) or ELISA as described in the Materials and Methods section [n=8–9, *p<0.05 or **p<0.01 vs. Gulo(+/+), †p<0.05 or ††p<0.01 or †††p<0.001 vs. Gulo(−/−)+VC]. (B) Increase of cytokine production from liver MNCs by Con A injection. Four hours after injection, liver MNCs were isolated and cultured as described in the Materials and Methods section. The levels of cytokine in the culture supernatant were measured by CBA or ELISA as described in the Materials and Methods section [n=10, *p<0.05 or ***p<0.001 vs. Gulo(+/+), †p<0.05 or ††p<0.01 or †††p<0.001 vs. Gulo(−/−)+VC]. MNCs, mononuclear cells; ND, not detected.
Vitamin C inhibits T-cell infiltration into the liver by Con A injection
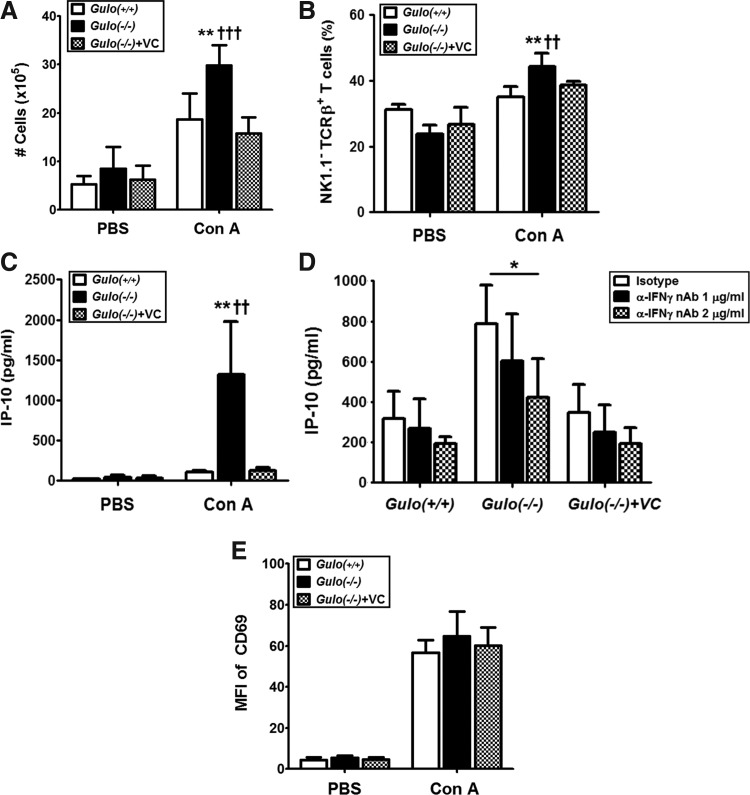
In relation to the increased production of proinflammatory cytokines followed by extensive liver injury, we examined the number of liver MNCs from mice with Con A injection. At 6 h after Con A injection, the number of liver MNCs increased in the Gulo(−/−)VC-insufficient mice, and T-cells were the major population in the liver-infiltrated MNCs (Fig. 3A, B). It is known that IFN-γ induces the production of IFN-inducible protein (IP)-10, which is important in the transmigration of effector T-lymphocytes through the hepatic endothelium under physiological conditions of blood flow (8, 38). Therefore, we measured IP-10 production from the isolated liver MNCs of Gulo(−/−)VC-insufficient mice. Extensive IP-10 production was induced by Con A injection in Gulo(−/−)VC-insufficient mice (Fig. 3C), but it was inhibited by IFN-γ neutralization with the treatment of anti-IFN-γ Abs (Fig. 3D). During these responses, early activation marker CD69 on the liver MNCs was at a similar level for the Gulo(−/−)VC-insufficient and vitamin C-sufficient control mice (Fig. 3E).
FIG. 3.
Increasing numbers of liver-infiltrating T-cells in vitamin C-insufficient Gulo(−/−) mice in response to Con A. After WT [Gulo(+/+)], vitamin C-insufficient [Gulo(−/−)], and vitamin C-sufficient [Gulo(−/−)+VC] mice were intravenously injected with 20 μg/g of Con A or phosphate buffered saline (PBS), liver MNCs were isolated at different time points. (A) Increase of the total number of liver MNCs 6 h after Con A injection [n=6, **p<0.01 vs. Gulo(+/+), †††p<0.001 vs. Gulo(−/−)+VC]. (B) Increase of the T-cell frequency in liver MNCs by Con A injection. Six hours after injection, the frequency of T-cells (NK1.1− TCRβ+) in liver MNCs upon Con A injection was analyzed by flow cytometry analysis as described in the Materials and Methods section [n=5, **p<0.01 vs. Gulo(+/+), ††p<0.01 vs. Gulo(−/−)+VC]. (C) Increased IP-10 production from liver MNCs by Con A injection. Four hours after injection, liver MNCs were isolated and cultured for 24 h as described in the Materials and Methods section. The amounts of IP-10 in the supernatants were determined by ELISA [n=10, **p<0.01 vs. Gulo(+/+), ††p<0.01 vs. Gulo(−/−)+VC]. (D) Suppression of IP-10 production by the neutralization of IFN-γ bioactivity. Four hours after injection, liver MNCs were isolated and cultured with anti-mouse IFN-γ (1 and 2 μg/ml) or isotype antibody (2 μg/ml). The amounts of IP-10 in the supernatants were determined by ELISA (n=5, *p<0.05). (E) The equal activation status of liver MNCs. Four hours after injection, liver MNCs were isolated, and surface expression of CD69 was measured by flow cytometry. The mean fluorescence intensity (MFI) of CD69 is represented as the mean±SD (n=5). IP-10, IFN-inducible protein-10.
Vitamin C insufficiency causes T-cells to excessively respond to Con A
To clarify if the immune cells in Gulo(−/−)VC-insufficient mice have intrinsically different potential responses to Con A, we examined whether Gulo(−/−)VC-insufficient, WT, and Gulo(−/−)VC-sufficient mice have an equal immune response to Con A. Splenocytes and splenic T-cells of Gulo(−/−)VC-insufficient had significantly increased the production of cytokines in response to Con A in vitro (Fig. 4A, B). There was also a significant increase in the proliferation of both cell populations from the Gulo(−/−)VC-insufficient mice (Fig. 4C, D). CD25 is generally expressed T-cells at 48 h after Con A stimulation. However, the frequency of CD25+ T-cells was similar in Gulo(−/−)VC-insufficient, WT, and Gulo(−/−)VC-sufficient mice (Fig. 4E). We found that splenocytes or splenic T-cells of the Gulo(−/−)VC-insufficient mice had an insufficient concentration of vitamin C (Supplementary Fig. S1C). It suggests that vitamin C insufficiency causes T-cells to excessively produce cytokines, especially proinflammatory cytokines, and proliferate in response to Con A.
FIG. 4.
The excessive responses of splenocyte and splenic T-cells in vitamin C-insufficient Gulo(−/−) mice in response to Con A. Splenocytes or splenic T-cells were isolated from WT [Gulo(+/+)], vitamin C-insufficient [Gulo(−/−)], and vitamin C-sufficient [Gulo(−/−)+VC] mice as described in the Materials and Methods section and stimulated with Con A (5 μg/1×106/ml) in vitro. (A, B) The excessive production of cytokines from splenocytes and splenic T-cells of vitamin C-insufficient Gulo(−/−) mice in response to Con A. After 48 h in vitro stimulation of Con A, the amounts of cytokines in the supernatants were determined by CBA or ELISA [n=10, *p<0.05 or **p<0.01 or ***p<0.001 vs. Gulo(−/−), †p<0.05 or †††p<0.001 vs. Gulo(−/−)+VC]. (C, D) The excessive proliferation of splenocytes and splenic T-cells from vitamin C-insufficient Gulo(−/−) mice in response to Con A. After 90 h in vitro stimulation of Con A, [3H]-thymidine incorporation was measured with a liquid scintillation counter as described in the Materials and Methods section [n=10, **p<0.01 vs. Gulo(−/−), †p<0.05 vs. Gulo(−/−)+VC]. (E) The frequency of the activated T-cells in splenocytes. After 48 h in vitro stimulation of Con A, the splenocytes were subjected to flow cytometry analysis of CD3+ CD25+ cells as described in the Materials and Methods section (n=5).
Vitamin C insufficiency increases IFN-γ production from the activated T-cells via the activation of p38MAPK
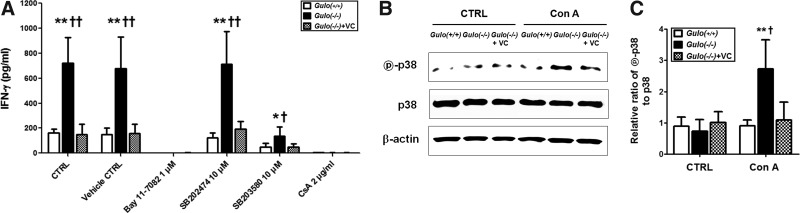
As the inflammatory responses were increased by vitamin C insufficiency, we examined what kinds of molecular pathway are involved. The molecular pathway was focused at the production of INF-γ from T-cells in vitamin C-insufficient Gulo(−/−) mice, because IFN-γ is major effector molecule during the process of the development of inflammation in vitamin C-insufficient Gulo(−/−) mice by Con A. The specific inhibitors for NF-κB (BAY 11-7082), NFAT (Cyclosporin A), and p38MAPK (SB203580) were pretreated to T-cells before treatment of Con A in vitro. As shown in Figure 5A, IFN-γ production was completely suppressed in all of the experimental groups by the treatment of BAY 11-7082 and Cyclosporin A. It assumes that the survival and activation of T-cells by Con A are mainly mediated by the activation of NF-κB and NFAT. However, the increase of IFN-γ production in T-cells isolated from vitamin C-insufficient Gulo(−/−) mice was definitely suppressed by the treatment of the specific inhibitors for p38MAPK and SB203580, but not by the treatment of its control compound, SB202474. To confirm whether the activation of p38MAPK is also modulated by vitamin C in vivo, the phosphorylation of p38MAPK in T-cells from each experimental group was examined. The phosphorylation of p38MAPK in T-cells from vitamin C-insufficient Gulo(−/−) mice was increased by the treatment of Con A, but it was inhibited by the supplementation of vitamin C (Fig. 5B, C). Taken together, vitamin C plays an important role as an anti-inflammatory agent through the downregulation of inflammatory mediator production.
FIG. 5.
Increase of p38MAPK activation in activated T-cells from vitamin C-insufficient
Gulo(−/−)
mice by Con A. Splenic T-cells were isolated from WT [Gulo(+/+)], vitamin C-insufficient [Gulo(−/−)], and vitamin C-sufficient [Gulo(−/−)+VC] mice as described in Materials and Methods. (A) Splenic T-cells were pretreated for 1 h for NF-κB (BAY 11-7082), NFAT (Cyclosporin A, CsA), and p38MAPK (SB203580 or its control compound SB202474), prior to the treatment of Con A (5 μg/1×106/ml) in vitro. After 48 hrs, the culture supernatant was collected, and the production of IFN-γ was determined by ELISA. [n=6, *p<0.05 or **p<0.01 vs. Gulo(−/−), †p<0.05 or ††p<0.01 vs. Gulo(−/−)+VC]. (B) After splenic T cells were treated with Con A (5 μg/1×106/ml) for 30 mins, proteins were extracted, and phosphorylated p38 (indicated as  -p38) or total p38 (indicated as p38) were determined by Western blot analysis as described in Materials and Methods. β-actin was used as a housekeeping control. (C) The density of the each band was measured by densitometry, and the values were expressed as the ratio of
-p38) or total p38 (indicated as p38) were determined by Western blot analysis as described in Materials and Methods. β-actin was used as a housekeeping control. (C) The density of the each band was measured by densitometry, and the values were expressed as the ratio of  -p38/p38 [n=5, **p<0.01 vs. Gulo(+/+), †p<0.05 vs. Gulo(−/−)+VC].
-p38/p38 [n=5, **p<0.01 vs. Gulo(+/+), †p<0.05 vs. Gulo(−/−)+VC].
Vitamin C insufficiency causes a defect in PI3K/Akt-dependent IL-22Rα expression and STAT3 activation
It is known that IL-22 has a role in the protection and regeneration of hepatocytes during liver injury (43, 61). In Gulo(−/−)VC-insufficient mice, IL-22 production was definitely increased by Con A injection, but its protective effect was not observed (Figs. 1 and 2). To address the reason why the increased IL-22 was not able to protect the liver from injury in Gulo(−/−)VC-insufficient mice, we investigated the expression IL-22Rα in hepatocytes. Interestingly, IL-22Rα expression was not induced in the Gulo(−/−)VC-insufficient mice by Con A injection, but it was induced in the WT and the Gulo(−/−)VC-sufficient mice (Fig. 6A, B). On the contrary, IL-22Rα expression increased in the primary hepatocyte cell line, FL83B, by treatment with vitamin C (Fig. 6C). Therefore, it seems that the defect in IL-22Rα expression is caused by vitamin C insufficiency in the Gulo(−/−) mice. Next, we investigated which kinds of signaling mediators are involved in vitamin C-induced IL-22Rα expression. From an inhibitor study that used several kinds of inhibitors against intracellular kinases, IL-22Rα expression in FL83B was suppressed by the treatment of LY294002, a specific inhibitor for PI3K/Akt, but not by the other inhibitors (Fig. 6D). In addition, vitamin C could not increase IL-22Rα expression in FL83B pretreated with LY294002 (Fig. 6E). This result indicates that the PI3K/Akt pathway is not only important in the maintenance of IL-22Rα expression, but also in its induction by vitamin C. The decrease of Akt phosphorylation in hepatocytes from Gulo(−/−)VC-insufficient mice was confirmed (Fig. 6F). Taken together, the defect in PI3K/Akt-dependent IL-22Rα expression is caused by vitamin C insufficiency.
FIG. 6.
Defects of PI3K/Akt-dependent IL-22Rα expression and its downstream STAT3 activation in the hepatocyte of vitamin C-insufficient
Gulo(−/−)
mice. (A) Defect on IL-22Rα expression in the liver of vitamin C-insufficient Gulo(−/−) mice. Two hours after Con A injection, the total liver lysate from WT (Gulo(+/+)), vitamin C-insufficient [Gulo(−/−)], and vitamin C-sufficient [Gulo(−/−)+VC] mice was prepared, and then IL-22Rα and GAPDH expression was determined by the Western blot analysis as described in the Materials and Methods section. (B) The density of the each band was measured by densitometry, and the values were expressed as the ratio of IL-22Rα/GAPDH [n=4, *p<0.05 vs. Gulo(+/+), †p<0.05 vs. Gulo(−/−)+VC]. (C) Increase of IL-22Rα expression on the murine hepatocyte cell line, FL83B, in a dose-dependent manner of vitamin C; FL83B was cultured with 0, 0.0625, 0.125, 0.25, 0.5, and 1 mM of vitamin C for 24 h, and then IL-22Rα or β-actin expression was determined by the Western blot analysis as described in the Materials and Methods section. Data represent four independent experiments. (D, E) PI3K/Akt-dependent IL-22Rα expression on the murine hepatocyte cell line, FL83B. FL83B was pretreated with PD98059 (20 μM), SP600125 (10 μM), SB203580 (20 μM), and LY294002 (5 μM) for 1 h and then incubated for another 24 h in the absence (D) or presence (E) of 1 mM of vitamin C, and then IL-22Rα and β-actin expression was determined by the Western blot analysis as described in Materials and Methods section. Data represent four independent experiments. (F) Defect on the phosphorylation of Akt in hepatocytes of vitamin C-insufficient Gulo(−/−) mice upon Con A stimulation. Two hours after Con A injection, hepatocytes were isolated from the liver as described in the Materials and Methods section. Phosphorylated Akt (indicated as  -Akt) or total Akt (indicated as Akt), and β-actin in the lysates were determined by the Western blot analysis as described in the Materials and Methods section. Data represent four independent experiments. (G) Defect on the activation of STAT3 and ERK1/2 in hepatocytes of vitamin C-insufficient Gulo(−/−) mice upon Con A stimulation. Two hours after Con A injection, hepatocytes were isolated from the liver as described in the Materials and Methods section. Phosphorylated STAT3 (indicated as
-Akt) or total Akt (indicated as Akt), and β-actin in the lysates were determined by the Western blot analysis as described in the Materials and Methods section. Data represent four independent experiments. (G) Defect on the activation of STAT3 and ERK1/2 in hepatocytes of vitamin C-insufficient Gulo(−/−) mice upon Con A stimulation. Two hours after Con A injection, hepatocytes were isolated from the liver as described in the Materials and Methods section. Phosphorylated STAT3 (indicated as  -STAT3) or total STAT3 (indicated as STAT3), ERK1/2, and β-actin in the lysates were determined by Western blot analysis as described in the Materials and Methods section. (H) The density of each band was measured by densitometry, and the values were expressed as the ratio phosphorylated STAT3/total STAT3 [n=3, ***p<0.001 vs. Gulo(+/+), †p<0.05 vs. Gulo(−/−)+VC]. (I) Defect on STAT3 activation in hepatocytes from vitamin C-insufficient Gulo(−/−) mice by recombinant IL-22 stimulation. After stimulation of isolated hepatocytes with or without recombinant IL-22 (20 ng/ml) for 30 min, phosphorylated STAT3 (indicated as
-STAT3) or total STAT3 (indicated as STAT3), ERK1/2, and β-actin in the lysates were determined by Western blot analysis as described in the Materials and Methods section. (H) The density of each band was measured by densitometry, and the values were expressed as the ratio phosphorylated STAT3/total STAT3 [n=3, ***p<0.001 vs. Gulo(+/+), †p<0.05 vs. Gulo(−/−)+VC]. (I) Defect on STAT3 activation in hepatocytes from vitamin C-insufficient Gulo(−/−) mice by recombinant IL-22 stimulation. After stimulation of isolated hepatocytes with or without recombinant IL-22 (20 ng/ml) for 30 min, phosphorylated STAT3 (indicated as  -STAT3) or total STAT3 (indicated as STAT3), and β-actin in the lysates were determined by the Western blot analysis as described in the Materials and Methods section. Data represent three independent experiments. ERK, extracellular signal-regulated kinases; IL-22, interleukin-22; STAT, signal transducers and activators of transcription.
-STAT3) or total STAT3 (indicated as STAT3), and β-actin in the lysates were determined by the Western blot analysis as described in the Materials and Methods section. Data represent three independent experiments. ERK, extracellular signal-regulated kinases; IL-22, interleukin-22; STAT, signal transducers and activators of transcription.
The activation of STAT3 and extracellular signal-regulated kinases (ERK)1/2, known as important signal transducing molecules downstream of IL-22Rα signaling in hepatic cells (28), was defective in the hepatocytes of Gulo(−/−)VC-insufficient mice (Fig. 6G, H). To clarify the defective activation of STAT3 through the stimulation of IL-22Rα by IL-22, the phosphorylation of STAT3 in hepatocytes upon IL-22 stimulation was examined in isolated hepatocytes in vitro. A severe defect of STAT3 phosphorylation in the hepatocytes of Gulo(−/−)VC-insufficient mice was observed by stimulation with IL-22 (Fig. 6I).
Discussion
The current study on Gulo(−/−)VC-insufficient mice provides several findings and implications for vitamin C in the pathogenesis of liver disease. After Con A injection, Gulo(−/−)VC-insufficient mice showed (i) increased infiltration of immune cells, especially T-cells, into the liver upon the increased production of IP-10 in the liver; (ii) the hyperactivation of infiltrated immune cells and increased production of proinflammatory cytokines, such as TNF-α and IFN-γ; and (iii) extensive hepatic damage through the induction of apoptosis in hepatocytes. The production of IL-22, known as a hepatic protective cytokine, was markedly increased in the Gulo(−/−)VC-insufficient mice, but protection against extensive liver injury and recovery of the liver did not occur due to the severe defects in the induction of IL-22Rα expression in hepatocytes and in the signal transduction pathway through the phosphorylation of STAT3.
During the development of inflammatory diseases in the liver, a remarkable increase in lymphocyte recruitment and intrahepatic localization is closely related with the nature and severity of the disease (40, 48). With Con A injection, there were no significant changes in B-cells, macrophages, and natural killer (NK) cells, whereas NK-T-cells decreased (Supplementary Fig. S4). However, the total numbers of liver-infiltrating MNCs and T-cells increased in the Gulo(−/−)VC-insufficient mice (Fig. 3A, B). IP-10 is very important in the migration of T-cells into the liver (8, 24, 53). Moreover, IP-10 production is related positively to the activity of chronic hepatitis (37). IP-10 is mainly produced by monocytes, endothelial cells, and fibroblasts in response to IFN-γ (30). In the Gulo(−/−)VC-insufficient mice, IFN-γ production was definitely increased by Con A injection (Fig. 2B). We confirmed the definite suppression of IP-10 production in the liver MNCs from Gulo(−/−)VC-insufficient mice by neutralizing with anti-IFN-γ antibodies (Fig. 3D). Therefore, high levels of IFN-γ in Gulo(−/−)VC-insufficient mice are responsible for the remarkable increase of IP-10 production. To relate with the increase of IP-10 production, it is already known that the activation of STAT1 plays a critical role (20, 33, 44). So, it assumed that STAT1 is also involved in the increase of IP-10 production upon vitamin C insufficiency, but further investigation is needed.
The activation status of T-cells in Gulo(−/−)VC-insufficient mice was examined by the expression of CD69 and CD25. CD69 is considered as an early activation marker that increased within 24 h by Con A stimulation, and CD25 expression follows CD69 expression within 48 h (7). There were no changes on CD69 expression in the liver MNCs within 4 h after in vivo Con A stimulation as well as the frequency of CD25+ T-cells at 48 h after in vitro Con A stimulation (Figs. 3E and 4E). Interestingly, we observed that most of the T-cells from the Gulo(−/−)VC-insufficient mice exhibited a hyperproliferating activity as well as hyperproduction of inflammatory cytokines. However, it was prevented by vitamin C supplementation (Fig. 4B, D). It implies that the characteristics of T-cells are diametrically opposed under vitamin C-sufficient and insufficient conditions, even though the expression of activation markers is similar for all experimental groups. That is to say, the increased ROS levels in Gulo(−/−)VC-insufficient mice seem to be involved the hyper-responsiveness to Con A (Supplementary Fig. S2). However, it is still unclear whether vitamin C causes the hyper-responsiveness of T-cells at the developmental stage in the bone marrow and thymus or the activation stage in the periphery. It is recently reported that ROS seem to be involved in the self-renewal, differentiation, and life span of hematopoietic stem cells (HSCs) (16, 18). In addition, there are many reports that ROS levels in HSCs and early progenitors are lower than their more-mature progeny (15, 16, 35, 55). Forkhead O family of transcription factors (FOXO) is known as the key gene for the transcriptional regulation by ROS in HSC. Interestingly, the activity of FOXO is regulated by the PI3K/AKT signaling pathway (12). Since we already presented the increase of ROS levels and defects on the PI3K/AKT signaling pathway in Gulo(−/−)VC-insufficient mice, it suggests that there must be a differences on the hematopoietic process between the Gulo(−/−)VC-insufficient and Gulo(−/−)VC-sufficient mice. To investigate whether the hyper-responsiveness of T-cells in Gulo(−/−)VC-insufficient mice is caused by a defect from the development of bone marrow precursor cells or from the reduced vitamin C concentration in peripheral T-cells, a study in which the transplantation of bone marrow cells from Gulo(−/−) mice to WT mice is currently in progress.
It is assumed that the infiltrated T-cells into the liver of Gulo(−/−)VC-insufficient mice by Con A injection play a pathogenic role. On the other hand, some of the T-cells, especially IL-22-producing T-cells, might have a protective role, based on a recent report regarding the role of IL-22 on liver protection and regeneration during hepatic inflammation (42, 43, 61). In patients with viral hepatitis, IL-22-producing immune cells were accumulated in the liver, and hepatic expression of IL-22 mRNA was upregulated (42). Therefore, we thought that the increased IL-22 production by Con A injection in our experiment coincides with the results from patients with viral hepatitis. However, it was very strange that liver injury was not attenuated in the Gulo(−/−)VC-insufficient mice, despite the high levels of IL-22 (Fig. 6A, B). IL-22 production is restricted to T- and NK cells, but no change in the frequency of hepatic NK cells in the Gulo(−/−)VC-insufficient mice by Con A injection was observed (Supplementary Fig. S4C). Therefore, it seems that NK cells are not responsible for increasing the IL-22 production in Gulo(−/−)VC-insufficient mice by Con A.
It has recently been reported that γδ T-cells produce IL-22 and protect against pathogenesis from lung fibrosis (49). In the liver of a patient with hepatitis, 95.3% of the T-cells had αβ-TCR, and the remaining had γδ TCR (21). It is well known that αβ T-cells play a major role in the pathogenesis of liver disease, but the role of γδ T-cells has not been clarified yet. Therefore, further investigation about the protective role of γδ T-cells in liver diseases is needed. It is widely recognized that IL-17-producing activated CD4+ T-cells are mainly responsible for IL-22 production. However, it seems that there is no protective role of IL-17 in liver injury, based on a report using IL-22 and IL-17 double-deficient mice (61). On the contrary, IL-17 appears to promote liver fibrosis through the activation of inflammatory and resident cells in the liver (34). In the Gulo(−/−)VC-insufficient mice after Con A injection, extensive liver injury was observed, despite increasing the IL-17 production. It suggests that IL-17 did not play a protective role in our experimental model. IL-6, a pleiotropic cytokine that is produced by T-cells and macrophages, has a protective effect through STAT3 activation at the early phase of liver injury. Since we observed increasing of IL-6 in the Gulo(−/−)VC-insufficient mice by Con A injection, IL-6 might also be contributed to the protection against liver injury in the Gulo(−/−)VC-insufficient mice. However, it has been reported that IL-6 cooperates with IFN-γ and TNF-α to kill hepatocytes, although it is induced by lipopolysaccharide (56). Moreover, its continuous production exerts harmful effects (51). Therefore, further investigation is needed to clarify whether IL-17 and IL-6 have harmful and protective effects, respectively, under a vitamin C insufficiency condition.
To clarify the reason why extensive liver injury appeared in the Gulo(−/−)VC-insufficient mice by Con A injection despite the increased IL-22, changes in IL-22 receptor expression and its related signaling pathways were examined. Among two subunits of the IL-22 receptor, IL-22Rα determines IL-22 responsiveness of cells (58). Interestingly, IL-22Rα expression, in a normal murine hepatocyte cell line FL83B, increased with treatment of vitamin C (Fig. 6C). In addition, we observed that IL-22Rα expression failed to increase by Con A injection in Gulo(−/−)VC-insufficient mice (Fig 6A, B). We present here that IL-22Rα expression in hepatocytes in Gulo(−/−) mice was regulated by the phosphorylation of PI3K/Akt (Fig. 6D, E). In addition, a severe defect in the phosphorylation of Akt was due to vitamin C insufficiency (Fig. 6F). Therefore, vitamin C maintains IL-22Rα expression in hepatocytes in the Gulo(−/−) mice through the PI3K/Akt signaling pathway. The JAK-STAT pathways have critical roles in various cellular functions in the immune and hepatic systems (11). Among six STATs, STAT3 is only involved in the protection of the liver injury (11). STAT3 activation downstream of IL-22R is an important process for protection against liver injury (43). Moreover, a severe defect in recovering from liver damage in liver-specific STAT3-deficient mice was reported (22). Interestingly, there was a remarkable reduction in the phosphorylation of STAT3 in the Gulo(−/−)VC-insufficient mice by Con A injection, but no difference in total STAT3 expression (Fig. 6G, H). Moreover, we observed that there was a severe defect in STAT3 activation in the hepatocytes of Gulo(−/−)VC-insufficient mice, in response to IL-22 stimulation (Fig. 6I). It seems that IL-22 production increases in Gulo(−/−)VC-insufficient to protect and recover from liver injury induced by Con A; however, it could not be achieved due to failure to increase IL-22Rα expression and to the defect in the STAT3 signaling pathways in the hepatocytes of Gulo(−/−)VC-insufficient mice.
Taken together, vitamin C appears to have beneficial effects on hepatitis by preventing the hepatocellular damage by regulating the immune response and IL-22-mediated STAT3 signaling. This observation suggests that a severe damage to the liver induced by autoimmune or chronic inflammation could be successfully prevented by a sufficient amount of supplemental vitamin C, which could be a potential therapeutic option for the treatment of hepatitis.
Materials and Methods
Cells
A murine hepatocyte cell line, FL83B, was purchased from ATCC (Manasass, VA) and grown per the supplier's instructions in an F-12K medium (ATCC) supplemented with 10% FBS, 2 mM glutamine, 1.5 g/L sodium bicarbonate, 100 U/ml penicillin, and 100 μg/ml streptomycin.
Mice
Gulo(+/−) breeding pairs were obtained from the Mutant Mouse Regional Resource Centers, University of California at Davis. Genotypes of the offspring were evaluated by PCR as recommended (32). To prevent death by vitamin C deficiency, Gulo(−/−) mice were given water supplemented with vitamin C (3.3 g sodium l-ascorbate/L; Sigma, St. Louis, MO). Gulo(−/−) and its background strain C57BL/6J wild-type (WT) mice were maintained in a specific pathogen-free condition in the animal facility at the Seoul National University College of Medicine. Wild types for Gulo(−/−) mice were used after obtaining through the mating of its heterozygous strain (Gulo(+/−). Nine- to 10-week-old male mice weighing 23 to 26 g were used for the experiments. Zoletil (25 mg/kg) and xylazine (10 mg/kg) were used for anesthetization. Buprenorphine (0.01 mg/kg s.c.) was conventionally used for analgesia.
The animal protocol for the experiments was reviewed and approved by the Ethics Committee of Seoul National University.
Withdrawal of vitamin C
Gulo(−/−) mice were maintained on the vitamin C-supplemented water (3.3 g ascorbate/L) for 6 to 7 weeks. The vitamin C-supplemented water was then replaced with normal water for 3 weeks. WT and vitamin C-supplemented Gulo(−/−) mice were used as the control mice.
Con A injection
Con A (20 μg/ml; Sigma) in pyrogen-free PBS was injected intravenously through the lateral tail vein at a dose of 20 μg/g body weight. The same volume of PBS was injected as the control.
Examination of liver injury
Eighteen hours after treatment with Con A, blood and liver tissues were collected. Liver injury was quantified by measuring the plasma enzyme activities of ALT and AST using a kit from Biotron Diagnostics, Inc. (Hemet, CA) according to the manufacturer's instructions. Liver tissues were fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned to thicknesses of 4 μm. The sections were stained with hematoxylin and eosin for histological examination. Apoptosis was detected using terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) staining according to the manufacturer's instructions (Millipore Corporation, Temecula, CA). The nuclei were counterstained with DAPI and observed with a confocal laser-scanning microscope (LSM 510 META; Carl Zeiss AG, Jena, Germany).
Isolation of liver MNCs
After the mice were anesthetized with zoletil (25 mg/kg) and xylazine (10 mg/kg), the portal vein was cannulated, and the liver was perfused with sterile PBS. The liver was aseptically removed and placed into the liver MNC washing medium containing cold DMEM supplemented with 2% horse serum (Gibco, Grand Island, NY). The liver homogenates were passed through 100-μm nylon meshes and centrifuged at 1500 rpm for 10 min. The cells were resuspended in 33% Percoll solution (Amersham, Piscataway, NJ) and centrifuged at 2700 rpm for 20 min. The resulting pellet was harvested as the liver MNCs and resuspended in an RBC lysis buffer. After washing with the liver MNC washing medium, the cells were counted and cultured (2×105/well) in a complete medium containing RPMI 1640 supplemented with 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% heat-inactivated fetal bovine serum (Gibco). After 24 h, the supernatants were harvested, and the amounts of cytokines were measured.
Isolation of splenocytes and splenic T-cells
Mice were euthanized, and the spleen was aseptically removed and placed into a splenocyte washing medium containing cold RPMI containing 10% heat-inactivated fetal bovine serum, 100 U/ml of penicillin, and 100 μg/ml of streptomycin. Spleen homogenates were passed through a 70-μm nylon mesh and centrifuged at 600 g for 10 min. The resulting pellet was harvested and resuspended in the RBC lysis buffer. After washing with the splenocyte washing medium, the cells were counted. Splenic T-cells were purified from splenocytes by an MACS negative selection system using the pan T-cell isolation kit (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). The purity of T-cells was >95%, as determined by flow cytometry using PE-labeled Abs to CD3 (BD Biosciences, San Jose, CA). Splenocytes or splenic T-cells were cultured (1×106/ml) in the complete medium, and stimulated with Con A (5 μg/ml). After 48 h, the supernatants were harvested. and the amounts of cytokines were measured.
Isolation and stimulation of hepatocytes
After Con A injection, mice were anesthetized with zoletil (25 mg/kg) and xylazine (10 mg/kg), and the portal vein was cannulated. The liver was perfused with a prewarmed (37°C) liver perfusion medium, followed by collagenase–dispase digestion with a liver digestion medium. The liver was aseptically removed and placed into an L-15 medium. The cells were released by gently mincing and pipetting with a large-bore pipette. The cell suspension was passed through a 100-μm nylon mesh and centrifuged at 50 g for 5 min. The pellet was resuspended and washed twice with the hepatocyte wash medium. The hepatocytes were purified by Percoll density-gradient separation and washed twice with the hepatocyte wash medium. Purified hepatocytes were cultured in hepatoZYME-SFM supplemented with 2 mM l-glutamine, 50 U/ml penicillin, and 50 μg/ml streptomycin, and stimulated for 30 min with recombinant IL-22 (20 ng/ml; R&D system, Minneapolis, MN) and subjected to analyze STAT3 activation by Western blot analysis. All media for the isolation and culturing of hepatocytes were purchased from the hepatocyte product line of GIBCO (Invitrogen).
Measurement of cytokines
Blood was collected from the intraorbital plexus with a heparinized capillary tube, and the plasma was obtained by centrifugation. The amounts of cytokines and IP-10 in plasma or culture supernatants were measured by cytometric bead array (CBA) or ELISA. IL-2, IL-6, IFN-γ, TNF-α, IL-22, and IP-10 ELISA kits were purchased from R&D system. The CBA Mouse Th1/Th2/Th17 Cytokine kit was purchased from BD Biosciences. ELISA and CBA were performed according to the manufacturer's instructions.
Neutralization of IFN-γ bioactivity
Four hours after Con A injection, liver MNCs were isolated and treated with the indicated concentration of anti-mouse IFN-γ or isotype antibody (R&D system) and cultured (2×105/well) in a complete medium. After 24 h, the supernatants were harvested, and the amounts of IP-10 were measured by ELISA according to the manufacturer's instructions (R&D system).
Flow cytometry analysis
Freshly isolated liver MNCs or splenocytes were resuspended in ice cold fluorescence activated cell sorting (FACS) buffer containing 0.5% BSA and blocked at 4°C for 10 min with an Fc blocking reagent (Miltenyi Biotec GmbH). For splenocyte isolation procedure, see the Supplementary Materials and Methods section. Then, the cells were stained with anti-CD3, CD25, CD69, NK1.1, and TCR β antibodies (BD Biosciences) on ice for 30 min. and washed twice with ice cold FACS buffer. For the staining of regulatory T-cells, liver MNCs were stained with anti-CD4 antibodies (BD Biosciences) on ice for 30 min and washed twice with ice cold FACS buffer. Cells were analyzed by FACSCalibur (BD Biosciences). FlowJo software (Tree Star, Inc., Ashland, OR) was used for the data analysis.
[3H]-Thymidine incorporation assay
Isolated splenocytes or splenic T-cells (2×105/200 μl) were seeded in a complete medium and stimulated with Con A (5 μg/ml) for 72 h, and then 1 μCi of [3H]-thymidine (American Radiolabeled Chemicals, Inc., St. Louis, MO) was added to each well. For the isolation procedure, see the Supplementary Materials and Methods section. After further incubation of 18 h, cells were harvested onto glass fiber filters using a cell harvester (Inotech biosystems International, Dietikon, Switzerland). When dry, these were sealed into polythene bags with the scintillation fluid (Betaplate Scint; PerkinElmer, Boston, MA), and the incorporated [3H]-thymidine was counted on MicroBeta Trilux 1450 (PerkinElmer).
Examination of the signaling pathway for cytokine production
Splenic T-cells (1×106/ml) were pretreated with specific inhibitors for NF-κB (Bay 11-7082), NFAT (Cyclosporin A), and P38MAPK (SB203580) for 1 h. After, the cells were stimulated with Con A (5 μg/ml), and then incubated for another 48 h. the culture supernatant was collected, and the production of IFN-γ was determined by ELISA (R&D system), according to the manufacturer's instructions. To exclude the nonspecific effect of SB203580, its control compound, SB202474, was used. SB203580 and SB202474 were purchased from Calbiochem (Darmstadt, Germany), and Bay 11-7082 and Cyclosporin A were purchased from Sigma.
Western blot analysis
The amounts of total proteins in the lysates were measured with the Bio-Rad Protein Assay Kit (Bio-Rad Laboratories, Hercules, CA). Equal amounts of protein were resolved over 10% polyacrylamide–SDS gels and transferred onto nitrocellulose membranes. The membranes were blocked with 5% nonfat milk in PBS-T (0.05% Tween-20 in PBS) for 1 h, washed with PBS-T, and then incubated with the primary antibodies at 4°C overnight. The primary antibodies were diluted 1:250 (STAT3 and phospho-STAT3; Santa Cruz Biotechnology), 1:500 (p38, ERK1/2, and phospho-ERK1/2; Santa Cruz Biotechnology), 1:500 (Akt and phospho-Akt; Cell signaling, Danvers, MA), 1:1000 (IL-22Rα; Abcam, Cambridge, MA), 1:1000 (GAPDH; Santa Cruz Biotechnology, Santa Cruz, CA), 1:2000 (phospho-p38; Cell signaling), and 1:4000 (β-actin; Santa Cruz Biotechnology) in 1% nonfat milk in PBS-T. After washing, the membranes were subsequently incubated with horseradish peroxidase-conjugated secondary antibody (1:5000; Cell Signaling) and detected with the ECL detection kit (Amersham).
Detection of IL-22Rα expression
After Con A injection, the total liver was harvested and homogenized with a lysis buffer. FL83B was stimulated in the presence of 0.0625, 0.125, 0.25, 0.5, and 1 mM of vitamin C for 24 h, and then vitamin C-treated FL83B cells were collected and homogenized with the lysis buffer. IL-22Rα and GAPDH expression was determined as described above in the Western blot analysis.
Examination of signaling pathways for IL-22Rα expression
FL83B was treated with the specific inhibitors for ERK (PD98059), JNK (SP600125), PI3K/Akt (LY294002), and p38 MAPK (SB203580) for 1 h. After wash with PBS, cells were cultured for another 24 h in the presence or absence of 1 mM of vitamin C, and then cells were collected and homogenized with the lysis buffer. The changes of IL-22Rα expression by the treatment of specific inhibitors were examined as described above in the Western blot analysis. All inhibitors were purchased from Sigma.
Detection of activated Akt, STAT3, and ERK1/2
At 2 h after intravenous injection of Con A (20 μg/g), the liver was obtained and hepatocytes were isolated, and then hepatocytes were homogenized with a lysis buffer. The activations of Akt, STAT3, and ERK1/2 by Con A in the absence or presence of vitamin C were examined as described above in the Western blot analysis.
Statistical analysis
Data were presented as the means±SDs. An unpaired two-tailed t-test was used to compare the two groups [WT vs. Gulo(−/−) or Gulo(−/−) vs. vitamin C-supplemented Gulo(−/−) mice]. p-values of <0.05 were considered statistically significant. Statistical tests were carried out using GraphPad InStat version 5.01 (GraphPad Software, Le Jolla, CA).
Supplementary Material
Abbreviations Used
- ALT
alanine transaminase
- AST
aspartate transaminase
- CBA
cytometric bead array
- Con A
concanavalin A
- ERK
extracellular signal-regulated kinases
- FACS
fluorescence activated cell sorting
- FOXO
Forkhead O family of transcription factors
- Gulo
l-gulonolactone-γ-oxidase
- HBSS
Hank's balanced salt solution
- HSC
hematopoietic stem cell
- IL-22
interleukin-22
- IP-10
IFN-inducible protein-10
- MNCs
mononuclear cells
- NK
natural killer
- PBS
phosphate buffered saline
- ROS
reactive oxygen species
- STAT
signal transducers and activators of transcription
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick-end labeling
- Vitamin C
l-ascorbic acid
- WT
wild-type
Acknowledgments
This work was supported by grants from the National R&D Program for Translational Research (A100216-1121-0000100), Ministry of Health, Welfare & Family Affairs, to J. S. K. We would like to offer special thanks to Prof. Sung-Tae Hong (Department of Parasitology and Tropical Medicine, Seoul National University College of Medicine) and Prof. Sung Joon Kim (Department of Physiology, Seoul National University College of Medicine) for the technical assistance and the comments for our experiments.
Author Disclosure Statement
The authors declare no conflict of interest.
References
- 1.Banerjee S, Hawksby C, Miller S, Dahill S, Beattie AD, and McColl KE. Effect of Helicobacter pylori and its eradication on gastric juice ascorbic acid. Gut 35: 317–322, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barja G, López-Torres M, Pérez-Campo R, Rojas C, Cadenas S, Prat J, and Pamplona R. Dietary vitamin C decreases endogenous protein oxidative damage, malondialdehyde, and lipid peroxidation and maintains fatty acid unsaturation in the guinea pig liver. Free Radic Biol Med 17: 105–115, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Bonham MJ, Abu-Zidan FM, Simovic MO, Sluis KB, Wilkinson A, Winterbourn CC, and Windsor JA. Early ascorbic acid depletion is related to the severity of acute pancreatitis. Br J Surg 86: 1296–1301, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Burek CL. and Rose NR. Autoimmune thyroiditis and ROS. Autoimmun Rev 7: 530–537, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Burns JJ, Moltz A, and Peyser P. Missing step in guinea pigs required for the biosynthesis of L-ascorbic acid. Science 124: 1148–1149, 1956 [DOI] [PubMed] [Google Scholar]
- 6.Burns JJ. Biosynthesis of L-ascorbic acid; basic defect in scurvy. Am J Med 26: 740–748, 1959 [DOI] [PubMed] [Google Scholar]
- 7.Cook G, Campbell JD, Carr CE, Boyd KS, and Franklin IM. Transforming growth factor beta from multiple myeloma cells inhibits proliferation and IL-2 responsiveness in T lymphocytes. J Leukoc Biol 66: 981–988, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Curbishley SM, Eksteen B, Gladue RP, Lalor P, and Adams DH. CXCR 3 activation promotes lymphocyte transendothelial migration across human hepatic endothelium under fluid flow. Am J Pathol 167: 887–899, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filippin LI, Vercelino R, Marroni NP, and Xavier RM. Redox signalling and the inflammatory response in rheumatoid arthritis. Clin Exp Immunol 152: 415–422, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher BJ, Seropian IM, Kraskauskas D, Thakkar JN, Voelkel NF, Fowler AA, 3rd, and Natarajan R. Ascorbic acid attenuates lipopolysaccharide-induced acute lung injury. Crit Care Med 39: 1454–1460, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Gao B. Cytokines, STATs and liver disease. Cell Mol Immunol 2: 92–100, 2005 [PubMed] [Google Scholar]
- 12.Greer EL. and Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene 24: 7410–7425, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Harrison SA, Torgerson S, Hayashi P, Ward J, and Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol 98: 2485–2490, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Hodges RE, Baker EM, Hood J, and Sauberlich HE, March SC. Experimental scurvy in man. Am J Clin Nutr 22: 535–548, 1969 [DOI] [PubMed] [Google Scholar]
- 15.Ito K, Hirao A, Arai F, Matsuoka S, Takubo K, Hamaguchi I, Nomiyama K, Hosokawa K, Sakurada K, Nakagata N, Ikeda Y, Mak TW, and Suda T. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature 431: 997–1002, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y, and Suda T. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med 12: 446–451, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Jaeckel E. Animal models of autoimmune hepatitis. Semin Liver Dis 22: 325–338, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Jang YY. and Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood 110: 3056–3063, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang JS, Kim HN, Jung da J, Kim JE, Mun GH, Kim YS, Cho D, Shin DH, Hwang YI, and Lee WJ. Regulation of UVB-induced IL-8 and MCP-1 production in skin keratinocytes by increasing vitamin C uptake via the redistribution of SVCT-1 from the cytosol to the membrane. J Invest Dermatol 127: 698–706, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Karonitsch T, Feierl E, Steiner CW, Dalwigk K, Korb A, Binder N, Rapp A, Steiner G, Scheinecker C, Smolen J, and Aringer M. Activation of the interferon-gamma signaling pathway in systemic lupus erythematosus peripheral blood mononuclear cells. Arthritis Rheum 60: 1463–1471, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Kasper HU, Ligum D, Cucus J, Stippel DL, Dienes HP, and Drebber U. Liver distribution of gammadelta-T-cells in patients with chronic hepatitis of different etiology. APMIS 117: 779–785, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Ki SH, Park O, Zheng M, Morales-Ibanez O, Kolls JK, Bataller R, and Gao B. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology 52: 1291–1300, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim H, Bae S, Yu Y, Kim Y, Kim HR, Hwang YI, Kang JS, Lee WJ. The analysis of vitamin C concentration in organs of gulo(−/−) mice upon vitamin C withdrawal. Immune Netw 12: 18–26, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kita H, Mackay IR, Van De Water J, and Gershwin ME. The lymphoid liver: considerations on pathways to autoimmune injury. Gastroenterology 120: 1485–1501, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Kotenko SV, Izotova LS, Mirochnitchenko OV, Esterova E, Dickensheets H, Donnelly RP, and Pestka S. Identification of the functional interleukin-22 (IL-22) receptor complex: the IL-10R2 chain (IL-10Rbeta) is a common chain of both the IL-10 and IL-22 (IL-10-related T cell-derived inducible factor, IL-TIF) receptor complexes. J Biol Chem 276: 2725–2732, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Ksontini R, Colagiovanni DB, Josephs MD, Edwards CK, 3rd, Tannahill CL, Solorzano CC, Norman J, Denham W, Clare-Salzler M, MacKay SL, and Moldawer LL. Disparate roles for TNF-alpha and Fas ligand in concanavalin A-induced hepatitis. J Immunol 160: 4082–4089, 1998 [PubMed] [Google Scholar]
- 27.Küsters S, Gantner F, Künstle G, and Tiegs G. Interferon gamma plays a critical role in T cell–dependent liver injury in mice initiated by concanavalin A. Gastroenterology 111: 462–471, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Lejeune D, Dumoutier L, Constantinescu S, Kruijer W, Schuringa JJ, and Renauld JC. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J Biol Chem 277: 33676–33682, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Li X, Qu ZC, and May JM. GSH is required to recycle ascorbic acid in cultured liver cell lines. Antioxid Redox Signal 3: 1089–1097, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Luster AD, Unkeless JC, and Ravetch JV. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature 315: 672–676, 1985 [DOI] [PubMed] [Google Scholar]
- 31.Machlin LJ. and Bendich A. Free radical tissue damage: protective role of antioxidant nutrients. FASEB J 1: 441–445, 1987 [PubMed] [Google Scholar]
- 32.Maeda N, Hagihara H, Nakata Y, Hiller S, Wilder J, and Reddick R. Aortic wall damage in mice unable to synthesize ascorbic acid. Proc Natl Acad Sci U S A 97: 841–846, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McInnis KA, Britain A, Lausch RN, and Oakes JE. Synthesis of alpha-chemokines IP-10, I-TAC, and MIG are differentially regulated in human corneal keratocytes. Invest Ophthalmol Vis Sci 46: 1668–1674, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Meng F, Wang K, Aoyama T, Grivennikov SI, Paik Y, Scholten D, Cong M, Iwaisako K, Liu X, Zhang M, Osterreicher CH, Stickel F, Ley K, Brenner DA, and Kisseleva T. Interleukin-17 signaling in inflammatory, kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology 143: 765–776e3, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyamoto K, Araki KY, Naka K, Arai F, Takubo K, Yamazaki S, Matsuoka S, Miyamoto T, Ito K, Ohmura M, Chen C, Hosokawa K, Nakauchi H, Nakayama K, Nakayama KI, Harada M, Motoyama N, Suda T, and Hirao A. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell 1: 101–112, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Mizuhara H, Uno M, Seki N, Yamashita M, Yamaoka M, Ogawa T, Kaneda K, Fujii T, Senoh H, and Fujiwara H. Critical involvement of interferon gamma in the pathogenesis of T-cell activation-associated hepatitis and regulatory mechanisms of interleukin-6 for the manifestations of hepatitis. Hepatology 23: 1608–1615, 1996 [DOI] [PubMed] [Google Scholar]
- 37.Narumi S, Tominaga Y, Tamaru M, Shimai S, Okumura H, Nishioji K, Itoh Y, and Okanoue T. Expression of IFN-inducible protein-10 in chronic hepatitis. J Immunol 158: 5536–5544, 1997 [PubMed] [Google Scholar]
- 38.Narumi S, Wyner LM, Stoler MH, Tannenbaum CS, and Hamilton TA. Tissue-specific expression of murine IP-10 mRNA following systemic treatment with interferon. J Leukocyte Biol 52: 27–33, 1992 [DOI] [PubMed] [Google Scholar]
- 39.Nishikimi M, Fukuyama R, Minoshima S, Shimizu N, and Yagi K. Cloning and chromosomal mapping of the human nonfunctional gene for L-gulono-gamma-lactone oxidase, the enzyme for L-ascorbic acid biosynthesis missing in man. J Biol Chem 269: 13685–13688, 1994 [PubMed] [Google Scholar]
- 40.Oo YH. and Adams DH. The role of chemokines in the recruitment of lymphocytes to the liver. J Autoimmun 34: 45–54, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Packer L, Traber MG, Kraemer K, and Frei B. (Eds). The Antioxidant Vitamin C and E. Champaign, IL: AOCS press, 2002, pp. 9–11 [DOI] [PubMed] [Google Scholar]
- 42.Park O, Wang H, Weng H, Feigenbaum L, Li H, Yin S, Ki SH, Yoo SH, Dooley S, Wang FS, Young HA, and Gao B. In vivo consequences of liver-specific interleukin-22 expression in mice: implications for human liver disease progression. Hepatology 54: 252–261, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Radaeva S, Sun R, Pan HN, Hong F, and Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology 39: 1332–1342, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Ramana CV, Gil MP, Han Y, Ransohoff RM, Schreiber RD, and Stark GR. Stat1-independent regulation of gene expression in response to IFN-gamma. Proc Natl Acad Sci U S A 98: 6674–6679, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reuter S, Gupta SC, Chaturvedi MM, and Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med 49: 1603–1616, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riemersma RA, Carruthers KF, Elton RA, and Fox KA. Vitamin C and the risk of acute myocardial infarction. Am J Clin Nutr 71: 1181–1186, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Sezikli M, Çetinkaya ZA, Güzelbulut F, Çimen B, Özcan Ö, Özkara S, Yeşil A, Gümrükçü G, Ipçioğlu OM, Sezikli H, and Ovünç AO. Effects of alpha tocopherol and ascorbic acid on Helicobacter pylori colonization and the severity of gastric inflammation. Helicobacter 17: 127–132, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Shetty S, Lalor PF, and Adams DH. Lymphocyte recruitment to the liver: molecular insights into the pathogenesis of liver injury and hepatitis. Toxicology 254: 136–146, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simonian PL, Wehrmann F, Roark CL, Born WK, O'Brien RL, and Fontenot AP. γδ T cells protect against lung fibrosis via IL-22. J Exp Med 207: 2239–2253, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sinclair AJ, Taylor PB, Lunec J, Girling AJ, and Barnett AH. Low plasma ascorbate levels in patients with type 2 diabetes mellitus consuming adequate dietary vitamin C. Diabet Med 11: 893–898, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Tagawa Y, Matthys P, Heremans H, Dillen C, Zaman Z, Iwakura Y, and Billiau A. Bimodal role of endogenous interleukin-6 in concanavalin A-induced hepatitis in mice. J Leukoc Biol 67: 90–96, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Tagawa Y, Sekikawa K, and Iwakura Y. Suppression of concanavalin A-induced hepatitis in IFN-gamma(−/−) mice, but not in TNF-alpha(−/−) mice: role for IFN-gamma in activating apoptosis of hepatocytes. J Immunol 159: 1418–1428, 1997 [PubMed] [Google Scholar]
- 53.Tamaru M, Nishioji K, Kobayashi Y, Watanabe Y, Itoh Y, Okanoue T, Murai M, Matsushima K, and Narumi S. Liver-infiltrating T lymphocytes are attracted selectively by IFN-inducible protein-10. Cytokine 12: 299–308, 2000 [DOI] [PubMed] [Google Scholar]
- 54.Tiegs G, Hentschel J, and Wendel A. A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J Clin Invest 90: 196–203, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, Armstrong SA, Passegué E, DePinho RA, and Gilliland DG. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 128: 325–339, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Tracey KJ. and Cerami A. Tumor necrosis factor, other cytokines and disease. Annu Rev Cell Biol 9: 317–343, 1993 [DOI] [PubMed] [Google Scholar]
- 57.Wolk K, Kunz S, Asadullah K, and Sabat R. Cutting edge: Immune cells as sources and targets of the IL-10 family members. J Immunol 168: 5397–5402 2002 [DOI] [PubMed] [Google Scholar]
- 58.Wolk K, Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, and Sabat R. IL-22 increases the innate immunity of tissues. Immunity 21: 241–254, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Xie MH, Aggarwal S, Ho WH, Foster J, Zhang Z, Stinson J, Wood WI, Goddard AD, and Gurney AL. Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J Biol Chem 275: 31335–31339, 2000 [DOI] [PubMed] [Google Scholar]
- 60.Yamamoto Y, Yamashita S, Fujisawa A, Kokura S, and Yoshikawa T. Oxidative stress in patients with hepatitis, cirrhosis, and hepatoma evaluated by plasma antioxidants. Biochem Biophys Res Commun 247: 166–170, 1998 [DOI] [PubMed] [Google Scholar]
- 61.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, and Flavell RA. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity 27: 647–659, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.