Abstract
The diaphragm is the most important inspiratory muscle in all mammals, and ventilatory insufficiency caused by diaphragm dysfunction is the leading cause of morbidity and mortality in many genetic and acquired diseases affecting skeletal muscle. Currently, pharmacological inhibitors, genetically modified animals, and invasive procedures are used to study disorders affecting the diaphragm. However, these methodologies can be problematic because of off-target drug effects and the possible nonphysiological consequences of lifelong genetic alterations. Therefore, alternative methods to study this important respiratory muscle are needed. To resolve this, we have developed a methodology to deliver recombinant adeno-associated virus (rAAV) vectors to the rat diaphragm via direct intramuscular injection. We hypothesized that by direct injection of rAAV into the muscle we can selectively target the diaphragm and establish a novel experimental method for studying signaling pathways and also provide a strategy for effectively using rAAV to protect the diaphragm against disease. This report describes the methods and evidence to support the use of rAAV as a therapeutic intervention to study rat diaphragm biology during conditions that promote diaphragm dysfunction.
Introduction
The diaphragm is the primary muscle of inspiration in all mammals, and significant impairment of diaphragm function results in inadequate pulmonary ventilation and gas exchange. In this regard, a disease (e.g., chronic obstructive pulmonary disease and sepsis) or other condition (e.g., aging, mechanical ventilation, and muscular dystrophy) that leads to diaphragm dysfunction and weakness can result in increased morbidity and mortality (Poole et al., 1997; Hussain, 1998; Powers et al., 2011; McCool and Tzelepis, 2012). Currently, two primary methods are utilized to study diaphragm muscle dysfunction (e.g., pharmacological inhibitors and transgenic animals). Unfortunately, both of these approaches have significant limitations. For example, pharmacological probes often possess off-target effects and transgenic animals can be problematic because of the nonphysiological consequences of lifelong genetic alterations. The problem of lifelong gene expression could be avoided by direct transduction into the diaphragm and acute expression of the gene of interest. Indeed, delivering a specific gene directly to the diaphragm to promote acute overexpression of the gene product could be a powerful experimental tool.
However, the diaphragm poses a problem for gene therapy because of its size and anatomical location. In this regard, several laboratories have demonstrated methods to transduce the mouse diaphragm using viruses as an alternative to previously used techniques (Gregorevic et al., 2004; Mah et al., 2004; Matecki et al., 2004; Grose et al., 2012; Falk et al., 2013). While these studies demonstrate gene expression in the mouse diaphragm (∼0.3–0.4 mm thick), the rat diaphragm represents a larger challenge to achieve whole-muscle transduction because of the thickness of the muscle (∼0.7–1 mm) (Boriek et al., 2001).
In this regard, recombinant adeno-associated virus (rAAV) is a small, single-stranded, DNA-containing human parvovirus. This nonpathogenic virus is being widely investigated as a therapeutic vector for a host of muscle disorders (Muzyczka, 1992; Kessler et al., 1996; Clark et al., 1997; Fisher et al., 1997). Currently, there are ongoing and completed clinical trials using rAAV as a therapeutic tool to treat diseases affecting skeletal muscle (Mendell et al., 2009, 2010; Bowles et al., 2012). For example, work with rAAV in animal models has led to the development of a gene therapy strategy for Pompe disease, which has resulted in a clinical trial of rAAV-mediated gene-based therapy for this disease (ClinicalTrials.gov Identifier: NCT00976352) (Byrne et al., 2011; Pacak and Byrne, 2011; Smith et al., 2013).
Currently, many rAAV serotypes exist that differ in tissue specificity as well as expression. In the current experiment, we utilized rAAV2/9 to express GFP in rat diaphragm muscle. This rAAV serotype was selected because of its ability to transduce striated muscle and because it has been shown to transduce all myofiber types, which is advantageous for use in rat diaphragm, which contains all four of the major muscle fiber types (Inagaki et al., 2006; Pacak et al., 2006; Sun et al., 2008). The objective of this study was to develop a safe and effective method to uniformly deliver rAAV to the entire costal region of the rat diaphragm. Our results indicate that we were successful in this regard; therefore, this is the first report to demonstrate that the rat diaphragm is of sufficient thickness to be directly injected and transfected with rAAV2/9 vectors. Based upon our successful results, we predict that this method will provide an effective experimental approach to specifically target the diaphragm to study genetic and signaling pathways related to diaphragm dysfunction.
Materials and Methods
Animals
Young adult (∼4–6 months old) female Sprague-Dawley rats were used in these experiments. Animals were maintained on a 12:12-hour light–dark cycle and provided food and water ad libitum throughout the experimental period. The Institutional Animal Care and Use Committee of the University of Florida approved these experiments.
Gene delivery methods
All surgery was done using aseptic surgical techniques. First, animals were anesthetized using isoflurane. Upon reaching a surgical plane of anesthesia, the animals were place supine and a region of their abdomen was shaved from the bottom of their ribcage extending down several inches. This area was then cleaned with a 4% chlorohexidine solution. An∼2-inch midline incision was made from the xyphoid process to the suprapubic region. Once the abdomen was opened, the diaphragm was accessed by lifting up the xyphoid process while sterile cotton-tipped applicators were used to retract the liver. The diaphragm was exposed and four injections were made directly into one side of the costal diaphragm using a 0.5-inch 29G needle. Before injection, the tip of the needle (∼5 mm) was bent to ∼75° to facilitate entry of the needle into the diaphragm. The four injections were evenly spaced across the hemidiaphragm. Animals were injected with AAV9-CMV-mCherry (n=2), AAV9-CMV-GFP (n=6), AAV1-LacZ (n=1), or lactated ringers (n=6). All AAV vectors were packaged and purified at the University of Florida Gene Therapy Center Vector Core Lab as previously described (Zolotukhin et al., 2002). About 1×1011 vg total was diluted in lactated ringers solution and injected per hemidiaphragm. A volume of 40 μl per injection was chosen based on preliminary experiments. Successful injection was visualized by the formation of a small bleb within the tissue. Figure 1A depicts a representative image of this procedure.
FIG. 1.
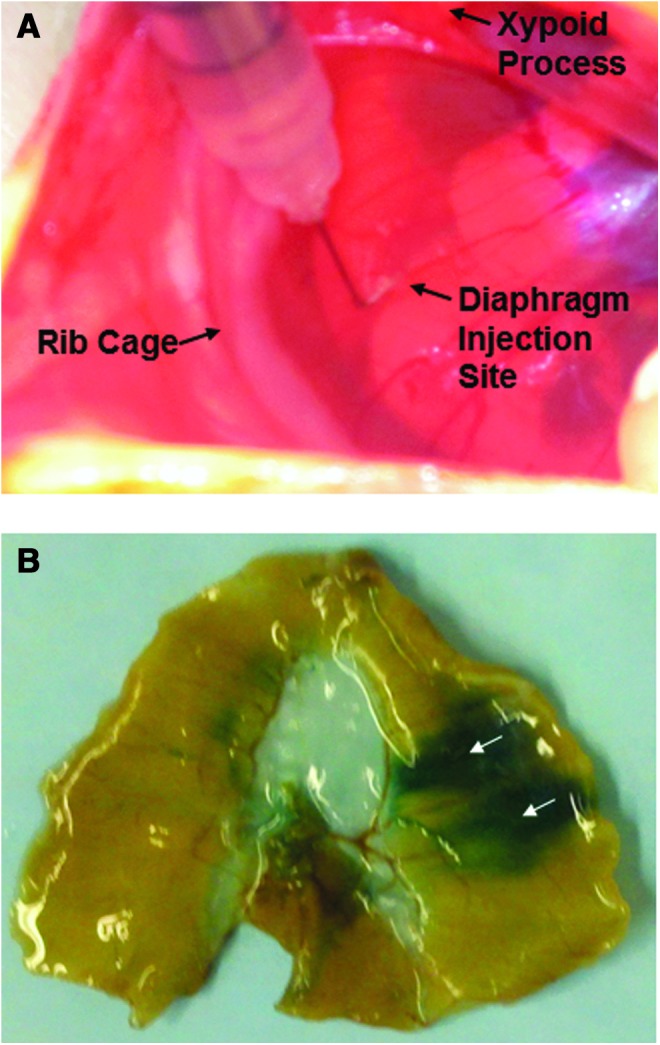
(A) Representative in vivo image depicting the procedure for a direct diaphragm injection. The left hemidiaphragm is exposed, and a 40 μl intramuscular injection is given in the diaphragm using a 29G needle with a bent tip. (B) LacZ-stained diaphragm depicting the area of distribution after direct intramuscular diaphragm injections of 1×1011 vg rAAV-LacZ. Arrows depict approximate injection sites. rAAV, recombinant adeno-associated virus. Color images available online at www.liebertpub.com/hgtb
Four weeks after rAAV administration, animals were sacrificed. Diaphragm, heart, spleen, liver, soleus, and lung tissues were immediately removed and either frozen in liquid nitrogen or placed in 4% paraformaldehyde solution.
Assay of LacZ enzyme activity
rAAV1-LacZ-treated diaphragm tissue was assayed to visualize LacZ expression according to manufacturer's instructions using the LacZ Tissue Staining Kit (InvivoGen, San Diego, CA). The percentage of the total diaphragm area expressing LacZ was quantified using ImageJ software (NIH).
Fluorescence optical imaging
Tissue fluorescence optical imaging was performed using the Xenogen system within the University of Florida Cell and Tissue Analysis Core. Appropriate imaging parameters were selected and implemented throughout the acquisition period. Excitation and emission filters were set at 535 and 580, respectively. Bright-field photographs were also obtained, and relative fluorescence of the tissues was measured.
Biochemical measures
Western blot analysis
Protein abundance was determined in diaphragm, heart, spleen, liver, soleus, and lung samples via Western blot analysis. Briefly, all tissue samples were homogenized 1:10 (wt/vol) in 5 mM Tris (pH 7.5) and 5 mM EDTA (pH 8.0) with a protease inhibitor cocktail (Sigma, St. Louis, MO) and centrifuged at 1,500 g for 10 min at 4°C. After collection of the resulting supernatant, protein content was assessed by the method of Bradford (Sigma). Proteins were separated via polyacrylamide gel electrophoresis via 4–20% gradient gels containing 0.1% sodium dodecyl sulfate for ∼1 hour at 200 V. After electrophoresis, the proteins were transferred to nitrocellulose membranes and incubated with GFP primary antibody (Santa Cruz Biotechnology, Santa Cruz, CA). After incubation, membranes were washed extensively with PBS-Tween and then incubated with secondary antibody (GE Healthcare, Piscataway, NJ). After washing, a chemiluminescent system was used to detect labeled proteins (GE Healthcare). Membranes were developed using autoradiography film, and images of the film were captured and analyzed using the 440CF Kodak Imaging System (Kodak, New Haven, CT). Ponceau staining was used to verify protein loading.
Histological measures
Tissues of interest were removed and immediately incubated in freshly made 4% paraformaldehyde in PBS for 12–24 hours. Tissues were then washed in PBS and incubated in PBS for 12–24 hours, followed by incubation in 30% sucrose in PBS. Tissues were subsequently frozen in OCT, and sections from frozen samples were cut at 10 μm using a cryotome (Shandon Inc., Pittsburgh, PA).
GFP staining
Sections were directly imaged using an inverted fluorescent microscope to visualize GFP expression using a 10× objective lens.
Hematoxylin and eosin staining
Sections were stained for hematoxylin and eosin for analysis of diaphragm muscle damage and imaged using an inverted microscope using a 10× objective lens.
Myofiber cross-sectional area
Diaphragms were removed and fixed in OCT and stored at −80°C. On the day of analysis, sections from frozen diaphragm samples were cut at 10 μm with a cryotome (Shandon Inc.) and allowed to air-dry at room temperature for 30 min. Sections were stained for dystrophin, myosin heavy chain (MHC) I, and MHC type IIa proteins for fiber cross-sectional area as previously described (McClung et al., 2007). Cross-sectional area was determined using Scion software (NIH).
Functional measures
Contractile properties
To determine if injections altered diaphragmatic function, we measured diaphragm contractile properties in vitro. Upon sacrifice, the entire diaphragm was removed and placed in a dissecting chamber containing Krebs-Hensleit solution equilibrated with 95% O2–5% CO2 gas. A muscle strip from the injected costal hemidiaphragm, including the tendinous attachments at the central tendon and rib cage, was dissected from the midcostal region. The strip was suspended vertically between two lightweight Plexiglas clamps with one end connected to an isometric force transducer (model FT-03; Grass Instruments, Quincy, MA) within a jacketed tissue bath. The force output was recorded via a computerized data acquisition system (Super Scope II; GW Instruments, Somerville, MA; Apple Computer, Cupertino, CA). The tissue bath was filled with Krebs-Hensleit saline and the buffer was aerated with gas (95% O2–5% CO2), pH was maintained at 7.4, and the osmolality of the bath was ∼290 mosmol/kg H2O. After a 15 min equilibration period (25°C), in vitro diaphragm contractile measurements were made (Segal et al., 1986; Reid, 2008).
Results
Systemic and biologic response
No significant differences existed in body weight between the experimental groups at any time point. Specifically, before surgery, animal body weight was 293±17 g. Four weeks after diaphragm injection, average animal body weight was 312±20 g. Therefore, there was no apparent weight loss caused by surgical stress. In fact, the animals continue to gain weight over the 4-week AAV vector incubation period. In addition, at the completion of the experimental period, there was no animal mortality, no visual abnormalities of the peritoneal cavity, and no evidence of infection, indicating that our aseptic surgical technique was successful.
Transduction efficiency
To determine the efficiency of rAAV delivery via direct intramuscular injection, we used lacZ as a reporter gene (Fig. 1B). The images obtained indicate the diaphragm area covered by each injection, and this analysis was used to determine the appropriate injection protocol to ensure gene uptake throughout the entire costal diaphragm. Specifically, our results indicate that ∼24% of the costal diaphragm was affected by the two side-by-side injections depicted in Fig. 1B.
Fluorescent imaging
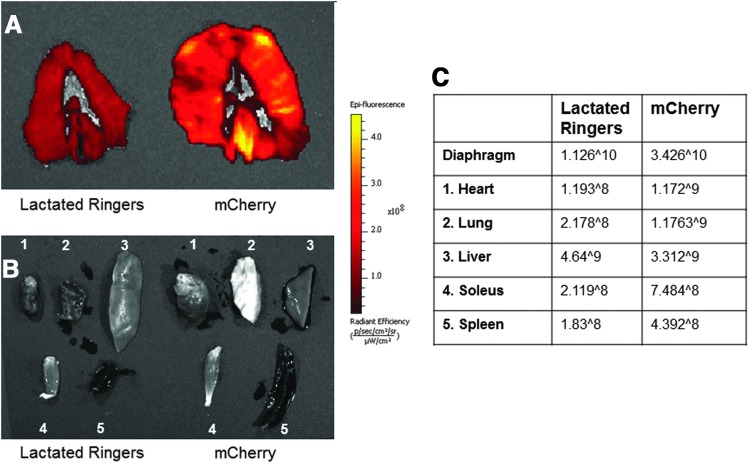
To detect fluorescence in the tissue of our AAV9-CMV-mCherry-injected animals, we used the Xenogen system (Fig. 2). Intramuscular injection in the diaphragm demonstrated an increase in fluorescence in the hemidiaphragm when compared with a lactated ringers-injected control animal (Fig. 2A). In addition, we also examined other tissues to examine the possible spread of AAV9-CMV-mCherry throughout the circulation. Specifically, we quantified the relative change in fluorescence of the heart, lung, liver, spleen, and soleus muscle (Fig. 2B and C). In general, there was no difference in tissue fluorescence in the mCherry animals when compared with the control animals except for the diaphragm. Therefore, this demonstrates that there was little to no detectable expression of the transgene in tissues other than the diaphragm.
FIG. 2.
Fluorescence optical imaging from lactated ringers-injected, control diaphragms and diaphragm tissue injected with AAV9-CMV-mCherry. (A) Diaphragm muscle. (B) 1, Cardiac muscle tissue; 2, lung tissue; 3, liver tissue; 4, soleus muscle tissue; 5, spleen tissue. (C) Relative fluorescence for all tissues.
GFP protein levels
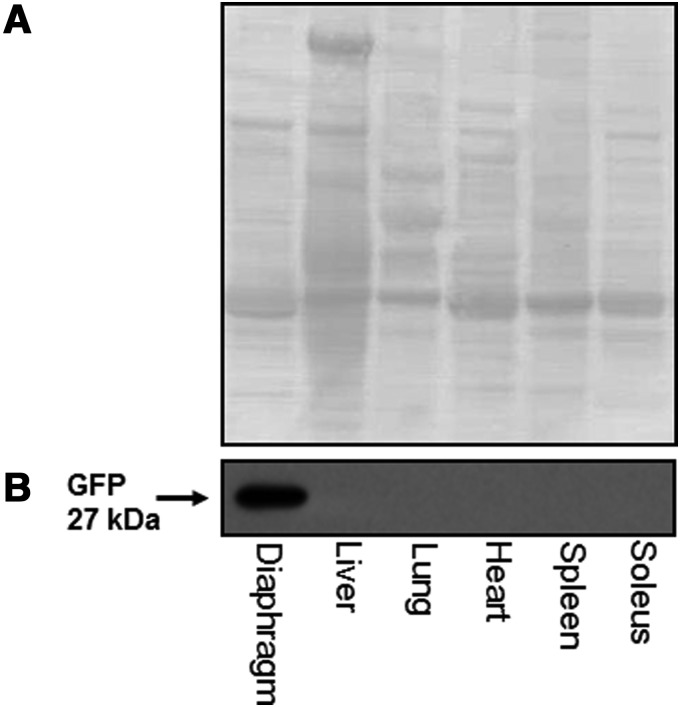
Change in the protein concentration of GFP in the tissues of interest was determined via Western blot. We determined that direct injection of AAV9-CMV-GFP into the costal diaphragm resulted in a significant increase in GFP protein concentration in the muscle. In addition, direct injection of the diaphragm appears to be specific to the diaphragm, and we see no increased GFP protein expression in any of the other tissues measured (Fig. 3B).
FIG. 3.
GFP expression. (A) Representative ponceau staining image demonstrating protein loading. (B) Representative Western blot image demonstrating that GFP expression after intramuscular diaphragm injections is localized to diaphragm tissue. GFP, green fluorescent protein.
GFP immunohistochemistry
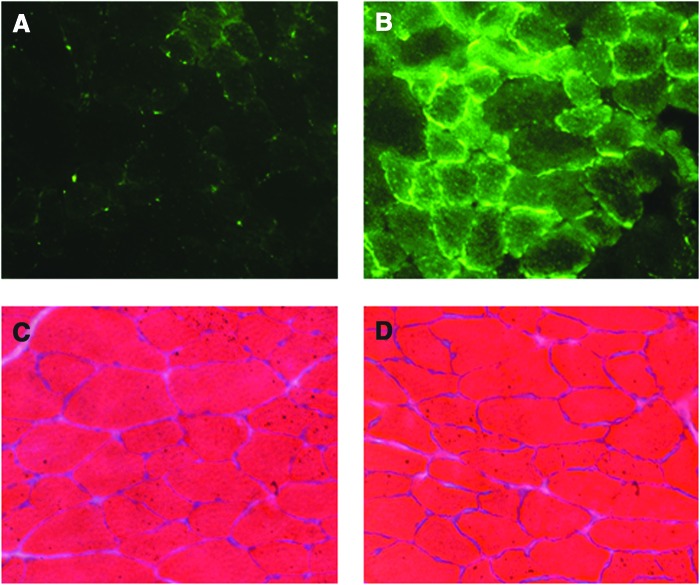
In animals injected with AAV9-CMV-GFP, we directly imaged the muscle fibers for GFP to see the relative distribution of GFP within the diaphragm cross section. Our data reveal that compared with control, noninjected animals, the injected animals expressed GFP in approximately 80% of the diaphragm muscle fibers (Fig. 4A and B).
FIG. 4.
(A and B) Representative direct GFP detection in diaphragm muscle. (A) Cross section of a noninjected, control diaphragm. (B) Cross section of a diaphragm injected with 1×1011 vg AAV9-CMV-GFP. (C and D) Representative hematoxylin and eosin staining of diaphragm muscle. (C) Noninjected, control diaphragm. (D) Injected (diaphragm intramuscular injection).
Direct intramuscular injection does not damage muscle fibers
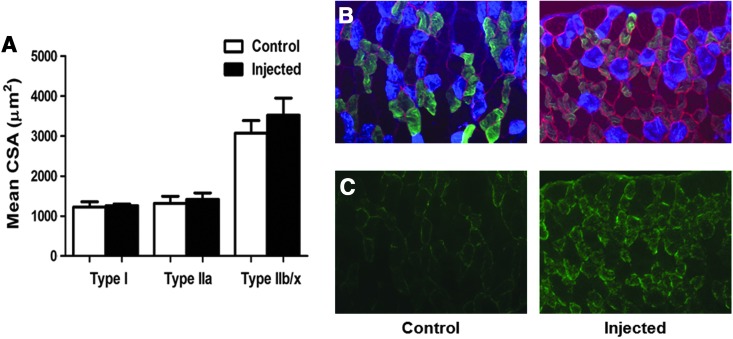
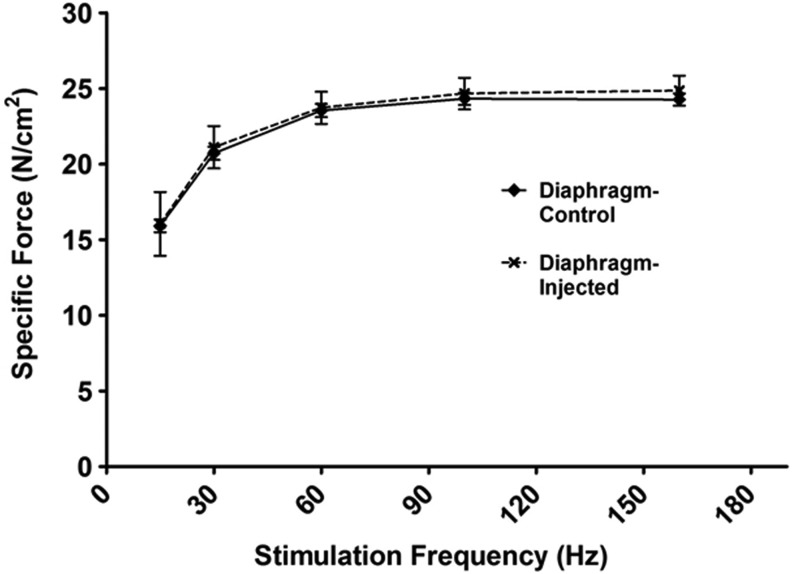
Diaphragm samples were stained with hematoxylin and eosin and were examined using light microscopy to determine whether injection of the diaphragm results in significant fiber damage. Our results reveal that 4 weeks after injection, the appearance of the diaphragm fibers from injected animals does not differ from fibers of animals that were not subjected to direct intramuscular injection (Fig. 4C and D). Additionally, we measured both diaphragm fiber cross-sectional area and the contractile properties of diaphragm strips to determine if direct intramuscular injection results in changes in muscle fiber size, fiber type distribution, and contractile function (Figs. 5 and 6). Our results confirm that direct injection of the diaphragm muscle results in no change in fiber size or in the fiber type distribution. Importantly, our results confirm that direct intramuscular injections does not alter diaphragm-specific force production at any stimulation frequency.
FIG. 5.
(A) Fiber cross-sectional area in diaphragm muscle myofibers expressing MHC I (type I), MHC IIa (type IIa), and MHC IIb/IIx (type IIb/IIx). Representative fluorescent staining of MHC I (DAPI filter/blue), MHC IIa (FITC filter/green), and dystrophin (Rhodamine filter/red) proteins in diaphragm samples are shown to the right of the graph (n=6/group). Control animals represent acutely anesthetized animals receiving no treatment. Injected animals represent animals receiving intramuscular diaphragm injections of GFP. Values are mean±standard error. No differences existed between groups. (B) Representative images depicting fiber type distribution of control and injected diaphragms. In these images, blue fibers represent MHC type I fibers, green fibers represent MHC type IIa fibers, and nonstained fibers represent MHC type IIb/x fibers. (C) Representative images demonstrating fiber type localization of GFP. MHC, myosin heavy chain.
FIG. 6.
Diaphragmatic force–frequency response (in vitro) (n=6/group). Control animals represent acutely anesthetized animals receiving no treatment. Injected animals represent animals receiving intramuscular diaphragm injections of GFP. Values are mean±standard error. No differences existed between groups.
Discussion
The diaphragm is the primary muscle of inspiration, and diaphragm dysfunction can result in increased morbidity and mortality in a variety of conditions. Therefore, identifying methods to directly treat diaphragm dysfunction is important. In this regard, the rat is an important animal model for performing experiments to explore diaphragm weakness because the anatomical features and function of the rat diaphragm are similar to the human diaphragm. In addition, the fiber type composition of the rat and human diaphragm is similar (Mizuno, 1991; Powers et al., 1997). Therefore, the rat is a useful animal model to develop effective treatment methods that can later be translated into human medicine. In this regard, we have demonstrated for the first time that the rat diaphragm can be effectively transduced by administering rAAV through direct intramuscular injection. This technique is simple and highly reproducible, results in high transduction, and does not induce significant damage to diaphragm muscle fibers.
Many conditions, including various forms of muscular dystrophy, chronic obstructive pulmonary disease, and prolonged mechanical ventilation, result in progressive muscle degeneration of the diaphragm (Poole et al., 1997; Hussain, 1998; Powers et al., 2009; Byrne et al., 2011). While gene transfer has attracted increasing attention as a component of treatment for many disorders and as a method for studying muscle-signaling pathways in limb skeletal muscle, there is currently no well-established technique to confer long-term gene expression into the rat diaphragm. A key consideration in gene modulation is the effectiveness of the method. In this regard, while gene transfer using viral and nonviral DNA vectors and oligonucleotides has been studied extensively, the efficiency of these methods is often limited (Davis et al., 1995; Aihara and Miyazaki, 1998; Mir et al., 1998). This handicap has caused many investigators to employ pharmacological inhibitors to study specific signaling pathways in the diaphragm. Unfortunately, many pharmacological inhibitors have off-target effects, which compromise the experimental findings. Additionally, many groups currently use plasmid-mediated gene transfection to increase expression of target genes within skeletal muscle. This method of gene transfection can result in several problems. In regard to the diaphragm, the anatomical structure and location of the diaphragm does not permit effective electroporation to optimize muscle delivery and expression. Finally, there are many novel genetic mouse models that utilize inducible muscle-specific promoters to upregulate or knockdown genes of interest in skeletal muscle that potentially reduces the risk of life-long genetic alterations. However, the disadvantage to this technique is the amount of time necessary to produce the animals as well as the cost associated with this process. In addition, the efficiency of this technique in the rat is not well characterized. Recently, zinc finger nucleases and transcription activator-like effector nuclease-mediated rat models have become available and have the potential to translate into clinical applications. However, these are high-cost methods that also have the potential for off-target effects (Gaj et al., 2013). Therefore, an alternative technique is necessary for use in rat experimental models.
In this regard, the use of virus-mediated transduction is a valuable alternative to the methods described above. Previous studies using mice have demonstrated that virus-mediated transduction is a practical and efficient technique to deliver transgenes to mice. Systemic administration of rAAV vectors has repeatedly shown diaphragm transduction; however, the serotype, promoter, and animal model have resulted in a wide range of success (Gregorevic et al., 2004, 2008; Yue et al., 2008; Wang et al., 2009; Yu et al., 2009; Kornegay et al., 2010). Regional delivery of rAAV has resulted in successful transduction of the murine diaphragm (Bish et al., 2008; Falk et al., 2013), and an alternative route using a gel-based delivery method directly administered to the diaphragm demonstrated high efficiency of gene transfer (Mah et al., 2004, 2010). For direct diaphragm administration, Matecki et al. (2004) demonstrated that injection of the mouse diaphragm with an adenovirus resulted in expression in∼20% of diaphragm skeletal muscle fibers. Finally, Grose et al. (2012) demonstrated transduction of dysferlin into the diaphragm of dysferlin-deficient mice using a single direct injection of rAAV5. This single injection resulted in some correction to the functional deficits in the diaphragm, which demonstrates the administration of a functional protein to the diaphragm via direct injection. These studies formed the basis for the development of a method of treatment to incorporate rAAV vectors to confer expression in the entire rat diaphragm muscle.
In this study, using an rAAV vector demonstrates that directly injecting the diaphragm is an effective way to achieve gene transduction throughout the entire costal diaphragm in the rat. Specifically, our work demonstrates that direct diaphragm injection using rAAV was highly effective, as ∼80% of the diaphragm muscle fibers expressed the transgene. Additionally, we determined the approximate area of the diaphragm affected by each injection and optimized the protocol to maximize gene expression throughout the costal diaphragm. Finally, we also demonstrated the specificity of our methods and established that direct injection of the diaphragm allows for high levels of gene expression in the diaphragm, while other tissues are not affected. These results confirm that this method allows for a safe, effective, and tissue-specific means to study both diseases that affect the diaphragm as well as key muscle signaling pathways in the rat diaphragm.
Finally, our data demonstrate that directly injecting the diaphragm with rAAV does not affect diaphragm fiber morphology, phenotype, or function. Indeed, animals whose diaphragms were directly injected with rAAV did not differ from diaphragms from noninjected control animals in diaphragm muscle fiber size and contractile function. These observations indicate that direct diaphragm injection of rAAV is a safe method of introducing a target gene into skeletal muscle.
Limitations and Conclusions
We have demonstrated for the first time that direct intramuscular injection into the diaphragm is an efficient way to administer rAAV vectors, which allows for specific treatment of the diaphragm. This study highlights the benefits of this technique compared with the current conventional approaches. Nonetheless, several limitations remain with the use of rAAV. Specifically, compared with plasmid DNA, rAAV has a relatively small packaging capacity (∼4.7 kb). This necessitates the use of truncated forms of many proteins to successfully package them (Ruegg and Glass, 2011). Additionally, while rAAV vectors are efficiently delivered into the nucleus and can target chromosomal loci to introduce defined sequence changes with high fidelity, they are relatively inefficient at targeting silent loci as well as unable to target nondividing cells and also have a low potential for random integration (Khan et al., 2011). Furthermore, while direct diaphragm injection appears to be tissue specific and safe, there is a small radius of diffusion of vector particles after intramuscular injection that restricts therapeutic gene transfer to an area surrounding the injection site, which necessitates multiple injections to deliver the vector to the entire diaphragm. However, we have shown that multiple injections are feasible and cause no alterations to the normal diaphragm structure or contractile function. Therefore, the evidence presented in this report indicates that direct diaphragm injection has distinct advantages compared with other currently utilized gene transfer methodologies. Indeed, key advantages include the ability of direct intramuscular diaphragm injections to specifically target the diaphragm along with the ability to express the gene of interest throughout the entire width and thickness of the rat diaphragm. This is beneficial as it is currently unknown whether previous gene delivery techniques (i.e., gel delivery) can effectively transduce the entire thickness of the rat diaphragm.
In conclusion, this study provides the first evidence that the rat diaphragm can be effectively transduced by directly injecting an rAAV vector. This technique provides a highly effective and safe way to alter gene expression within the diaphragm of rats to study both disease and signaling pathways. In addition, gene transfer methodology creates the option to specifically target pathways by providing a means to upregulate or knock down key targets. This method represents a novel way in which animal studies can mirror clinical trials and represents an efficient means for delivery of therapeutic genes to the diaphragm to combat disuse and disease-induced diaphragm weakness.
Acknowledgments
This work was supported by the National Institutes of Health RZ1 AR063805 awarded to SKP.
Author Disclosure Statement
No competing financial interests exist.
References
- Aihara H., and Miyazaki J. (1998). Gene transfer into muscle by electroporation in vivo. Nat. Biotechnol. 16, 867–870 [DOI] [PubMed] [Google Scholar]
- Bish L.T., Sleeper M.M., et al. (2008). Percutaneous transendocardial delivery of self-complementary adeno-associated virus 6 achieves global cardiac gene transfer in canines. Mol. Ther. 16, 1953–1959 [DOI] [PubMed] [Google Scholar]
- Boriek A.M., Rodarte J.R., et al. (2001). Shape and tension distribution of the passive rat diaphragm. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280, R33–R41 [DOI] [PubMed] [Google Scholar]
- Bowles D.E., McPhee S.W., et al. (2012). Phase 1 gene therapy for Duchenne muscular dystrophy using a translational optimized AAV vector. Mol. Ther. 20, 443–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne B.J., Falk D.J., et al. (2011). Pompe disease gene therapy. Hum. Mol. Genet. 20, R61–R68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark K.R., Sferra T.J., et al. (1997). Recombinant adeno-associated viral vectors mediate long-term transgene expression in muscle. Hum. Gene Ther. 8, 659–669 [DOI] [PubMed] [Google Scholar]
- Davis H.L., Michel M.L., et al. (1995). Use of plasmid DNA for direct gene transfer and immunization. Ann. NY Acad. Sci. 772, 21–29 [DOI] [PubMed] [Google Scholar]
- Falk D.J., Mah C.S., et al. (2013). Intrapleural administration of AAV9 improves neural and cardiorespiratory function in Pompe disease. Mol. Ther. 21, 1661–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher K.J., Jooss K., et al. (1997). Recombinant adeno-associated virus for muscle directed gene therapy. Nat. Med. 3, 306–312 [DOI] [PubMed] [Google Scholar]
- Gaj T., Gersbach C.A., et al. (2013). ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 31, 397–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorevic P., Blankinship M.J., et al. (2004). Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat. Med. 10, 828–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorevic P., Blankinship M.J., et al. (2008). Systemic microdystrophin gene delivery improves skeletal muscle structure and function in old dystrophic mdx mice. Mol. Ther. 16, 657–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose W.E., Clark K.R., et al. (2012). Homologous recombination mediates functional recovery of dysferlin deficiency following AAV5 gene transfer. PLoS One 7, e39233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain S.N. (1998). Respiratory muscle dysfunction in sepsis. Mol. Cell Biochem. 179, 125–134 [DOI] [PubMed] [Google Scholar]
- Inagaki K., Fuess S., et al. (2006). Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol. Ther. 14, 45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler P.D., Podsakoff G.M., et al. (1996). Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc. Natl. Acad. Sci. USA 93, 14082–14087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan I.F., Hirata R.K., et al. (2011). AAV-mediated gene targeting methods for human cells. Nat. Protoc. 6, 482–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornegay J.N., Li J., et al. (2010). Widespread muscle expression of an AAV9 human mini-dystrophin vector after intravenous injection in neonatal dystrophin-deficient dogs. Mol. Ther. 18, 1501–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah C., Fraites T.J. Jr., et al. (2004). A new method for recombinant adeno-associated virus vector delivery to murine diaphragm. Mol. Ther. 9, 458–463 [DOI] [PubMed] [Google Scholar]
- Mah C.S., Falk D.J., et al. (2010). Gel-mediated delivery of AAV1 vectors corrects ventilatory function in Pompe mice with established disease. Mol. Ther. 18, 502–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matecki S., Dudley R.W., et al. (2004). Therapeutic gene transfer to dystrophic diaphragm by an adenoviral vector deleted of all viral genes. Am. J. Physiol. Lung Cell Mol. Physiol. 287, L569–L576 [DOI] [PubMed] [Google Scholar]
- McClung J.M., Kavazis A.N., et al. (2007). Caspase-3 regulation of diaphragm myonuclear domain during mechanical ventilation-induced atrophy. Am. J. Respir. Crit. Care Med. 175, 150–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool F.D., and Tzelepis G.E. (2012). Dysfunction of the diaphragm. N. Engl. J. Med. 366, 932–942 [DOI] [PubMed] [Google Scholar]
- Mendell J.R., Rodino-Klapac L.R., et al. (2009). Limb-girdle muscular dystrophy type 2D gene therapy restores alpha-sarcoglycan and associated proteins. Ann. Neurol. 66, 290–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell J.R., Rodino-Klapac L.R., et al. (2010). Sustained alpha-sarcoglycan gene expression after gene transfer in limb-girdle muscular dystrophy, type 2D. Ann. Neurol. 68, 629–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir L.M., Bureau M.F., et al. (1998). Long-term, high level in vivo gene expression after electric pulse-mediated gene transfer into skeletal muscle. C. R. Acad. Sci. III 321, 893–899 [DOI] [PubMed] [Google Scholar]
- Mizuno M. (1991). Human respiratory muscles: fibre morphology and capillary supply. Eur. Respir. J. 4, 587–601 [PubMed] [Google Scholar]
- Muzyczka N. (1992). Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr. Top. Microbiol. Immunol. 158, 97–129 [DOI] [PubMed] [Google Scholar]
- Pacak C.A., and Byrne B.J. (2011). AAV vectors for cardiac gene transfer: experimental tools and clinical opportunities. Mol. Ther. 19, 1582–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacak C.A., Mah C.S., et al. (2006). Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo. Circ. Res. 99, e3–e9 [DOI] [PubMed] [Google Scholar]
- Poole D.C., Sexton W.L., et al. (1997). Diaphragm structure and function in health and disease. Med. Sci. Sports Exerc. 29, 738–754 [DOI] [PubMed] [Google Scholar]
- Powers S.K., Demirel H.A., et al. (1997). Myosin phenotype and bioenergetic characteristics of rat respiratory muscles. Med. Sci. Sports Exerc. 29, 1573–1579 [DOI] [PubMed] [Google Scholar]
- Powers S.K., Kavazis A.N., et al. (2009). Prolonged mechanical ventilation alters diaphragmatic structure and function. Crit. Care Med. 37, S347–S353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers S.K., Hudson M.B., et al. (2011). Mitochondria-targeted antioxidants protect against mechanical ventilation-induced diaphragm weakness. Crit. Care Med. 39, 1749–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid M.B. (2008). Free radicals and muscle fatigue: of ROS, canaries, and the IOC. Free Radic. Biol. Med. 44, 169–179 [DOI] [PubMed] [Google Scholar]
- Ruegg M.A., and Glass D.J. (2011). Molecular mechanisms and treatment options for muscle wasting diseases. Annu. Rev. Pharmacol. Toxicol. 51, 373–395 [DOI] [PubMed] [Google Scholar]
- Segal S.S., White T.P., et al. (1986). Architecture, composition, and contractile properties of rat soleus muscle grafts. Am. J. Physiol. 250, C474–C479 [DOI] [PubMed] [Google Scholar]
- Smith B.K., Collins S.W., et al. (2013). Phase I/II trial of adeno-associated virus-mediated alpha-glucosidase gene therapy to the diaphragm for chronic respiratory failure in Pompe disease: initial safety and ventilatory outcomes. Hum. Gene Ther. 24, 630–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B., Young S.P., et al. (2008). Correction of multiple striated muscles in murine Pompe disease through adeno-associated virus-mediated gene therapy. Mol. Ther. 16, 1366–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Li J., et al. (2009). Systemic human minidystrophin gene transfer improves functions and life span of dystrophin and dystrophin/utrophin-deficient mice. J. Orthop. Res. 27, 421–426 [DOI] [PubMed] [Google Scholar]
- Yu C.Y., Yuan Z., et al. (2009). A muscle-targeting peptide displayed on AAV2 improves muscle tropism on systemic delivery. Gene Ther. 16, 953–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y., Ghosh A., et al. (2008). A single intravenous injection of adeno-associated virus serotype-9 leads to whole body skeletal muscle transduction in dogs. Mol. Ther. 16, 1944–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolotukhin S., Potter M., et al. (2002). Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods 28, 158–167 [DOI] [PubMed] [Google Scholar]