Abstract
We tested the performance of DNA barcoding in Acridoidea and attempted to solve species boundary delimitation problems in selected groups using COI barcodes. Three analysis methods were applied to reconstruct the phylogeny. K2P distances were used to assess the overlap range between intraspecific variation and interspecific divergence. “Best match (BM)”, “best close match (BCM)”, “all species barcodes (ASB)” and “back-propagation neural networks (BP-based method)” were utilized to test the success rate of species identification. Phylogenetic species concept and network analysis were employed to delimitate the species boundary in eight selected species groups. The results demonstrated that the COI barcode region performed better in phylogenetic reconstruction at genus and species levels than at higher-levels, but showed a little improvement in resolving the higher-level relationships when the third base data or both first and third base data were excluded. Most overlaps and incorrect identifications may be due to imperfect taxonomy, indicating the critical role of taxonomic revision in DNA barcoding study. Species boundary delimitation confirmed the presence of oversplitting in six species groups and suggested that each group should be treated as a single species.
Introduction
Acridoidea is the largest group in Orthoptera, with more than 1000 species known from China to date. However, imperfect taxonomy may exist in many species groups, especially in those with reduced tegmina. Many brachypterous and apterous new species have been described from China during the last thirty years based on minor differences which might in fact be normal intraspecific variations, and some of them are extremely close geographically to their nearest relatives, resulting in a possible oversplitting, i.e. morphotypes are inappropriately recognized as species [1]. Some efforts have been made to resolve these issues based on morphological study [2]–[4]. Of course, cryptic species may exist in some groups and additional evidence is needed to distinguish them from known close relatives.
Acrididae is the largest family in Acridoidea, including 24 subfamilies and some genera unassigned to any subfamily [5]. However, some family names, such as Catantopidae [6], Oedipodidae, Arcypteridae [7] and Gomphoceridae [8], have been defined from those subfamilies. Molecular evidence has long been used to resolve the phylogeny, evolutionary history and taxonomy of Acridoidea at different hierarchical levels. Besides some researches that discussed the monophyly of Caelifera as well as Acridoidea [9]–[21], there were also many investigations exploring the phylogenies and origins of their subordinate categories, including Acrididae [22]–[23], Pamphagidae [24]–[25], Catantopidae [26]–[28], Oedipodidae [29]–[30], Arcypteridae [31], Melanoplinae [32]–[35], Podisminae [36], Oedipodinae [37]–[39], Gomphocerinae [39]–[41], Proctolabinae [42], and so on. Because of the difference in taxon sampling, selection of genetic markers and analytical strategy, the results were frequently inconsistent to some extent among different case studies and the phylogenies remain unresolved in many taxa.
DNA barcoding is a diagnostic technique in which short DNA sequence(s) can be used for species identification [43]. It has attracted extensive attention from taxonomists all over the world. Using the standard 658-base fragment of the 5′ end of the mitochondrial gene cytochrome c oxidase subunit I (COI, cox1) as the barcode region for animals [44], the proponents of the approach suggest that at least 98% of congeneric species pairs of animals can be correctly identified through DNA barcoding [45]. Besides assigning specimens to known species, DNA barcoding also aids to highlight potential cryptic, synonymous and extinct species [46]–[49] as well as to match adults with immature specimens [50]. However, a lot of factors can affect the success rate of DNA barcoding, for example, the selection of gene fragment(s) [51], control of numts [52]–[53], monophyly of the known species [1], analytical methods [54]–[59], success rate of barcode amplification and sequencing [60], and so on. The effectiveness of barcoding is critically dependent upon species delineation [1]. Funk and Omland [61] found that about 23% of surveyed metazoan species were genetically polyphyletic or paraphyletic. Nearly all failures of species identification through DNA barcoding in cowries could be attributed to imperfect taxonomy (overlumping or overspliting) or incomplete lineage sorting [1], indicating that comprehensive taxonomic revision (species boundary delimitation) is crucial in the DNA barcoding studies.
Although there are a lot of successful examples in most insect orders using DNA barcoding to identify species [62]–[66], we have found only four studies concerning DNA barcoding of Acridoidea, one with positive result [67], one with a failed result [68], and two cases focused on the distribution patterns and coamplification of numts and their effects on DNA barcoding [52]–[53]. López et al [69] discussed the species boundary delimitation of threatened grasshopper species from the Canary Islands using both mitochondrial COI and nuclear ITS2 sequences.
To test the performance of DNA barcoding in Acridoidea on a larger scale, we examined patterns of CO1 divergence in 72 species/subspecies of grasshoppers mostly from China (Schistocerca americana and Chorthippus parallelus are not distributed in China and their sequences were downloaded from GenBank).
Taxon sampling was mainly focused on the following eight morphologically similar but taxonomically questionable species groups: (1) Sinopodisma houshana+S. lushiensis+S. qinlingensis; (2) Pedopodisma tsinlingensis+P. funiushana+P. wudangshanensis; (3) Fruhstorferiola kulinga+F. huayinensis; (4) Shirakiacris shirakii+F. yunkweiensis; (5) Spathosternum prasiniferum prasiniferum+S. p. sinense; (6) Calliptamus italicus+C. abbreviatus; (7) Oedaleus decorus+O. asiaticus; (8) Oedaleus infernalis+O. manjius.
For the first four groups, there is nearly no morphological difference found between species within each group, and the only criterion that can be used to identify species is geographical distribution information. Each group should be regarded as the same species according to the extremely high morphological similarity. Shirakiacris yunkweiensis was synonymized with S. shirakii [70], but this has not yet been accepted by Chinese acridologists, although there is no additional evidence to support their decision to retain the species status of S. yunkweiensis [6]. Pedopodisma, a genus endemic to China, was synonymized with Sinopodisma [71], but Storozhenko's treatment has not been accepted by Chinese acridologists [6] because tegmina are completely absent in Pedopodisma but distinct though reduced in Sinopodisma.
Spathosternum sinense was originally described as an independent species [72], then reduced to a race of S. prasiniferum [73], and even synonymized with the latter [74]. However, Grunshaw's synonymy was not accepted by other orthopterists, and S. sinense has been recognized as a subspecies of S. prasiniferum until now [5]. S. prasiniferum prasiniferum and S. p. sinense are usually distinguished from each other by their tegmen length, the former with tegmina reaching or slightly exceeding the apices of hind femora and the latter usually only reaching the middle of the hind femora. They may be allopatric and it remains unclear whether there is an overlap distributional zone. However, we did collect a few female individuals with long tegmina from the population of S. p. sinense, but never found males with this phenotype. It is not clear to which species/subspecies, sinense or prasiniferum, these rare individuals with long tegmina but from the population of S. p. sinense should be assigned. Whether these two taxa are species or subspecies requires clarification.
Calliptamus italicus is widely distributed in Europe, north Africa, central and west Asia as well as northwest China (Xinjiang and Qinghai Province). C. abbreviatus is distributed in north Asia, Korea and most provinces of China [6]. It seems that there is nearly no geographical overlap between these two species and geographical information can be used to assign individuals to species correctly most of the time. However, incompatibility occurred when using external morphological characters to identify the specimens. Generally, the length of tegmina is the main character distinguishing these two species from each other. C. italicus has tegmina reaching or exceeding the apices of the hind femora, but C. abbreviatus has tegmina much shorter and distinctly not reaching the apices of the hind femora [6]. When examining specimens from all over China, we found that most specimens from localities south of theYangtze River had their tegmina obviously not reaching the apices of the hind femora and can be assigned to C. abbreviatus without any doubt. However, those from localities north of the Yangtze River had their tegmina reaching or distinctly exceeding the apices of the hind femora, and should be assigned to C. italicus. Therefore, either the distribution range of C. italicus should be extended to most provinces of north China from Xinjiang according to the morphological identification, or the taxonomic status of C. abbreviatus represented by populations in north China should be supported by additional evidence to clarify the incompatibility between the implications of morphological identification and geographical distribution ranges.
For the remaining two groups, the minor morphological differences between species within each group have usually been considered as normal variations among individuals from different geographical populations.
In this study, the overlap range between intraspecific variation and interspecific divergence was analyzed, the success rate of species identification was tested using “best match (BM)”, “best close match (BCM)”, “all species barcodes (ASB)” and “back-propagation neural networks (BP-based method)”, and species boundary delimitations of the eight species group were discussed based on the COI barcode sequences.
Materials and Methods
Taxon sampling
A total of 466 sequences from different individuals representing 4 superfamilies 5 families 49 genera and 72 species/subspecies were analyzed. Of these sequences, 20 were downloaded from GenBank (http://www.ncbi.nlm.nih.gov/) (Table S1), either COI fragments completely overlapped with the 658-bp Folmer region [75] or extracted from the published complete mitochondrial genome. At least five individuals from each population and as many populations as possible of the widespread species were sampled whenever the specimens were available (Table S2). However, sometimes only one or two individuals per species (or locality) could be collected. All specimens were preserved in 100% ethanol and stored at room temperature.
DNA extraction, PCR amplification and sequencing
Whole genomic DNA was extracted from muscle tissue of the hind femur using a routine phenol/chloroform method [76]. Using the degenerated primer pair designed for Orthoptera [67], COBU (5′-TYTCAACAAAYCAYAARGATATTGG-3′) and COBL (5′-TAAACTTCWGGRTGWCCAAARAATCA-3′), the 658-bp fragments were amplified from the 5′ region of the cytochrome c oxidase 1 (COI) gene that has been adopted as the standard barcode for members of the animal kingdom [44].
The 25 µl PCR reaction mixture contained 13.875 µl of ultrapure water, 2.5 µl of 10× PCR buffer (Mg2+ free), 2.5 µl of MgCl2 (25 mM), 2 µl of dNTP (2.5 mM), 1.5 µl of each primer (0.01 mM), 0.125 µl of TaKaRa r-Taq polymerase, and 1 µl of DNA template. Amplifications were performed using a TaKaRa PCR Thermal Cycler. The cycling protocol consisted of an initial denaturation step at 95°C for 5 min, followed by 30–35 cycles of denaturation at 94°C for 45 s, annealing at 48°C for 45 s and extension at 70°C for 1 min 30 s, and a final extension at 72°C for 10 min and then held at 4°C. PCR products were used directly for sequencing after purification. Sequencing primers were the same as those for PCR amplification. Products were labeled using the BigDye® Terminator v.3.1 Cycle Sequencing Kit (Applied Biosystems, Inc.) and sequenced bidirectionally using an ABI PRISM™ 3100-Avant Genetic Analyzer.
Sequence assembly and alignment
The raw sequence files were assembled using the Staden Package [77]. The assembled sequences were aligned using Clustal X [78], and both ends of the sequences matching the primer sequences were excised to remove artificial nucleotide similarity introduced by PCR amplification, resulting in the final length of 658 bp for both phylogenetic and barcoding analysis. COI nucleotide sequences were translated to amino acid sequences to check for stop codons and shifts in reading frame that might indicate the presence of nuclear mitochondrial copies (numts) [68]. Haplotype nucleotide sequences are deposited in GenBank (KC139803—KC140101, Table S3). Each haplotype was blasted using MEGABLAST option against the nucleotide collection (nr/nt), available on the NCBI website (http://blast.ncbi.nlm.nih.gov/Blast.cgi?CMD=Web&PAGE_TYPE=BlastHome). Only haplotypes that blasted within the correct suborder with E-values≤1.00E-30 were included in this study [53].
Phylogeny reconstruction
To test the resolution of COI barcode sequences in reconstructing the phylogeny of grasshoppers at different taxonomic levels, we performed analyses in both parsimony and Bayesian frameworks with the following 4 species as outgroups: Yunnanites coriacea and Atractomorpha sinensis (Pyrgomorphoidea), Bennia multispinata (Eumastacoidea) and Criotettix bispinosus (Tetrigoidea).
Within the parsimony framework, the aligned sequence data were analyzed by using heuristic search algorithms with 100 random addition replicates implemented in PAUP 4.0 beta 10 win [79]. To assess support, we calculated standard bootstrap values based on 100 replicates (100 random-addition TBR replicates each).
Within the Bayesian framework, we analyzed the data sets by using the program MrBayes 3.1.2 [80] after selecting best-fit models of nucleotide evolution under the AIC criteria by using MrModeltest 3.7 [81]. The analyses consisted of running four simultaneous chains for 20 million generations (TVM+I+G), sampling every 1000 generations. Two independent identical Bayesian runs were performed to ensure convergence on similar results and the nodal support was assessed by using the posterior probability generated from a consensus tree of the trees sampled after burn-in. The amount of burn-in is the number of samples that will be discarded at the start of the run. Tracer 1.5 (http://beast.bio.ed.ac.uk) was used to view the point where the chain reached stationary and then the burn-in was determined by the number of samples before this point.
To provide a profile for the setup of taxa and groups for calculating genetic distances, a neighbor-joining (NJ) tree of K2P distances was created to provide a graphic representation of the patterning of divergence between species [82] because of its strong track record in the analysis of large species assemblages [83]. NJ tree building with 1000 bootstrap replicates was implemented in MEGA4.1 [84].
Network analysis
Traditional bifurcating trees are less powerful for resolving relationship among intraspecific populations and closely related species than haplotype networks which can provide significant inferences about evolutionary relationships [85]–[86]. Therefore, we constructed haplotype networks for the eight aforementioned species groups. The construction of haplotype networks was implemented in TSC1.21 [87].
Intraspecific variation, interspecific divergence and DNA barcoding overlap
Sequence divergences were calculated using the Kimura two parameter (K2P) distance model [88]–[89]. The calculation of the sequence divergences was implemented in MEGA4.1 [84]. From the sequence divergence data, the extent of DNA barcoding gap/overlap was then explored as typically done in barcoding studies [1].
Species assignment with distance-based methods and the neural network approach
Distance-based methods of species assignments in conjunction with computer simulations are capable of determining the statistical significance of species identification success rates. We therefore performed the “best match (BM)” and “best close match (BCM)” [90] for the species with more than three individuals sampled, utilizing “single-sequence-omission” or “leave-one-out” simulation. The BCM identification protocol first identifies the best barcode match of a query, but only assigns the species name of that barcode to the query if the barcode is sufficiently similar. This approach requires a threshold similarity value that defines how similar a barcode match needs to be before it can be identified. Such a value could be estimated for a given data set by obtaining a frequency distribution of all intraspecific pairwise distances and determining the threshold distance below which 95% of all intraspecific distances are found. After the BCM analysis, a more rigorous application of the best close match strategy, all species barcodes (ASB), was implemented. Here information from all intraspecies barcodes in the reference data set was utilized instead of just focusing on the barcode that is most similar to the query. A list of all barcodes sorted by similarity to the query using the same threshold as for BCM was assembled for each query. Queries were considered a success when they were followed by all intraspecies barcodes as long as there were at least two barcodes for the species. With this approach, the identifier will be more confident about assigning this species name to the query than in cases where multiple species names are found on the list of best matches. Indeed, a conservative identifier would probably only assign a species name if the query is followed by all known barcodes for a particular species and insist that there are at least two intraspecies matches. The BM, BCM and ASB approaches are implemented in the computer program TaxonDNA (available at http://taxondna.sf.net/) [90].
Taking both accuracy and precision into account, we calculated an ad hoc distance threshold for our data set using a simple linear regression [91]. The query results were subdivided into (1) true positives (TP), (2) false positives (FP), (3) true negatives (TN), and (4) false negatives (FN) [91]. The performances of BCM were quantified by calculating accuracy ((TP+TN)/total number of queries) and precision (TP/(number of not-discarded queries)) as well as the overall identification (ID) error ((FP+FN)/total number of queries) and the relative ID error (FP/number of not-discarded queries). Variations in the proportions of TP, TN, FP, FN, accuracy, precision, overall and relative ID errors were quantified for 30 arbitrary K2P distance thresholds (THRK2P) ranging from THRK2P = the largest query-best match K2P distance in a library (all queries are accepted as being correctly identified, i.e. none is discarded as in the BM criterion) to THRK2P = 0.00 (only identical sequences are accepted as being correctly identified, all the others are discarded). Relationships between relative ID errors and K2P distance thresholds were investigated through linear regression. The regression equation was then used to infer an ad hoc distance threshold corresponding to the K2P distance yielding a relative ID error<0.05 (THRK2P_0.05). This ad hoc threshold corresponds to the K2P distance at which 95% of the not-discarded queries are expected to be correctly identified. The analysis was performed by Dr. Gontran Sonet using a script in R by himself.
Back-propagation neural network-based species identification (BP-based method) is a recently proposed method for DNA barcoding [92]–[93]. The BP-based method proved to be powerful in species assignments via DNA sequences, especially for closely related species [93]. We performed BP analysis for the same data set as we did BCM analysis. The data set was randomly divided into a reference data set and a query data set. The reference data set was used to train a BP-neural network model, while the query data set was used as a test data set. The ratio of reference sequences to query sequences was about 1∶1 since increasing the reference sequences did not significantly improve species identification success rate for the COI barcode [60]. For all these simulations, the learning rate was set to 0.2, moment value to 0.5 and training goal to 0.00001, as implemented in the program BPSI2.0 [92].
Results
Phylogeny reconstruction
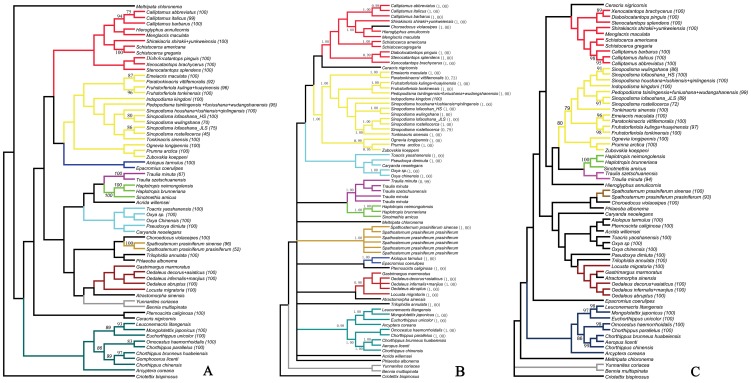
Both parsimony and Bayesian analyses of our data set consistently recovered several monophyletic clades above genus levels (Fig. 1A, 1B). However, the relationships among these clades could not be resolved well, and none of the Catantopidae, Oedipodidae, Arcypteridae or Gomphoceridae were supported as monophyletic (Fig. S1). This result is consistent with earlier studies [13]–[15].
Figure 1. Phylogenetic analysis based on mtDNA COI in parsimony, Bayesian and neighbour-joining (K2P) frameworks.
Subclades within a species with more than one individual sampledare collapsed. The number in parentheses indicates its bootstrap support values or posterior probabilities. Colored blocks of branches indicate the clades consistently recovered in parsimony, Bayesian and NJ analyses. Bootstrap values of <75 and posterior probability of <0.95 are not recorded on the tree. A. Cladogram from parsimony analysis. B. Cladogram from Bayesian analysis. C. Cladogram from NJ analysis.
Excepting the Spathosternum and Calliptamus groups, each of the species groups we focused on was comprised of species that did not form reciprocally monophyletic clades. However, each of the six species groups usually formed a monophyletic clade most having high posterior probability and/or bootstrap values. In addition, Sinopodisma lofaoshana formed two distantly separated clades, with the clade of individuals from Jiulianshan population as a sister to the S. rostellocerca clade, and the clade of individuals from Hengshan population as a sister to the S. wulingshana clade. Traulia minuta included the single representative of T. szetschuanensis in the Bayesian tree but excluded it in the MP tree with a bootstrap value of less than 75%. Monophyletic clades of Paratonkinacris vittifemoralis were recovered both within the Bayesian and parsimony frameworks with posterior probability of 0.73 and bootstrap support of 92%.
Congeneric species formed monophyletic clades most of time in the case that two or more species were sampled from a genus. The exceptions were Sinopodisma into which the genus Tonkinacris fell, and Chorthippus which is a very difficult large grasshopper genus and needs a comprehensive revision (Fig. 1A, 1B). The genus Pedopodisma was recovered either as a sister to the Sinopodisma+Tonkinacris clade in the MP tree or as a sister to the Fruhstorferiola clade in the Bayesian tree.
The NJ analysis recovered a topology similar to those from parsimony and Bayesian analyses, with small differences in placements of a few species (Fig. 1C). All of the eight species groups we focused on formed monophyletic clades respectively, but individuals within each group, excluding groups of Spathosternum and Calliptamus, clustered neither by species nor populations (See the section on species boundary delimitation for the detailed clades).
To explore the further implications of the COI barcode fragment in reconstructing phylogeny at higher-levels, the data set was reanalyzed twice under MP framework with exclusion of third base data or both first and third base data. The results suggested that it seemed to have a little improvement in resolving higher-level relationships (Figs. S1, S2, S3). Although the higher-level relationships in the topologies were still not strongly supported and the monophylies of Catantopidae, Oedipodidae, Arcypteridae and Gomphoceridae were still not completely supported, most members of Catantopidae and Oedipodidae did show a closer relationship within family than between families (Figs. S2, S3).
Intraspecific variation, interspecific divergence and DNA barcoding gap
Based on the neighbor-joining (NJ) tree of K2P distances, taxa or groups were set up to calculate the intraspecific variations and interspecific divergences. The results showed that variations within population were mostly distinctly less or slightly larger than 1%, intraspecific variations between populations were usually less than 3% (Table S4), a putative threshold for species assignment proposed by previous study [44], with Sinopodisma lofaoshana as the single exception which had much higher intraspecific variation (5.06–5.56%, average 5.25%) between interpopulation individuals. Two populations of S. lofaoshana, one from Hengshan and the other from Jiulianshan, were sampled; the variations within population were less than 1% but those between populations were slightly higher than 5%. Consequently, S. lofaoshana was divided into two groups to calculate intergroup divergences. The results showed that the divergence between Jiulianshan populations of S. lofaoshana and S. rostellocerca was as low as 2.17–3.12% (average 2.53%), indicating a closer relationship between them than between the two groups of S. lofaoshana.
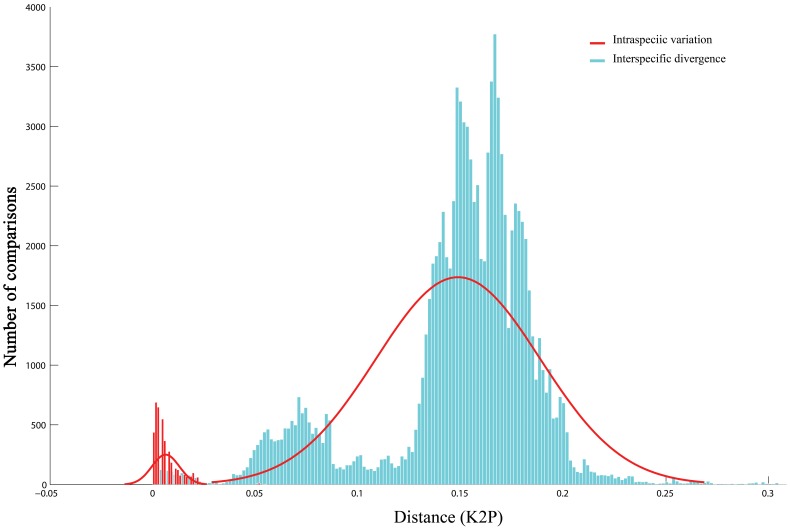
The interspecific divergences within each of the six species groups abovementioned (Table S4) and 22.5% of interspecific divergences within a genus were less than 3%, 77.5% of them more than 3%, and 68.19% of them more than 5% (Table S5), resulting in a total overlap of 5.55% (from 0.0% to 5.55%) between intraspecific variations and interspecific divergences (Fig. 2).
Figure 2. Distribution pattern of intraspecific variation and interspecific divergence.
Species assignment through BCM and BP-based methods
In the case of identification with the BCM method, we assembled a data set including 432 sequences belonging to 43 species, with each species including at least three sequences to meet the requirement for “all species barcodes (ASB)” analysis. The threshold value for the identification simulation was calculated from the data set. Using the 95th percentile of intraspecific distances (2%) computed from pairwise summary as the threshold for the BCM simulation, the correct identification according to “best close match (BCM)” is 91.66%, but that according to “all species barcodes (ASB)” is only 60.64% (Table 1). The 5.32% ambiguous and 2.77% incorrect identifications in BCM were all caused by the sequences from the six questionable species groups (Table S6). However, the much higher ambiguous identification in ASB analysis (38.88%) occurred also in query sequences of Sinopodisma lofaoshana and S. rostellocerca as well as in those of the six questionable species groups.
Table 1. Success rate of identification through “Best Match”, “Best Close Match” and “All Species Barcode”.
| Identification method | Best Match | Best Close Match | All Species Barcodes |
| Correct identifications | 397 (91.89%) | 396 (91.66%) | 262 (60.64%) |
| Ambiguous identifications | 23 (5.32%) | 23 (5.32%) | 168 (38.88%) |
| Incorrect identifications | 12 (2.77%) | 12 (2.77%) | 1 (0.23%) |
| Sequences without any match closer than 2.0% | ---- | 1 (0.23%) | 1 (0.23%) |
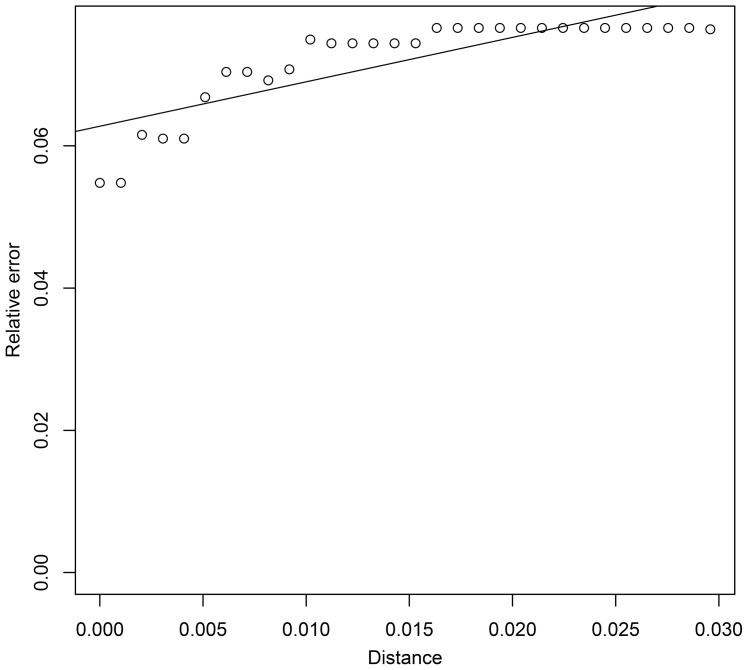
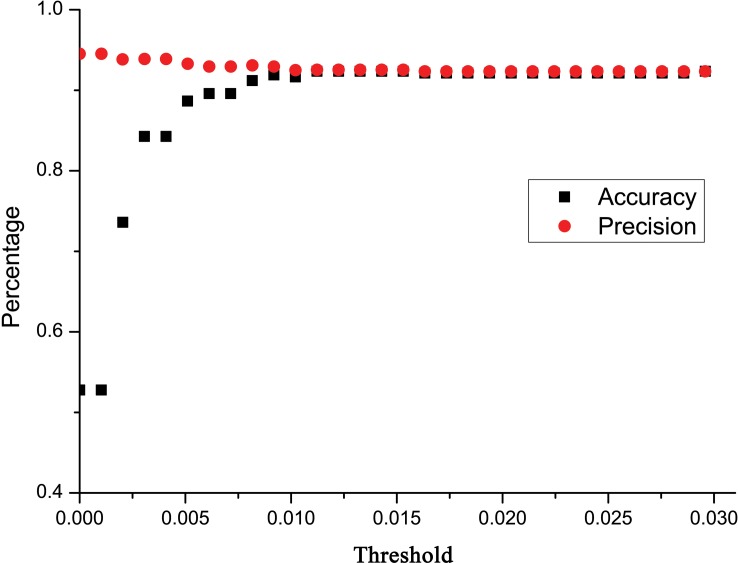
Taking both accuracy and precision into account, we calculated an ad hoc distance threshold for the data set using a simple linear regression [91]. Unfortunately, according to the analysis, we could not obtain an estimated relative identification error lower than 0.06 with the current data set (Fig. 3). According to the regression line, we would have to use a distance threshold of −0.02 to obtain an estimated relative identification error of 0.05, but a negative threshold is impossible to apply. By applying a threshold distance of 0, we can get the lowest estimated relative identification error possible with this data set, i.e. the intercept = 0.06. More restrictive distance thresholds will improve precision to some extent, yet negatively affect accuracy due to the higher proportions of queries discarded [91]. The use of more restrictive distance thresholds in our data set scarcely improved precision, but heavily decreased the accuracy when the threshold used was less than 0.01 (Fig. 4).
Figure 3. Relative identification errors at thirty arbitrary distance thresholds in BCM analysis.
Figure 4. Accuracy and precision of identification at thirty distance thresholds ranging from K2P = 0.0295 to K2P = 0.000 in BCM analysis.
Instead of using the leave-one-out simulation for BCM methods, we used randomly selected reference and query sequences for BP-based method to investigate the performance of the barcode. This strategy was employed due to the slow training process which hinders the utility of the BP-based method in large scale simulation studies. Using the same sequence data as in BCM analysis, we assembled a training data set with 230 barcode sequences and a query data set with 202 barcode sequences. As a result, 10 query sequences were identified incorrectly with extremely high probability, 1 identified incorrectly with low probability, and 3 identified correctly with low probability, resulting in a success rate of 94.55%. All incorrect or inaccurate identification occurred in the six questionable species groups (Table S7).
Species boundary delimitations for selected species groups
Calliptamus italicus species group
Since there were some doubts on the relationship between Calliptamus italicus and C. abbreviatus, we first assigned a species name to each sequence according to the localities of materials under study, i.e. the materials from Xinjiang and Qinghai were provisionally considered as C. italicus, and those from other areas of China were considered as C. abbreviatus. The results showed that C. bararus and individuals of C. italicus from Xinjiang and Europe (NC_011305) formed monophyletic clades in the NJ tree respectively, both with 100% bootstrap value. However, the individuals of C. italicus from Qinghai did not cluster with those from Xinjiang and Europe, but formed another monophyletic clade with the specimens of C. abbreviatus. Individuals in the clade of C. abbreviatus did not cluster by populations (Fig. 5A).
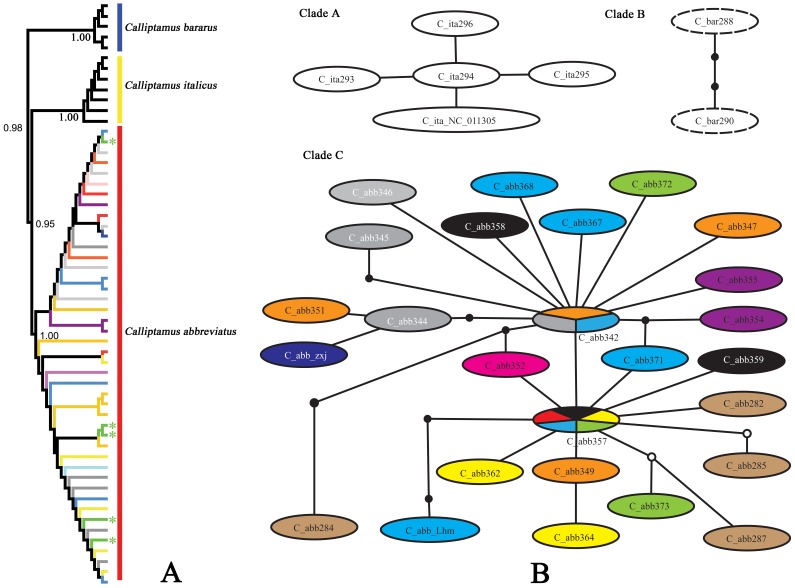
Figure 5. NJ tree subclade and haplotype network of three Calliptamus species.
A. NJ tree subclade. Different colors of the edges indicate individuals from different populations, the green asterisks indicate the individuals from Xunhua county, Qinghai Province. B. Haplotype network. Different colors indicate haplotypes from different populations, the ovals filled with multiple colors indicate haplotypes shared by several populations, and the green color represents haplotypes from Qinghai Province.
Analysis with haplotype network led to a similar result. The network was divided into three separate clades, Clade A, Clade B and Clade C (Fig. 5B). Clade A was composed of haplotypes of C. italicus from Xinjiang and Europe, Clade B consisted of haplotypes of C. barbarus. However, the haplotypes of C. italicus from Qinghai (marked with green color) formed clade C together with those of C. abbreviatus, suggesting that the individuals sampled from Qinghai in this study actually belong to C. abbreviatus. Nearly no haplotypes of C. abbreviatus from the same population formed a relatively independent subclade, and two haplotypes were found shared by multiple populations, one shared by three populations (Siping, Jilin Province; Tongliao, Inner Mongolia Autonomous Region; Qin'an, Gansu Province) and the other by five populations (Anji, Zhejiang Province; Zhuolu, Hebei Province; Zhidan, Shaanxi Province; Qin'an, Gansu Province; Xunhua, Qinghai Province) (Fig. 5B).
Therefore, it is clear that C. italicus and C. abbreviatus are two separate species. However, the tegmina length should not be regarded as distinguishing character between these two species since wing-length polymorphism is quite common in Acrididae [94]–[95]. As for the distribution of C. italicus in Qinghai, study with larger scale sampling is needed to clarify it. Possibly, C. italicus is prevented from extending its distribution to Qinghai by the barrier of the Altai Mountains and Qilian Mountains. It is also impossible for C. italicus to spread from Xinjiang to neighboring provinces such as Gansu and Inner Mongolia because of the barriers imposed by the Altai Mountains and deserts.
Sinopodisma houshana and Pedopodisma tsinlingensis groups
The species are extremely similar within each group. Nearly no morphological difference can be found among the species within each group except that the body size of Sinopodisma houshana is slightly larger than those of S. lushiensis and S. qinlingensis. All materials were collected from the type locality of each species except for those of S. houshana, which has a much more widespread distribution than S. lushiensis and S. qinlingensis. Therefore, there is no problem in assigning a morphospecies name to sampled individuals.
The topology of the NJ tree showed that species of S. houshana group were slightly structured genetically but no species formed independent reciprocally-monophyletic clades (Fig. 6A). The intraspecific variations and interspecific divergences formed a complete overlap, with the latter falling completely within the range of the former (Table 2). The relationship among species within the Pedopodisma tsinlingensis group was even more confused; sequences of the three species scattered messily in the NJ tree and no species showed conspicuous genetic structure. The intraspecific genetic variations and interspecific divergences formed a broad overlap of 2.17% (Table 3).
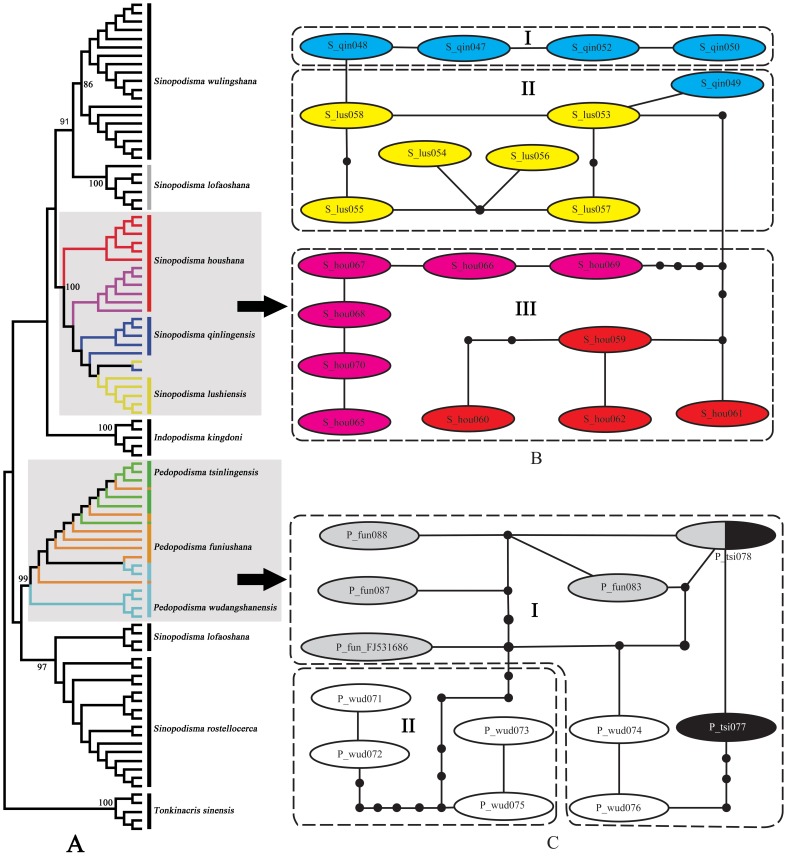
Figure 6. NJ tree subclade and haplotype network of the genera Sinopodisma and Pedopodisma.
A. NJ tree subclade. B. Haplotype network of the Sinopodisma houshana species group; red color indicates Yinshan population and pink indicates Mulanshan population of S. houshana. C. Haplotype network of the Pedopodisma tsinlingensis species group.
Table 2. Intraspecific variation and interspecific divergence of the Sinopodisma houshana group.
| Species | Intraspecific variation | Interspecific divergence | |
| S. houshana | S. lushiensis | ||
| S. houshana | 0∼2.17% | ||
| S. lushiensis | 0.15∼0.61% | 0.77∼1.85% | |
| S. qinlingensis | 0∼0.61% | 0.92∼1.70% | 0.15∼1.07% |
Table 3. Intraspecific variation and interspecific divergence of the Pedopodisma tsinlingensis group.
| Species | Intraspecific variation | Interspecific divergence | |
| P. tsinlingensis | P. funiushana | ||
| P. tsinlingensis | 0∼0.15% | ||
| P. funiushana | 0∼0.76% | 0∼0.92% | |
| S. wudangshanensis | 0.15∼2.17% | 0.61∼2.33 | 0.46∼2.33% |
In the networks, haplotypes of each group formed a separate clade (Fig. 6B, 6C). The clade of S. houshana group can be divided into three subclades, with subclade I composed of haplotypes of S. qinlingensis, subclade II composed of haplotypes of S. lushiensis and one of S. qinlingensis, and subclade III composed of haplotypes of S. houshana. Haplotypes of S. lushiensis had a minimum of one mutational step to those of S. qinlingensis and at least five mutational steps to those of S. houshana, indicating the much closer relationship of this species with S. qinlingensis than with S. houshana. The haplotypes of S. houshana formed a so-called independent subclade, the haplotypes from Yingshan population have a minimum of five mutational steps and those from Mulanshan population have a minimum of six mutational steps to those of S. lushiensis. However, there was a minimum of seven mutational steps between haplotypes from Yinshan and Mulanshan populations, indicating that both of them have a much closer relationship to S. lushiensis than between themselves. The clade of the Pedopodisma tsinlingensis group can be divided into two subclades, with subclade I containing haplotypes from all three species and subclade II containing haplotypes only from P. wudangshanensis. One haplotype was found shared by P. tsinlingensis and P. funiushana. Haplotypes of P. wudangshanensis in subclade I had a minimum of three mutational steps to P. funiushana and a minimum of four mutational steps to P. tsinlingensis, but had a minimum of nine mutational steps to those of the conspecifics in subclade II.
Therefore, the validities of S. lushiensis, S. qinlingensis, P. funiushana and P. wudangshanensis are questionable. It is reasonable to consider each group as a single species since they have no conspicuous difference in either morphological or molecular characters.
Fruhstorferiola kulinga species group
Fruhstorferiola kulinga and F. huayinensis are extremely similar to each other, and the characters used to distinguish them have been found to be substantially variable even within populations, so geographical distribution was used as the main criterion for preliminary identification of the materials under study. In the NJ tree, the sampled individuals of these two species displayed weak genetic structure to some extent, but did not form reciprocally monophyletic clades, with one individual of F. huayinensis falling into the clade of F. kulinga and two individuals of F. kulinga falling into the clade of F. huayinensis (Fig. 7A). The intraspecific variation of F. kulinga and F. huayinensis were 0∼2.97% and 0∼1.85% respectively, but the pairwise distances between the two species are 0.15∼2.96%, leading to a complete overlap, i.e. the interspecific divergences fell completely within the range of intraspecific variation of F. kulinga. The haplotype network of these two species can be divided into two subclades, with subclade I containing only haplotypes from F. kulinga and subclade II containing mainly haplotypes from F. huayingensis but two from F. kulinga (Fig. 7B).
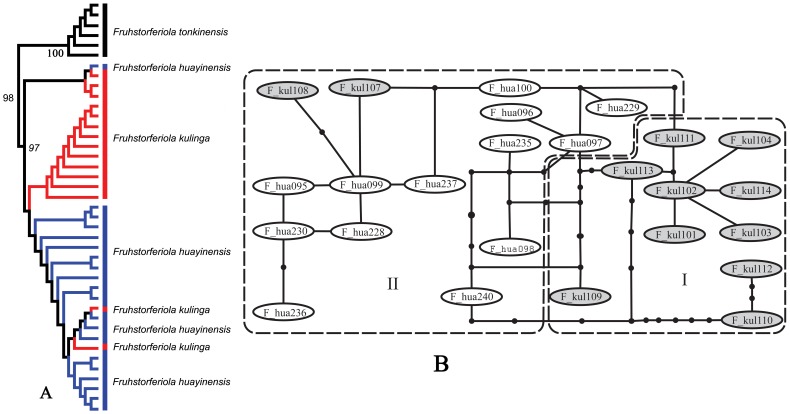
Figure 7. NJ tree Subclade and haplotype network of the genus Fruhstorferiola.
A. NJ tree subclade. B. Haplotype network of the Fruhstorferiola kulinga group. F_kul indicates Fruhstorferiola kulinga (filled with grey color), F_hua indicates Fruhstroferiola huayinensis.
Since the present molecular data do not separate these two species from each other, and the morphological characters used to distinguish them have been found to be unstable, we would like to treat them provisionally as a single species before further phylogeographical research with much larger scale samples.
Shirakiacris shirakii species group
There are different opinions on the validity of Shirakiacris yunkweiensis [6], [70]. In this study, the limited samples provided additional evidence on the relationship between these two species. In the NJ tree (Fig. 8A), one individual of S. shirakii fell into the clade of S. yunkweiensis. The ranges of intraspecific variation within S. shirakii and S. yunkweiensis were 0∼1.7% and 0∼1.54% respectively, and the interspecific pairwise distances were 0.61∼2.01%, leading to a overlap of as much as 1.09% between intraspecific variation and interspecific divergence. The haplotype network can be divided into two subclades (Fig. 8B), with subclade I containing haplotypes from S. shirakii and subclade II containing haplotypes from S. yunkweiensis, but one haplotype of S. shirakii had a minimum of four mutational steps to S. yunkweiensis, much smaller than the minimum of ten mutational steps to other haplotypes of S. shirakii.
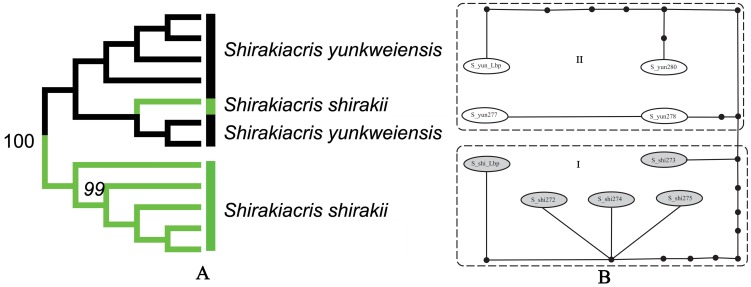
Figure 8. NJ tree subclade and haplotype network of the Shirakiacris shirakii group.
A. NJ tree subclade. B. Haplotype network.
Since no results from any analysis supported the validity of S. yunkweiensis, it should be regarded as a junior synonym of S. shirakii [70].
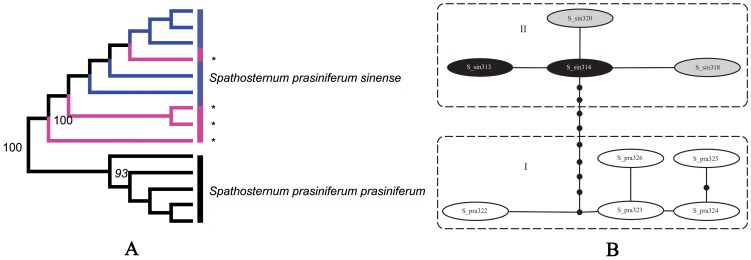
Spathosternum prasiniferum species group
While Spathosternum prasiniferum sinense and S. p. prasiniferum are regarded at present as two subspecies of the same species, they formed reciprocally monophyletic clades in the NJ tree (Fig. 9A) and there was a conspicuous gap of 0.93% (from 0.61% to 1.54%) between intraspecific variations (0∼0.3% and 0.15∼0.61% respectively; Table S4) and interspecific divergences (pairwise distances varying between 1.54∼1.85%). The haplotype network displayed a similar relationship and there was a minimum of ten mutational steps between the nominal subspecies (Fig. 9B). Interestingly, there were four female individuals with long tegmina collected together with S. p. sinense from Guilin. Judged solely according to morphology, these four females should be recognized as S. p. prasiniferum, but they formed a monophyletic clade in the gene tree with the short-winged individuals from the same population (Fig. 9A). The pairwise distances between these four long-winged individuals and short-winged individuals varied between 0∼0.3%, indicating that they were much closer genetically to and may be aberrant individuals of S. p. sinense. Furthermore, there were haplotypes shared by long- and short-winged individuals from the same population.
Figure 9. NJ tree subclade and haplotype network of the Spathosternum prasiniferum species group.
A. NJ tree subclade. The asterisks and pink color indicate the female individuals of S. prasiniferum sinense from Guilin with long tegmina. B. Haplotype network. S_pra represents S. prasiniferum prasiniferum and S_sin represents S. prasiniferum sinense. Black color indicates the haplotypes from the normal individuals of S. prasiniferum sinense with reduced tegmina. Grey color indicates the haplotypes from the female individuals of S. prasiniferum sinense from Guilin with long tegmina.
Therefore, S. p. sinense and S. p. prasiniferum are at least two distinct subspecies since they have conspicuous morphological differences and molecular divergences. Considering the common presence of polymorphism in wing length in Acrididae [94]–[95], the relatively low genetic distances between the two subspecies (1.54∼1.85%) and the broad distribution of S. prasiniferum, data from more populations will certainly facilitate making a categorical decision. Possibly, the taxonomic status of S. p. sinense could even be raised to species-level based on futher evidences from phylogeographical study.
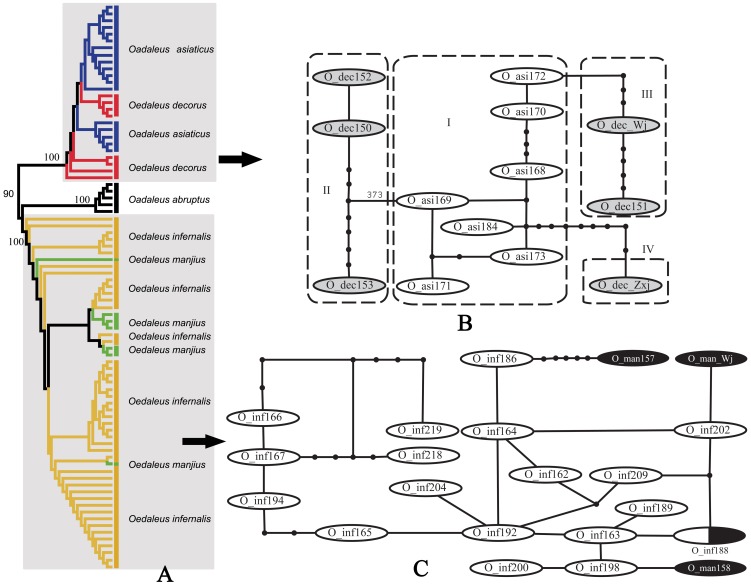
Oedaleus decorus and Oedaleus infernalis species groups
Oedaleus decorus differs from O. asiaticus mainly in having the dark transverse band of hind wing broader and not interrupted at the first anal vein. Eight individuals of O. decorus from three populations of Xinjiang and Gansu and eighteen individuals of O. asiaticus from six populations were sampled in this study. They did not form reciprocally monophyletic clades in the NJ tree (Fig. 10A). The ranges of intraspecific variations within O. decorus and O. asiaticus were 0∼2.17% and 0∼1.23% respectively, and the interspecific pairwise distances were 1.39∼2.01%, leading to a complete overlap between intraspecific variations and interspecific divergences. The haplotypes of O. decorus were connected to the central network of O. asiaticus through three subclades, and there was a minimum of four mutational steps between the haplotypes of the two species (Fig. 10B).
Figure 10. NJ tree subclade and haplotype network of Oedaleus.
A. NJ tree subclade. B. Haplotype network of the Oedaleus decorus group. O_dec indicates O. decorus and O_asi indicates O. asiaticus. C. Haplotype network of the Oedaleus infernalis group. O_inf indicates O. infernalis and O_man indicates O. manjius, the oval filled with white-black color indicates the haplotype shared by O. infernalis and O. manjius.
Oedaleus infernalis can be distinguished from O. manjius mainly by its yellowish brown tibiae and the narrower dark transverse band of the hind wing. Forty-four individuals of O. infernalis from 11 populations and seven individuals of O. manjius from 2 populations were sampled in this study. However, they did not form reciprocally monophyletic clades in the NJ tree (Fig. 10A). The intraspecific variations of O. infernalis and O. manjius ranged from 0 to 2.47% and 0 to 1.7% respectively, and the interspecific pairwise distances from 0 to 2.63%, leading to a broad overlap between intraspecific variations and interspecific divergences within this group. The haplotypes of O. manjius were connected to the network of O. infernalis through three subclades, and one haplotype was found shared by them (Fig. 10C).
Therefore, the minor morphological differences between the nominal species within each group may be attributed to adaptation to different environments and each group should be actually considered as comprising a single widely-distributed species. Further phylogeographical study may reveal much more precise genetic structure pattern in these two groups, including the color pattern polymorphism that commonly exist in Acrididae [94], [96].
Discussion
Performance of COI barcode sequence in reconstructing higher-level phylogeny
Although the primary aim of our study was to investigate DNA barcoding, the phylogeny inferred from COI barcode sequence sheds new light on the relationship among taxa within Acrididae. Given that the genus Pedopodisma did not fall within Sinopodisma either in the MP tree or in the Bayesian tree, it likely be an independent genus [6] rather than a junior synonym of Sinopodisma [71]. Further study with thorough sampling will certainly facilitate a better understanding of the relationship between Sinopodisma and Pedopodisma. Since the monophylies of most genera and well delineated species were supported by our data set, it seemed that COI barcode region performed much better in phylogenetic reconstruction at genus and species levels than at higher levels. It is reasonable to assume that the success of species assignment may be higher where the reconstructed evolution of the gene reflects speciation events, particularly where closely related species are under study [97].
Although the phylogeny at higher levels were not well resolved using COI barcode sequence, a little improvement was gained when the third base data or both first and third base data were excluded. This limited improvement may be due to: (1) the fact that some groups may be paraphyletic or polyphyletic; (2) the bias in taxon sampling; and (3) the use of the partial fragment of the single COI gene. It is expected that combining COI barcode data with other gene sequences (especially suitable nuclear genes), more thorough sampling and the exploration of new analytical strategies will certainly improve our understanding of the phylogeny of Acridoidea at higher-levels.
Efficacy of DNA barcoding in Acridoidea
The most important goal of DNA barcoding is to facilitate the species discovery process by increasing the speed, objectivity, and efficiency of species identification. However, barcoding failure associated with non-monophyly is very likely at the traditionally recognized species level [1]. Such a mismatch of taxonomy and genealogy was also observed in the New Zealand grasshopper genus Sigaus [68]. In our data set, when the traditional species names were used, interspecific divergences extended at their lower end well into the range of intraspecific variation (Fig. 2). Species assignment through each of the “best close match (BCM)”, “all species barcodes (ASB)” and “BP-based neural network” methods produced error rates of more than 5% (Tables 1). Since nearly all incorrect identifications occurred in the sequences of the six questionable species groups (Table S6, S7), and the species boundary delimitation supported our presumption on the relationships among species within each species group, this error rate appeared to be mainly a result of imperfect taxonomy. Furthermore, no ad hoc threshold was available to obtain an estimated relative identification error of 0.05. This result might be due to the high proportion of false positives caused by too high genetic similarities among extremely close species. Such a high genetic similarity can derive from either incomplete lineage sorting among newly diverged species or imperfect taxonomy. Therefore, it is expected that a much higher success rate could be obtained when the revised species names are applied to the data set. It is obvious that perfect taxonomy will greatly increase the success rate of identification through DNA barcoding, and that taxonomic revision plays an important role in DNA barcoding studies [1].
The single representative of Traulia szetschuanensis was resolved within Traulia minuta in Bayesian analysis (Fig. 1B) but outside of the latter in both the MP tree (Fig. 1A) and the NJ tree (Fig. 1C). It is possible that T. szetschuanensis will form a monophyletic clade in phylogenetic trees when more individuals are sampled. The divergence between them was 4%, much higher than the threshold of 3% and the 95th percentile distance threshold (2%), indicating that they can be correctly identified through DNA barcoding.
While Tonkinacris sinensis fell into the genus Sinopodisma in both of the MP and Bayesian analyses, it was recovered as a monophyletic clade that was the sister of the clade of Sinopodisma rostellocerca+S. lofaoshana (Jiulianshan population) (Fig. 1A, 1B). It fell outside of Sinopodisma in the NJ tree (Fig. 1C) and had an interspecific divergence of 5.89% from Sinopodisma rostellocerca and of 6.48% from the Jiulianshan population of Sinopodisma lofaoshana. That is to say, Tonkinacris sinensis can be correctly identified under a completely sampled phylogeny through DNA barcoding using either tree-based or threshold or other species identification approaches.
While the average interspecific divergence between Spathosternum prasiniferum prasiniferum and S. p. sinense is only 1.7%, they were reciprocally monophyletic in the MP and NJ trees, and both have much constrained intraspecific variation (Table S4), giving rise to a distinctive gap between intraspecific variation and interspecific divergence. Furthermore, eight purely diagnostic positions, nearly half of the total variable positions were found within the barcode sequence alignment (Fig. 11), and bases private to one of the two subspecies existed in all the other variable positions, suggesting a conspicuous genetic divergence between these two taxa which may be very recently diverged species. Therefore, molecular data derived from much more extensive sampling should serve as evidence to revive the species status of S. sinense and they can be correctly identified most of time by using either morphological characters (the length of tegmina) or the COI barcoding fragment through a thoroughly sampled phylogeny. As for the four females of S. p. sinense with long tegmina from Guilin, their similarity to S. p. prasiniferum in tegmen length may be a result induced by environmental factors. Such aberrant individuals occur extremely rarely and have nearly no genetic difference from the abundant normal individuals with short tegmina. In such a case, it is DNA barcoding but not a traditional morphological approach that provides a more accurate identification for the aberrant individuals. Of course, geographical information will help us in identifying such individuals if further study can support their allopatry. While the intraspecific variation of Sinopodisma lofaoshana exceeded the interspecific divergences of S. p. prasiniferum against S. p. sinense, they were in different parts of the tree. Although having a substantial impact during the discovery phase (i.e., in an incompletely sampled group), such overlap will not affect identification of unknowns in a thoroughly sampled tree [1].
Figure 11. Alignment of Spathosternum, showing the purely diagnostic positions (position 70, 151, 190, 247, 298, 316, 346, 548, marked with red boxes) and the total variable positions.
S_sin313∼321 indicate sequences of S. prasiniferum sinense (including the four long-winged individuals from the Guilin population)and S_pra322∼326 indicate S. prasiniferum prasiniferum.
Sinopodisma lofaoshana is the most difficult taxon in our data set. Two populations were sampled in this study and the result showed that the intraspecific variations between individuals of different populations (5.06–5.56%, average 5.25%) distinctly overlapped with the interspecific divergence between individuals of Jiulianshan population of S. lofaoshana and those of S. rostellocerca (2.17–3.12%, average 2.53%). This overlap did not lead to any incorrect or ambiguous identification in either BCM or BP-based analysis because each population has more than one individual sampled and the intraspecific variation within population was below 1% (0–0.92%). However, the ambiguous identification occurred when performing the ASB simulation because of the presence of the genetic distance overlap between these two species, suggesting that ASB, as a more rigorous barcoding method, can reveal much more sensitively the presence of a possible taxonomic problem in some groups. Both S. lofaoshana and S. rostellocerca have widespread distributions in south China and appear to have an overlap zone. Although both populations of S lofaoshana formed monophyletic clades, further study with thorough sampling is needed to reveal the geographical genetic structures and to clarify its relationship to S. rostellocerca.
Nearly all erroneous identifications in the BCM and BP-based analyses were caused by the six questionable species groups abovementioned. If it can be confirmed by further evidence that inappropriate morphological classification does exist in these groups, then a comprehensive revision, i.e. the synonymy for each group based on morphology and supported by other evidence, will lead to more accurate identification through DNA barcoding. In any case, accurate taxon identification is extremely important for molecular studies [98].
In a word, the 91% of correct identification for the BCM method and the 94% for the BP-based method in Acridoidea are acceptable at the moment and consistent with the results reported in previous studies [58], [90]. While mtDNA barcoding cannot offer good resolution at higher taxonomic levels, the completeness of the DNA barcode database against which unknown sequences are compared will certainly increase the accuracy of species identification [99].
Implications of DNA barcoding in species boundary delimitation
The accuracy of a threshold-based approach critically depends upon the level of overlap between intraspecific variation and interspecific divergence across a phylogeny. However, the ranges of both intraspecific variation and interspecific divergence will change in response to species delineation, thus leading to a variation of the overlap [1]. Since many traditional species will appear to be genetically non-monophyletic because of imperfect taxonomy [61], it is necessary to make a comprehensive revision of any group before or in combination with DNA barcoding study. Nearly all traditional species were established based only on morphological character sets, and many species, especially those described much earlier, were not compared with relevant type specimens of closely-related species when newly described. Thus all species, especially if they are closely-related, should firstly be revised to confirm the presence of morphological differences among them, and this will resolve directly to some extent the issue of oversplitting caused by the lack of type comparison [1].
In this study, the barcoding sequence data set not only tested the success rate of identification but also provided additional data for the morphologically questionable species groups. DNA barcoding supported our doubts about taxonomic accuracy in six species groups and the validity of species in two groups, and facilitated a better understanding of the relationship among species within each species group. Furthermore, ASB analysis also revealed the particular relationship between Sinopodisma lofaoshana and S. rostellocerca, which needs further study with more thorough sampling. Therefore, DNA barcoding can not only increase the speed and accuracy of species identification, but also help us highlight or resolve some taxonomic issues.
As an identification tool, DNA barcoding should be used in conjunction with other information [52]. Species with distinct morphological differences observed should still be corroborated with other sources of data, including geographical, biological, ecological, reproductive, behavioral and DNA sequence information [54]. If no additional non-morphological difference can be found among species established by morphology alone, the conclusion should be that only one species exists with morphological polymorphism, and synonymy should be proposed. Similarly, we should re-examine morphology or move on to some other source of information once fixed DNA differences are observed among aggregates in which morphological differences have not previously been observed. This may show whether the DNA sequence divergences represent truly morphologically cryptic species or genetic polymorphism within a single species. In other words, from the barcoding perspectives, substantial sequence differentiation can be explained as heteroplasmy or due to numts if it is not confirmed with other sources of information. Conversely, even as few as one nucleotide of consistent differentiation can serve as a diagnostic character to identify and delineate a valid species if it is distinctly different morphologically, or ecologically, or in other aspects from its closely related congeneric species [100]. Putative cryptic species detected by mtDNA barcoding merit closer investigation via analysis of, or more in-depth examination of, ecology and taxonomy [100]–[101]. Nuclear genetic data can be used as a check when mtDNA barcoding reveals unexpected results, because deep genetic divisions found during mtDNA barcoding are not always reflected in the nuclear genome in some groups [102]–[103].
In conclusion, despite the problems of sampling size [104]–[105] and the criticisms on methodological [106], theoretical [107] and empirical grounds [1], [108]–[110], the prospect of DNA barcoding is still promising if it is based on solid foundations of comprehensive taxonomy. The exploration on new analytical methods [59], [93], [99], [105], [111]–[117] and the use of nuclear genes as additional effective DNA barcodes [60], [103] will certainly promote the progress in DNA barcoding.
Supporting Information
MP tree infferred with complete base data. Members of Catantopidae are marked with red, those of Oedipodidae with deep blue, those of Arcypteridae with yellow, those of Gomphoceridae with pink, those of Acrididae with green, those of Pamphagidae with bright blue and other groups with black.
(TIF)
MP tree infferred with exclusion of third base data. Members of Catantopidae are marked with red, those of Oedipodidae with deep blue, those of Arcypteridae with yellow, those of Gomphoceridae with pink, those of Acrididae with green, those of Pamphagidae with bright blue and other groups with black.
(TIF)
MP tree infferred with exclusion of both third and first base data. Members of Catantopidae are marked with red, those of Oedipodidae with deep blue, those of Arcypteridae with yellow, those of Gomphoceridae with pink, those of Acrididae with green, those of Pamphagidae with bright blue and other groups with black.
(TIF)
Sequences downloaded from NCBI.
(DOC)
Materials used in this study.
(DOC)
Cross reference list between GenBank accession numbers and numbers of vouchers sharing the same haplotype.
(DOC)
ranges of intraspecific genetic variations.
(DOC)
Distribution of intraspecific variations and interspecific divergences.
(DOC)
Sequences identified as ambiguous or incorrect in BCM analysis.
(DOC)
Assignment of species through BP-based method.
(DOC)
Acknowledgments
We would like to thank Dr. Jerzy A. Lis for kindly sending us his publication, to thank Dr. Gontran Sonet for helping us calculate the ad hoc threshold. We are deeply indebted to Dr. Ioana C. Chintauan-Marquier and the other anonymous reviewers for their constructive academic suggestions and detailed grammatical polish on the manuscript.
Funding Statement
This study is supported by National Natural Science Foundation of China (No. 30970346, 31172076, 31260523), the Fundamental Research Funds for the Central Universities (GK201001004), China Postdoctoral Science Foundation (20070421105) and Guangxi Natural Science Foundation (2010GXNSFA013070). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Meyer CP, Paulay G (2005) DNA barcoding: error rates based on comprehensive sampling. PLoS Biol 3: e422, 2229–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang JH, Fu P, Huang Y (2007) Redescription of Caryanda jiuyishana Fu & Zheng, 2000 (Orthoptera: Acrididae: Oxyinae: Oxyini) with proposal of a new synomym. Zootaxa 1436: 55–60. [Google Scholar]
- 3. Huang JH, Zheng ZM, Huang Y, Zhou SY (2009) New synonymies in Chinese Oxyinae (Orthoptera: Acrididae). Zootaxa 1976: 39–55. [Google Scholar]
- 4. Wei T, Huang JH (2012) To the synonymy of Traulia brachypeza Bi, 1986 (Orthoptera: Acrididae, Catantopinae). Far East Entomol 255: 11–15. [Google Scholar]
- 5.Eades DC, Otte D, Cigliano MM, Braun H (2013) Orthoptera species file online. Version 2.0/4.1. Available from http://orthoptera.speciesfile.org/HomePage.aspx (accessed 3 October 2013).
- 6.Li HC, Xia KL (2006) Fauna Sinica, Insecta, volume 43, Orthoptera, Acridoidea, Catantopidae. Science Press, Beijing, China, 736 pp. [Google Scholar]
- 7.Zheng ZM, Xia KL (1998) Fauna Sinica. Insecta Volume 10. Orthoptera, Acridoidea, Oedipodidae and Arcypteridae. Science Press, Beijing, China, 616 pp.
- 8.Yin XC, Xia KL (2003) Fauna Sinica. Insecta Volume 32. Orthoptera, Acridoidea, Gomphoceridae and Acrididae. Science Press, Beijing, China, 280 pp.
- 9. Flook PK, Rowell CHF (1998) Inferences about orthopteroid phylogeny and molecular evolution from small subunit nuclear ribosomal RNA sequences. Insect Mol Biol 7: 163–178. [DOI] [PubMed] [Google Scholar]
- 10. Flook PK, Klee S, Rowell CHF (1999) A combined molecular phylogenetic analysis of the Orthoptera and its implications for their higher systematics. Syst Biol 48: 233–253. [DOI] [PubMed] [Google Scholar]
- 11. Lu HM, Ye WP, Huang Y (2001) Phylogenetic relationship among orthopteran superfamilies derived from 16S gene sequences. J Northwest Univers (Nat Sci Ed) 31 (special issue) 7–9. [Google Scholar]
- 12. Zhou ZJ, Ye HY, Huang Y, Shi F (2010) The phylogeny of Orthoptera inferred from mtDNA and description of Elimaea cheni (Tettigoniidae: Phaneropterinae) mitogenome. J Genet Genomics 37: 315–324. [DOI] [PubMed] [Google Scholar]
- 13. Wang XY, Zhou ZJ, Huang Y, Shi FM (2011) The phylogenetic relationships of higher Orthopteran categories inferred from 18S rRNA gene sequences. Acta Zootax Sinica 36: 139–150. [Google Scholar]
- 14. Cui AM, Huang Y (2012) Phylogenetic relationships among Orthoptera insect groups based on complete sequences of 16S ribosomal RNA. Hereditas (Beijing) 4: 597–608. [DOI] [PubMed] [Google Scholar]
- 15. Lv HJ, Huang Y (2012) Phylogenetic relationship among some groups of orthopteran based on complete sequences of the mitochondrial cox1 gene. Zool Res 33: 319–328. [DOI] [PubMed] [Google Scholar]
- 16. Flook PK, Rowell CHF (1997) The phylogeny of the Caelifera (Insecta, Orthoptera) as deduced from mitochondrial rRNA gene sequences. Mol Phylogen Evol 8: 89–103. [DOI] [PubMed] [Google Scholar]
- 17. Rowell CHF, Flook PK (1998) Phylogeny of the Caelifera and the Orthoptera as derived from ribosomal RNA gene sequences. J Orthoptera Res 7: 147–156. [Google Scholar]
- 18. Ren ZM, Ma EB, Guo YP (2002) The studies of the phylogeny of Acridoidea based on mtDNA cyt b sequences. Acta Genet Sinica 29: 314–321. [PubMed] [Google Scholar]
- 19. Yin H, Zhang DC, Bi ZL, Yin Z, Liu Y, et al. (2003) Molecular phylogeny of some species of the Acridoidea based on 16S rDNA. Acta Genet Sin 30: 766–772. [PubMed] [Google Scholar]
- 20. Yin H, Li XJ, Wang WQ, Yin XC (2004) Inferences about Acridoidea phylogenetic relationships from small subunit nuclear ribosomal DNA sequence. Acta Entomol Sinica 47: 809–814. [Google Scholar]
- 21. Liu DF, Jiang G (2005a) Molecular phylogenetic analysis of Acridoidea based on 18S rDNA with a discussion on its taxonomic system. Acta Entomol Sinica 48: 232–241. [Google Scholar]
- 22. Sun ZL, Jiang GF, Huo GM, Liu DF (2006) A phylogenetic analysis of six genera of Acrididae and monophyly of Acrididae in China using 16S rDNA sequences (Orthoptera, Acridoidea). Acta Zool Sinica 52: 302–308. [Google Scholar]
- 23. Wang NX, Feng X, Jiang GF, Fang N, Xuan WJ (2008) Molecular phylogenetic analysis of five subfamilies of the Acrididae (Orthoptera: Acridoidea) based on the mitochondrial cytochrome b and cytochrome c oxidase subunit I gene sequences. Acta Entomol Sinica 51: 1187–1195. [Google Scholar]
- 24. Zhang DC, Li XJ, Wang WQ, Yin H, Yin Z, et al. (2005) Molecular phylogeny of some genera of Pamphagidae (Acridoidea, Orthoptera) from China based on mitochondrial 16S rDNA sequences. Zootaxa 1103: 41–49. [Google Scholar]
- 25. Zhang DC, Han HY, Yin H, Li XJ, Yin Z, et al. (2011) Molecular phylogeny of Pamphagidae (Acridoidea, Orthoptera) from China based on mitochondrial cytochrome oxidase II sequence. Insect Science 18: 234–244. [Google Scholar]
- 26. Liu DF, Jiang GF, Shi H, Sun ZL, Huo GM (2005) Monophyly and the taxonomic status of subfamilies of the Catantopidae based on 16S rDNA sequences. Acta Entomol Sinica 48: 759–769. [Google Scholar]
- 27. Ma L, Huang Y (2006) Molecular phylogeny of some subfamilies of Catantopidae (Orthoptera: Caelifera: Acridoidea) in China based on partial sequence of mitochondrial COII gene. Acta Entomol Sinica 49: 982–990. [Google Scholar]
- 28. Lu RS, Huang Y, Zhou ZJ (2010) Phylogenetic analysis among the nine subfamilies in Catantopidae (Orthoptera, Acridoidea) in China inferred from cyt b, 16s rDNA and 28s rDNA sequences. Acta Zootax Sinica 35: 782–789. [Google Scholar]
- 29. Lu HM, Huang Y (2006) Phylogenetic relationship of 16 Oedipodidae species (Insecta: Orthoptera) based on the 16S rRNA gene sequences. Insect Science 13: 103–108. [Google Scholar]
- 30. Ding FM, Huang Y (2008) Molecular evolution and phylogenetic analysis of some species of Oedipodidae (Orthoptera: Caelifera) in China based on complete mitochondrial nad2 gene. Acta Entomol Sinica 51: 55–60. [Google Scholar]
- 31. Huo GM, Jiang GF, Sun ZL, Liu DF, Zhang YL, et al. (2007) Phylogenetic reconstruction of the family Acrypteridae (Orthoptera: Acridoidea) based on mitochondrial cytochrome b gene. J Genet Genom 34: 294–306. [DOI] [PubMed] [Google Scholar]
- 32. Chapco W, Litzenberger G, Kuperus WR (2001) A molecular biogeographic analysis of the relationship between North American melanoploid grasshoppers and their Eurasian and South American relatives. Mol Phylogen Evol 18: 460–466. [DOI] [PubMed] [Google Scholar]
- 33. Amédégnato C, Chapco W, Litzenberger G (2003) Out of South America? Additional evidence for a southern origin of melanopline grasshoppers. Mol Phylogen Evol 29: 115–119. [DOI] [PubMed] [Google Scholar]
- 34. Litzenberger G, Chapco W (2003) The North American Melanoplinae (Orthoptera: Acrididae): a molecular phylogenetic study of their origins and taxonomic relationships. Ann Entomol Soc America 96: 491–497. [Google Scholar]
- 35. Chintauan-Marquier IC, Jordan S, Berthier P, Amédégnato C, Pompanon F (2011) Evolutionary history and taxonomy of a short-horned grasshopper subfamily: The Melanoplinae (Orthoptera: Acrididae). Mol Phylogen Evol 58: 22–32. [DOI] [PubMed] [Google Scholar]
- 36. Litzenberger G, Chapco W (2001) Molecular phylogeny of selected Eurasian podismine grasshoppers (Orthoptera: Acrididae). Ann Entomol Soc America 94: 505–511. [Google Scholar]
- 37. Chapco W, Martel RKB, Kuperus WR (1997) Molecular phylogeny of North American band-winged grasshoppers (Orthoptera: Acrididae). Ann Entomol Soc America 90: 555–562. [Google Scholar]
- 38. Fries M, Chapco W, Contreras D (2007) A molecular phylogenetic analysis of the Oedipodinae and their intercontinental relationships. J Orthoptera Res 16: 115–125. [Google Scholar]
- 39. Chapco W, Contreras D (2011) Subfamilies Acridinae, Gomphocerinae and Oedipodinae are “fuzzy sets”: a proposal for a common African origin. J Orthoptera Res 20: 173–190. [Google Scholar]
- 40. Bugrov A, Novikova O, Mayorov V, Adkison L, Blinov A (2006) Molecular phylogeny of Palaearctic genera of Gomphocerinae grasshoppers (Orthoptera, Acrididae). System Entomol 31: 362–368. [Google Scholar]
- 41. Contreras D, Chapco W (2006) Molecular phylogenetic evidence for multiple dispersal events in gomphocerines grasshoppers. J Orthoptera Res 15: 91–98. [Google Scholar]
- 42. Rowell CHF, Flook PK (2004) A dated molecular phylogeny of the Proctolabinae (Orthoptera: Acrididae), especially the Lithoscirtae, and the evolution of their adaptive traits and present biogeography. J Orthoptera Res 13: 35–56. [Google Scholar]
- 43. Savolainen V, Cowan RS, Vogler AP, Roderick GK, Lane R (2005) Towards writing the encyclopaedia of life: an introduction to DNA barcoding. Philos Trans R Soc Lond, B, Biol Sci 360: 1805–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Herbert PDN, Cywinska A, Ball SL, deWaard JR (2003a) Biological identifications through DNA barcodes. Proc R Soc Lond, B, Biol Sci 270: 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hebert PDN, Ratnasingham S, deWaard JR (2003b) Barcoding animal life: cytochrome c oxidase subunit 1 divergences among closely related species. Proc R Soc Lond, B, Biol Sci (Suppl.) 270: S96–S99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Baker AJ, Huynen LJ, Haddrath O, Millar CD, Lambert DM (2005) Reconstructing the tempo and mode of evolution in an extinct clade of birds with ancient DNA: The giant moas of New Zealand. Proc Natl Acad Sci USA 102: 8257–8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lambert DM, Baker A, Huynen L, Haddrath O, Hebert PDN, et al. (2005) Is a large-scale DNA-based inventory of ancient life possible? J Hered 96: 279–284. [DOI] [PubMed] [Google Scholar]
- 48. Campbell DC, Johnson PD, Williams JD, Rindsberg AK, Serb JM, et al. (2008) Identification of ‘extinct’ freshwater mussel species using DNA barcoding. Mol Ecol Res 8: 711–724. [DOI] [PubMed] [Google Scholar]
- 49. Lawrence HA, Millar CD, Imber MJ, Crockett DE, Robins JH, et al. (2009) Molecular evidence for the identity of the Magenta petrel. Mol Ecol Res 9: 458–461. [DOI] [PubMed] [Google Scholar]
- 50. Waugh J (2007) DNA barcoding in animal species: progress, potential and pitfalls. BioEssays 29: 189–197. [DOI] [PubMed] [Google Scholar]
- 51. Hajibabaei M, Singer GAC, Hebert PDN, Hickey DA (2007) DNA barcoding: how it complements taxonomy, molecular phylogenetics and population genetics. Trends Genet 23: 167–172. [DOI] [PubMed] [Google Scholar]
- 52. Song H, Buhay JE, Whiting MF, Crandall KA (2008) Many species in one: DNA barcoding overestimates the number of species when nuclear mitochondrial pseudogenes are coamplified. Proc Natl Acad Sci USA 105: 13486–13491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Moulton MJ, Song H, Whiting MF (2010) Assessing the effects of primer specificity on eliminating numt coamplification in DNA barcoding: a case study from Orthoptera (Arthropoda: Insecta). Mol Ecol Resour 10: 615–627. [DOI] [PubMed] [Google Scholar]
- 54. DeSalle R, Egan MG, Siddall M (2005) The unholy trinity: taxonomy, species delimitation and DNA barcoding. Philos Trans R Soc Lond, B, Biol Sci 360: 1905–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Meier R, Zhang G, Ali F (2008) The use of mean instead of smallest interspecific distances exaggerates the size of the “barcoding gap” and leads to misidentification. Syst Biol 57: 809–813. [DOI] [PubMed] [Google Scholar]
- 56. Austerlitz F, David O, Schaeffer B, Bleakley K, Olteanu M (2009) DNA barcode analysis: a comparison of phylogenetic and statistical classification methods. BMC Bioinformatics 10 (Suppl. 14) S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Monaghan MT, Wild R, Elliot M, Fujisawa T, Balke M (2009) Accelerated species inventory on Madagascar using coalescent based models of species delineation. Syst Biol 58: 298–311. [DOI] [PubMed] [Google Scholar]
- 58. Virgilio M, Backeljau T, Nevado B, Meyer M De (2010) Comparative performances of DNA barcoding across insect orders. BMC Bioinformatics 11: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rosa ML, Fiannaca A, Rizzo R, Urso A (2013) Alignment-free analysis of barcode sequences by means of compression-based methods. BMC Bioinformatics 14 (Suppl7) S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dai QY, Gao Q, Wu CS, Chesters D, Zhu CD (2012) Phylogenetic reconstruction and DNA barcoding for closely related pine moth species (Dendrolimus) in China with multiple gene markers. PlosONE 7: e32544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Funk DJ, Omland KE (2003) Species-level paraphyly and polyphyly: Frequency, causes, and consequences, with insights from animal mitochondrial DNA. Annu Rev Ecol Evol Syst 34: 397–423. [Google Scholar]
- 62. Monaghan MT, Balke M, Pons J, Vogler AP (2006) Beyond barcodes: complex DNA taxonomy of a south Pacific island radiation. Proc R Soc Lond B Biol Sci 273: 887–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. deWaard JR, Landry JF, Schmidt BC, Derhousoff J, McLean JA, et al. (2009) In the dark in a large urban park: DNA barcodes illuminate cryptic and introduced moth species. Biodivers Conserv 18: 3825–3839. [Google Scholar]
- 64. Foottit RG, Maw HEL, Havill NP, Ahern RG, Montgomery ME (2009) DNA barcodes to identify species and explore diversity in the Adelgidae (Insecta: Hemiptera: Aphidoidea). Mol Ecol Resour 9 (Suppl. 1)188–195. [DOI] [PubMed] [Google Scholar]
- 65. Rivera J, Currie DC (2009) Identification of Nearctic black flies using DNA barcodes (Diptera: Simuliidae). Mol Ecol Resour 9 (Suppl. 1)224–236. [DOI] [PubMed] [Google Scholar]
- 66. Sheffield CS, Hebert PDN, Kevan PG, Packer L (2009) DNA barcoding a regional bee (Hymenoptera: Apoidea) fauna and its potential for ecological studies. Mol Ecol Resour 9 (Suppl. 1)196–207. [DOI] [PubMed] [Google Scholar]
- 67. Pan CY, Hu J, Zhang X, Huang Y (2006) The DNA barcoding application of mtDNA COI gene in seven species of Catantopidae (Orthoptera). Entomotaxonomia 28: 103–110. [Google Scholar]
- 68. Trewick SA (2008) DNA Barcoding is not enough: mismatch of taxonomy and genealogy in New Zealand grasshoppers (Orthoptera: Acrididae). Cladistics 24: 240–254. [Google Scholar]
- 69. López H, Contreras-Díaz H, Oromí P, Juan C (2007) Delimiting species boundaries for endangered Canary Island grasshoppers based on DNA sequence data. Conserv Genet 8: 587–598. [Google Scholar]
- 70.Storozhenko SY (1983) Review of grasshoppers of the subfamily Catantopinae (Orthoptera, Acrididae) from the Sovjet Far East. In: Bodrova, Y.D., Soboleva, R.G., Meshcherkyakov, A.A. [eds] Systematics and ecological-faunistic reviews of the various orders of Insecta of the Far East. Academy of Sciences USSR Far-East Science Centre, Vladivostok, USSR, Rusian, 154 pp. 48–63. [In Russian] [Google Scholar]
- 71. Storozhenko SY (1993) To the knowledge of the tribe Melanoplini (Orthoptera, Acrididae, Catantopinae) of the Eastern Palearctica. Articulata 8: 1–22. [Google Scholar]
- 72. Uvarov BP (1931) Some Acrididae from South China. Lingnan Sci J 10: 217–221. [Google Scholar]
- 73. Tinkham ER (1936) Spathosternum sinense Uvarov considered to be a race of S. prasiniferum (Walker) (Orth.: Acrididae). Lingnan Sci J 15: 47–54 plate 6. [Google Scholar]
- 74. Grunshaw JP (1988) A taxonomic revision of the grasshopper genus Spathosternum (Orthoptera: Acrididae). J East Afr Nat Hist Soc Natl Mus 78: 1–21. [Google Scholar]
- 75. Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3: 294–297. [PubMed] [Google Scholar]
- 76. Tian YF, Huang G, Zheng ZM, Wei ZM (1999) A simple method for isolation of insect total DNA. J Shaanxi Norm Univ (Nat Sci Ed) 27: 82–84. [Google Scholar]
- 77. Staden R, Beal KF, Bonfield JK (2000) The Staden Package, 1998. Methods Mol Biol 132: 115–130. [DOI] [PubMed] [Google Scholar]
- 78. Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X window interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25: 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Swofford DL (2002) PAUP*. Phylogenetic analysis using parsimony (and other methods). Version 4. Sinauer Associates, Sunderland, Massachusetts.
- 80. Ronquist F, Huelsenbeck JP (2003) MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- 81. Posada D, Crandall KA (1998) Modeltest: testing the model of DNA substitution. Bioinformatics 14: 817–818. [DOI] [PubMed] [Google Scholar]
- 82. Saitou N, Nei M (1987) The neighbour-joining method: a new method for reconstructing evolutionary trees. Mol Biol Evol 4: 406–425. [DOI] [PubMed] [Google Scholar]
- 83. Kumar S, Gadagkar SR (2000) Efficiency of the neighbor-joining method in reconstructing deep and shallow evolutionary relationships in large phylogenies. J Mol Evol 51: 544–553. [DOI] [PubMed] [Google Scholar]
- 84. Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24: 1596–1599. [DOI] [PubMed] [Google Scholar]
- 85. Templeton AR, Crandall KA, Sing CF (1992) A cladistic analysis of phenotypic associations with haplotypes inferred from restriction endonuclease mapping and DNA sequence data. III. Cladogram estimation. Genetics 132: 619–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Templeton AR (2001) Using phylogeographic analyses of gene trees to test species status and processes. Mol Evol 10: 779–791. [DOI] [PubMed] [Google Scholar]
- 87. Clement M, Posada D, Crandall KA (2000) TCS: a computer program to estimate gene genealogies. Mol Ecol 9: 1657–1659. [DOI] [PubMed] [Google Scholar]
- 88. Kimura M (1980) A simple method of estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16: 111–120. [DOI] [PubMed] [Google Scholar]
- 89.Nei M, Kumar S (2000) Molecular evolution and phylogenetics. Oxford University Press, 348 pp. [Google Scholar]
- 90. Meier R, Shiyang K, Vaidya G, Ng PKL (2006) DNA barcoding and taxonomy in Diptera: a tale of high intraspecific variability and low identification success. Syst Biol 55: 715–728. [DOI] [PubMed] [Google Scholar]
- 91. Virgilio M, Jordaens K, Breman FC, Backeljau T, De Meyer M (2012) Identifying insects with incomplete DNA barcode libraries, African fruit flies (Diptera: Tephritidae) as a test case. PlosONE 7: e31581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zhang AB, Savolainen P (2008) BPSI2.0: A C/C++ interface program for species identification via DNA barcoding with a BP-neural network by calling the Matlab engine. Mol Ecol Resour 9: 104–106. [DOI] [PubMed] [Google Scholar]
- 93. Zhang AB, Sikes DS, Muster C, Li SQ (2008) Inferring species membership via DNA barcoding with back-propagation neural networks. Syst Biol 57: 202–215. [DOI] [PubMed] [Google Scholar]
- 94. Dearn JM (1978) Polymorphisms for wing length and colour pattern in the grasshopper Phaulacridium vittatum (Sjöst.). J Aust Entomol Soc 17: 135–137. [Google Scholar]
- 95. Gaines SB (1991) Body-size and wing-length variation among selected grasshoppers (Orthoptera: Acrididae) from Nebraska's Sandhills Grasslands. Trans Nebraska Acad Sci Affil Soc 18: 67–72. [Google Scholar]
- 96. Dearn JM (1981) Latitudinal cline in a colour pattern polymorphism in the Australian grasshopper Phaulacridium vittatum . Heredity 47: 111–119. [Google Scholar]
- 97. Hendrich L, Pons J, Ribera I, Balke M (2010) Mitochondrial cox1 sequence data reliably uncover patterns of insect diversity but suffer from high lineage-idiosyncratic error rates. PlosONE 5 (12) e14448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lis JA, Lis B (2011) Is accurate taxon identification important for molecular studies? Several cases of faux pas in pentatomoid bugs (Hemiptera: Heteroptera: Pentatomoidea). Zootaxa 2932: 47–50. [Google Scholar]
- 99. Luo AR, Zhang AB, Ho SYW, Xu W, Zhang Y, et al. (2011) Potential efficacy of mitochondrial genes for animal DNA barcoding: a case study using eutherian mammals. BMC Genomics 12: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Burns JM, Janzen DH, Hajibabaei M, Hallwachs W, Hebert PDN (2007) DNA barcodes of closely related (but morphologically and ecologically distinct) species of skipper butterflies (Hesperiidae) can differ by only one to three nucleotides. J Lepid Soc 61: 138–153. [Google Scholar]
- 101. Smith MA, Woodley NE, Janzen DH, Hallwachs W, Hebert PDN (2006) DNA barcodes reveal cryptic host-specificity within the presumed polyphagous members of a genus of parasitoid flies (Diptera: Tachinidae). Proc Natl Acad Sci USA 103: 3657–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Yassin A, Amédégnato C, Cruaud C, Veuille M (2009) Molecular taxonomy and species delimitation in Andean Schistocerca (Orthoptera: Acrididae). Mol Phylogenet Evol 53: 404–411. [DOI] [PubMed] [Google Scholar]
- 103. Dasmahapatra KK, Ellas M, Hill RI, Hoffmans JI, Mallet J (2010) Mitochondrial DNA barcoding detects some species that are real, and some that are not. Mol Ecol Resour 10: 264–273. [DOI] [PubMed] [Google Scholar]
- 104. Moritz C, Cicero C (2004) DNA barcoding: promise and pitfalls. PLoS Biol 2 (10) e354, 1529–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zhang AB, He LJ, Crozier RH, Muster C, Zhu CD (2009) Estimating sample sizes for DNA barcoding. Mol Phylogenet Evol 54: 1035–1039. [DOI] [PubMed] [Google Scholar]
- 106. Will KW, Rubinoff D (2004) Myth of the molecule: DNA barcodes for species cannot replace morphology for identification and classification. Cladistics 20: 47–55. [DOI] [PubMed] [Google Scholar]
- 107. Hickerson M, Meyer CP, Moritz C (2006) DNA barcoding will often fail to discover new animal species over broad parameter space. Syst Biol 55: 729–739. [DOI] [PubMed] [Google Scholar]
- 108. Hurst GDD, Jiggins FM (2005) Problems with mitochondrial DNA as a marker in population, phylogeographic and phylogenetic studies: the effects of inherited symbionts. Proc R Soc Lond, B Biol Sci 272: 1525–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Elias M, Hill RI, Willmott KR, Dasmahapatra KK, Brower AVZ, et al. (2007) Limited performance of DNA barcoding in a diverse community of tropical butterflies. Proc R Soc Lond, B, Biol Sci 274: 2881–2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Wiemers M, Fiedler K (2007) Does the DNA barcoding gap exist? A case study in blue butterflies (Lepidoptera: Lycaenidae). Front Zool 4: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Chu KH, Xu M, Li CP (2009) Rapid DNA barcoding analysis of large data sets using the composition vector method. BMC Bioinformatics 10 (Suppl 14) S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kuksa P, Pavlovic V (2009) Efficient alignment-free DNA barcode analytics. BMC Bioinformatics 10 (Suppl 14) S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Feng J, Hu Y, Wan P, Zhang AB, Zhao W (2010) New method for comparing DNA primary sequences based on a discrimination measure. J Theor Biol 266: 703–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Luo A, Qiao H, Zhang Y, Shi W, Ho SYW, et al. (2010) Performance of criteria for selecting evolutionary models in phylogenetics: a comprehensive study based on simulated data sets. BMC Evol Biol 10: 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zhang AB, Feng J, Ward RD, Wan P, Gao Q, et al. (2012a) A new method for species identification via protein-coding and non-coding DNA barcodes by combining machine learning with bioinformatic methods. PlosONE 7 (2) e30986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Zhang AB, Muster C, Liang HB, Zhu CD, Crozier R, et al. (2012b) A fuzzy-set-theory-based approach to analyze species membership in DNA barcoding. Mol Ecol 21: 1848–1863. [DOI] [PubMed] [Google Scholar]
- 117. Puillandre N, Lambert A, Brouillet S, Achaz G (2012) ABGD, automatic barcode gap discovery for primary species delimitation. Mol Ecol 21: 1864–1877. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MP tree infferred with complete base data. Members of Catantopidae are marked with red, those of Oedipodidae with deep blue, those of Arcypteridae with yellow, those of Gomphoceridae with pink, those of Acrididae with green, those of Pamphagidae with bright blue and other groups with black.
(TIF)
MP tree infferred with exclusion of third base data. Members of Catantopidae are marked with red, those of Oedipodidae with deep blue, those of Arcypteridae with yellow, those of Gomphoceridae with pink, those of Acrididae with green, those of Pamphagidae with bright blue and other groups with black.
(TIF)
MP tree infferred with exclusion of both third and first base data. Members of Catantopidae are marked with red, those of Oedipodidae with deep blue, those of Arcypteridae with yellow, those of Gomphoceridae with pink, those of Acrididae with green, those of Pamphagidae with bright blue and other groups with black.
(TIF)
Sequences downloaded from NCBI.
(DOC)
Materials used in this study.
(DOC)
Cross reference list between GenBank accession numbers and numbers of vouchers sharing the same haplotype.
(DOC)
ranges of intraspecific genetic variations.
(DOC)
Distribution of intraspecific variations and interspecific divergences.
(DOC)
Sequences identified as ambiguous or incorrect in BCM analysis.
(DOC)
Assignment of species through BP-based method.
(DOC)