Abstract
Optical based analysis in microfluidic and lab-on-a-chip systems are currently considered the gold standard methodology for the determination of end point reactions for various chemical and biological reaction processes. Typically, assays are performed using bulky ancillary apparatus such as microscopes and complex optical excitation and detection systems. Such instrumentation negates many of the advantages offered by device miniaturisation, particularly with respect to overall portability. In this article, we present a CO2 laser ablation technique for rapidly prototyping on-chip planar lenses, in conjunction with capillary action based autonomous microfluidics, to create a miniaturised and fully integrated optical biosensing platform. The presented self-aligned on-chip optical components offer an efficient means to direct excitation light within microfluidics and to directly couple light from a LED source. The device has been used in conjunction with a miniaturised and bespoke fluorescence detection platform to create a complete, palm sized system (≈60 × 80 × 60 mm) capable of performing fluoro-immunoassays. The system has been applied to the detection of cardiac Troponin I, one of the gold standard biomarkers for the diagnosis of acute myocardial infarction, achieving a lower detection limit of 0.08 ng/ml, which is at the threshold of clinically applicable concentrations. The portable nature of the complete system and the biomarker detection capabilities demonstrate the potential of the devised instrumentation for use as a medical diagnostics device at the point of care.
INTRODUCTION
Lab-on-a-chip and microfluidic based systems hold considerable promise for a vast range of chemical and biological processing applications. For such systems fluorescence based optical analysis has become the gold standard technique by which end point reactions are determined, typically using a laser excitation source with imaging microscopy.1, 2 In such setups, optical detection apparatus and its associated instrumentation greatly increase the overall size of the complete system, negating the many benefits of device miniaturisation and limiting the portable nature of such a technology. In addition laser systems are relatively expensive, can have relatively high power consumption, can be bulky for variants in specific wavelengths for use with prominent fluorophores and require safety protocols for their use within a laboratory. Increasingly for microdevice applications, LEDs have proven to be a cost effective alternative to laser sources as they are readily available off the shelf, produce high luminosities across a wide range of optical wavelengths, have relatively low power consumption and are be readily integrated into miniaturised platforms. Such sources are broadband and have large half angles of emission, therefore requiring focusing lenses and band-pass optical filters. The resulting optical excitation setup can therefore be several orders of magnitude larger than the microfluidic chip itself and require careful manual configuration to optimise the setup.
To reduce the size of the overall system, much attention has been focused on the integration of on-chip light guiding structures. Various light delivery and focusing components ranging from planar/3D lenses3, 4, 5, 6, 7, 8, 9, 10, 11, 12 to waveguides13, 14 have been demonstrated in integrated microfluidic and lab-on-a-chip devices. The primary advantages to such on-chip integration, in addition to the reduced size of key optical elements, are the self-alignment of the components and the ability to more tightly focus light onto microfluidic channels. However, most demonstrated formats require the use of larger ancillary equipment, e.g., laser excitation sources, light guiding fibres, light collimators or fluorescence microscopes, which negates many of the benefits from device miniaturisation. Of all the demonstrated strategies, planar light guiding structures have demonstrated a high efficiency for light focusing within microsystems, with greatly reduced overall design complexity and modest increases in the total device footprint.3, 4, 5, 6, 7, 8, 9, 10, 12 In the work conducted by Seo et al., for example, significant consideration was paid to complete device miniaturisation, where a disposable format was created that operated directly from an LED excitation source, with no additional devices required for light coupling.9 Additionally, it was found that 2D Polydimethylsiloxane (PDMS) based microlenses confined the excitation emission within the chip and yielded no detectable excitation light orthogonal to the chip plane, resulting in a 7-fold increase in the detected fluorescence intensity from a sample of fluorescent nanospheres. This work was not exploited further however in more advanced systems and currently no biomicrofluidic system has demonstrated the advantageous use of integrated light guides by which to perform any biological or chemical based assays.
In this article, we assess the performance of various 2D planar lenses combined with capillary microfluidics and biosensing elements, all manufactured within PMMA substrate using CO2 laser thermal processing. As a demonstration of the complete system, fluoro-immunoassays were performed on-chip within the fabricated microfluidic channels to investigate the detection of the cardiac biomarker Troponin I (cTnI), which is considered the gold standard clinical biomarker for the diagnosis of acute myocardial infarction (AMI).15 The complete system of the microfluidic channels and planar lenses are fabricated in a single process by the CO2 laser. The lenses are then post-processed by polishing to remove imperfections. The entire chip is then thermally bonded to seal the microfluidic channels and further remove residual defects on the lens surface. This article presents the first demonstration of capillary microfluidic systems with integrated planar lens optics for biosensing purposes. The integration of on-chip light guiding structures onto autonomous capillary microfluidic devices unlocks the potential of such detection systems to be utilised as a complete working platform for alternative reaction processes requiring fluorescence excitation and/or detection such as DNA hybridisation assays16, 17 and Bioluminescence Resonance Energy Transfer (BRET) assays.18
EXPERIMENTAL
Materials and fabrication
Laser thermal processing is an attractive alternative to established micro manufacturing techniques as such a technology has a rapid turnaround time and does not require fixed photomasks, embossing tools or clean room environment.19, 20, 21, 22, 23, 24, 25, 26 However, the quality of manufactured microfluidics and the surface quality of the lenses are better defined, with fewer manufacturing defects using techniques such as photolithography.
All structures demonstrated in this work were manufactured using a class 2 CO2 laser etching system (Epilog, UK), which allows for repeatable micro feature sizes above 150 μm. The laser system has a maximum power of 40 W, maximum scan speed of 96 mm/s, operates in the near infrared region with a wavelength of 10.6 μm for a spot size of 76–127 μm. All PMMA substrates were purchased precast from Weatherall Equipment & Instruments Ltd (Bucks, UK). All devices were manufactured from CLAREX® precision thin sheet PMMA, made by a specialist cell-casting technique which ensures very high surface uniformity. Designs of the microfluidic systems and 2D planar lenses were carried out using Corel Draw X4 (Corel Software, USA) interfaced directly with the CO2 laser.
The type of substrate that can be laser ablated depends highly on the material absorption properties, the properties of the laser system (spot size, power, etc) and the fine control of the laser/substrate translational stage. PMMA is manufactured based on its physical ability to absorb the radiation produced by the laser, with the power required to etch a given depth depending on the melting and vaporisation temperatures of the polymer. The scan speed and power of the laser can be adjusted across a percentage scale from 0 to 100% of the maximum scan speed and laser power, in 1% increments, across either high or low power modes, as described previously.19 For the final manufacturing phase, the lenses and microfluidic channels were made in a single pass using a laser scan speed of 50% and power of 30%, with the laser set to high power mode.
This work presents the very first instance by which microlenses have been fabricated by CO2 laser ablation, in conjunction with simple backend post processing, and embedded for use within a microfluidic system. Additionally, we described a novel new manufacturing process by which to create microfluidic systems using CO2 laser ablation of PMMA. All microfluidic channels and planar lenses were fabricated by the laser thermally cutting through a given sheet of PMMA, followed by lamination with a modified top layer, containing entry and exit ports and a blank bottom PMMA layer, thereby sealing the microfluidic channel. 500 μm thick PMMA sheets we used in the fabrication process of each layer, thereby producing microfluidic channel and the planar lens of a height dictated by this thickness. The final chip device, comprising a triple laminate design, has a total thickness of 1.5 mm and an approximate surface area of 30 × 30 mm.
In our previous work, microfluidics were of a 2-layer design, with the channels etched into one substrate and sealed with a secondary cover PMMA layer.19, 20, 21 Fabricating the microfluidic channels by the new methodology fundamentally reduces defect formation on the upper and lower surfaces of the microchannels, restricting defects to the side walls only. We have found through the testing phase of the device that the fabrication route of etching the PMMA substrate to form microchannels, where defects are found in the bulk of the channel, results in significant scattering of the excitation and emitted light. Therefore, the newly presented microfluidic manufacturing process is critical to improving the overall detection sensitivity of the fluoro-immunoassay. An added benefit is that the capillary flow characteristics are also improved due to the fewer defects leading to a reduced flow resistance on a migrating fluid.
Thermal bonding was utilised to create the final devices using a methodology described previously.19, 20 Briefly, the stacked laminate layers were placed between two borosilicate glass microscope slides, which in turn were compressed between two 10 mm thick steel plates. The whole structure was placed within a programmable convection oven (Norbetherm, Germany) and heated above the glass transition temperature of the PMMA (170 °C) for approximately 40 min. The oven was then cooled to below the glass transition temperature (80–90 °C) and held at that temperature for 30 min before allowing the stack to cool to room temperature, with the complete process requiring approximately 1.5–2 h. Due to the high surface uniformity of the borosilicate glass and its much higher glass transition temperature relative to PMMA, use of the slides ensures an even compressive pressure across the chip surface and that the surfaces of the chip remain uniform during the bonding procedure, preserving the chips clarity for optical analysis.
Manufacturing limitations
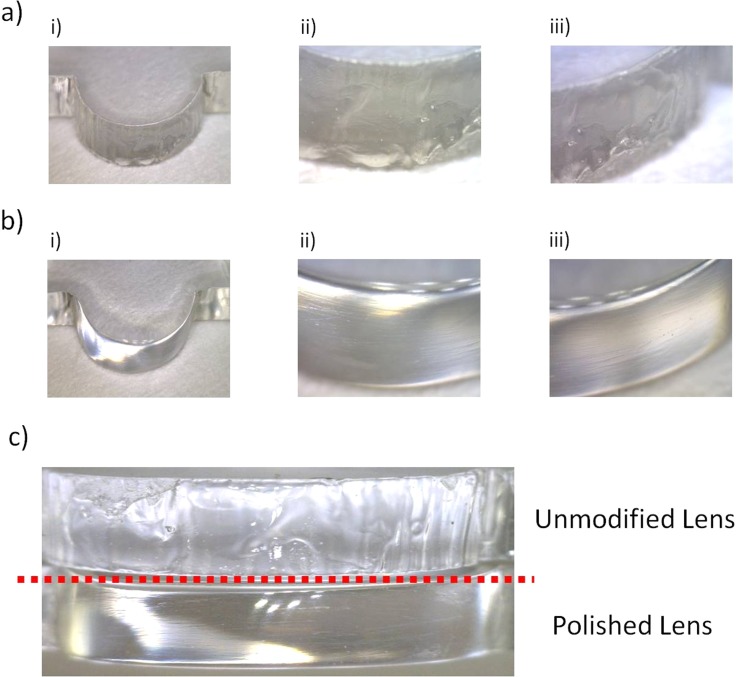
Defects were observed on the surface of the lenses due to the nature of the ablation process and the limitations of the stepper motor used to move the laser beam. The PMMA is effectively vaporised or liquefied under the spot area of the laser. The pattern is formed as the vapours drive out the molten material.22 This results in rough edges on the lenses and microchannel walls, and microbubble formation when vapours become trapped within the molten material. As lenses comprise circular edges, the minimum incremental movement of the translational stage can become a major limiting factor to lens quality. The x-y translational movement of the laser system has a minimum translation of 100 μm, which resulted in the surface of the lens being effectively rippled post processing as the laser beam attempted to form a curved pattern. Both forms of defects found on the surfaces of the lens can be observed in Fig. 1a.
Figure 1.
Photographs of the manufactured 2D lenses and their surface profiles (a) post laser ablation, (b) after polishing and (c) a side by side comparison of the two lens surfaces.
The defects on the lens surface are an inevitable consequence of the laser manufacturing process. However, such defects can be almost entirely removed using an abrasive polishing of the PMMA surface. Initially, the lens surfaces are lightly filed to remove any loose debris and the rippled edges resulting from the laser translation stage. Following this, the surface is sequentially sanded with various grade wet and dry sandpaper, comprising 240, 600, 1200, 2400, and 4000 grit, to remove larger scratches and to improve surface clarity. Finally, the surface clarity is improved further and minor scratches removed using two acrylic polishing compounds (Glass Polish LTD, UK). Post polishing, as the PMMA is heated during bonding above the glass transition temperature, the lens edges liquefy, and upon re-solidification, many of the remaining defects are smoothed out due to surface tension effects of the molten PMMA.
2D planar microlenses
The fabrication route for 2D planar lenses proposed in this article has multiple advantages: the lenses can be fabricated in the same process stage as the microfluidic features; they are self-aligned, require a relatively small footprint on a microchip device and can be easily coupled directly to excitation sources such as LEDs in comparison to optical fibre based waveguides which are numerical aperture dependent on their collection and emission. It is worth noting here that the construction of embedded, rigid waveguides, irrespective of the manufacturing process, have fixed focal points following fabrication and therefore it is critically important to optimise the planar lenses to match the bespoke application.
The optical characteristics of various 2D single and compound lens configurations were examined here using optical ray-tracing modelling. The most promising of these designs was subsequently fabricated and its capability experimentally validated through direct fluorescence excitation and detection tests, as described in Sec. 2E. All optical simulations, performed in OptiCAD 7.0 (OptiCAD Corporation, USA), modelling the focusing of light from a single LED source onto a virtual excitation area of 0.5 mm2 of a microchannel placed within a block of PMMA. This target area directly corresponds to an excitation of a 1 mm long, 500 μm deep channel. In all simulations, a refractive index for PMMA of 1.49 was used.
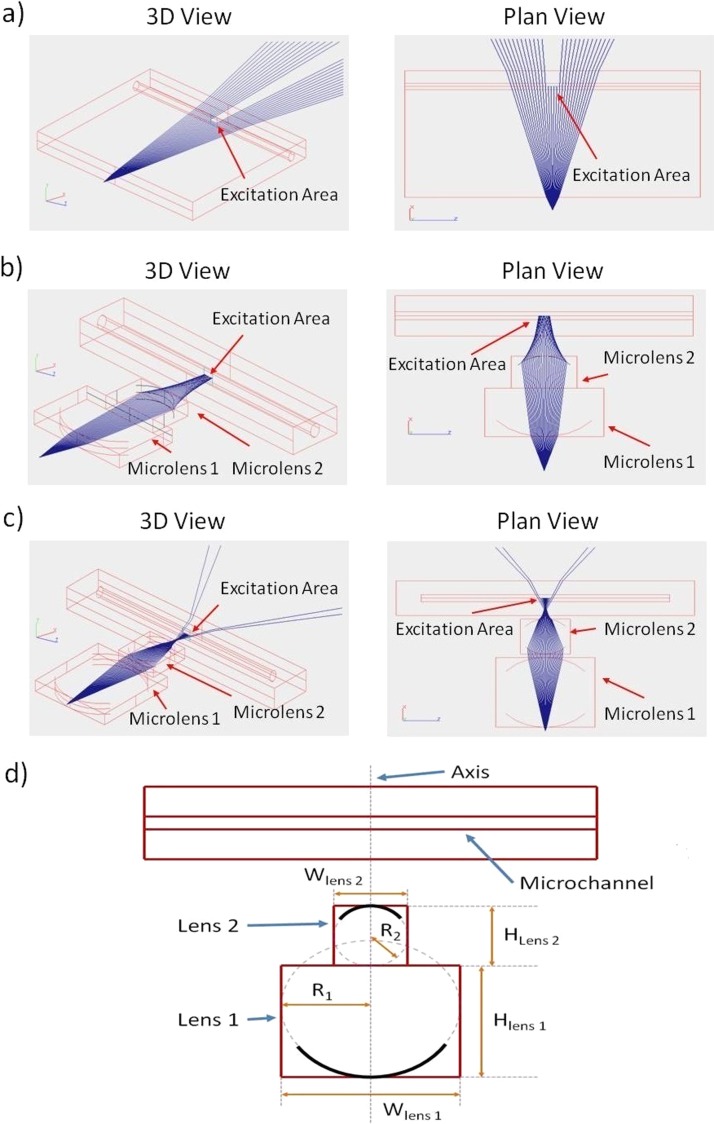
This work only investigated the potential of a single or up to 4 multiple planoconvex or planar concave 2D lens configurations, using selected lens radii of greater than or equal to 2 mm, a geometry that could readily be manufactured by the processes described in this study. Based on the design constraints, the qualitative results from various ray path simulations yielded a set of physical geometries by which both the biconvex and twin biconvex configurations could be implemented and experimentally investigated. Figure 2 shows a direct comparison of ray path simulations for three configurations labelled a to c and the lens parameters of the two final configurations (b,c) are summarised in Table TABLE I..
Figure 2.
Optical path simulation results, illustrating the emission from a single LED light source and the propagation of the resulting light ray as they pass through (a) a blank sheet of PMMA, (b) a single biconvex microlens and (c) a 2 biconvex microlens configuration. In all simulations light is focused onto an area of 0.5mm2, which represents the excitation area within a microfluidic channel/chamber. (d) A diagram of a generic example illustrating the various lens parameters, where R1 is the radius of the lens surface closest to the light source, R2 is the radius of the lens surface farthest from the light source, W represents the lens width in the chip plane and H represents the lens height in the chip plane.
TABLE I.
Summary of the lens properties.
| Lens configuration | Microlens type | R1 (mm) | R2 (mm) | W (mm) | H (mm) | Approximate simulated focal length (mm) |
|---|---|---|---|---|---|---|
| Biconvex | Planoconvex 01 | −10 | 0 | 10 | 15 | |
| Planoconvex 02 | 0 | 4 | 4 | 8 | 7.5 | |
| Dual Biconvex | Planoconvex 01 | −10 | 0 | 5 | 10 | |
| Planoconvex 02 | 0 | 10 | 5 | 10 | ||
| Planoconvex 03 | −5 | 0 | 10 | 20 | ||
| Planoconvex 04 | 0 | 5 | 10 | 20 | 6 |
Fluorescence detection
The two most promising configurations (Figures 2b, 2c) were fabricated using the CO2 laser ablation system. Each configuration was characterised by the detection sensitivity for serial dilutions of fluorescein isothiocyanate (FITC) dye and compared against the no-lens configuration. FITC has excitation and emission wavelengths at λex = 494 nm and λem = 518 nm, respectively, and was made fresh in a stock solution combined on the first instance with ethanol, following the manufacturer's recommendations, and then subsequently diluted with deionised water. This was required as ethanol degrades PMMA and so dilution in water ensured the integrity of the microfluidic channels.
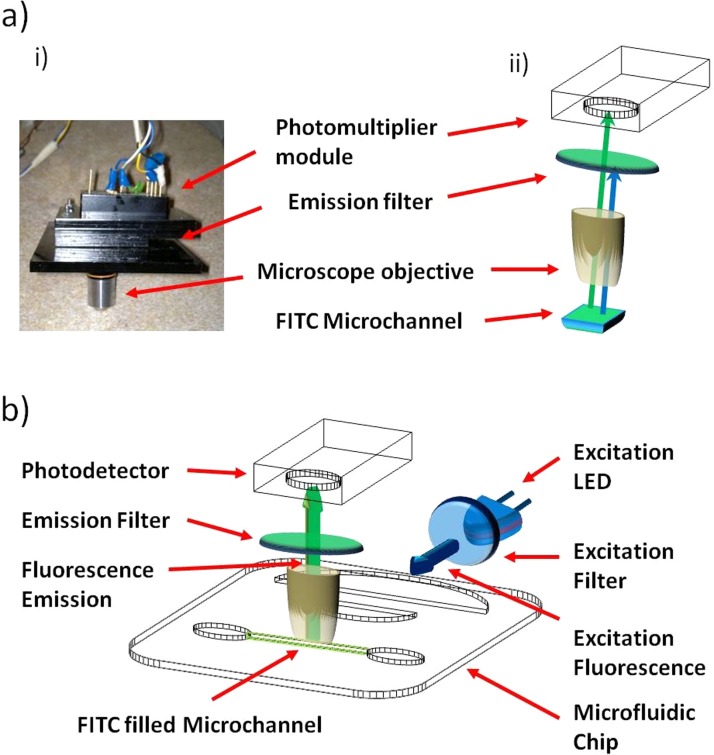
The fluorescence measurements were performed using a custom-made fluorescence detection rig as shown in Figure 3. The LED excitation source comprised a Nichia ultra bright blue LED (NSPB300A, RS Components, Glasgow, UK) and a band pass excitation filter (FB490-10, Thorlabs, UK) with λTrans = 490 ± 5 nm (approximately 48% transmission). The detection apparatus comprised a 10 x microscope objective (Thorlabs, UK), a FITC optimised band pass emission filter (HQ535-50 m, Chroma, USA), λTrans = 535 ± 25 nm (approximately 75–80% transmission), and a H9858 photosensor module (Hamamatsu, UK). All components were housed within a bespoke black PMMA encasing to preserve the alignment of the components relative to each other, while additionally reducing the background light that could be measured by the photodetector. The Microscope objective was placed directly above the microfluidic chip at a distance of approximately 500 μm from the surface of the antibody modified biosensing area, as seen in Figure 3. Such close proximity of the detection optics maximises the collection efficiency, providing a near 2π coverage of the sensing area. Such a methodology is therefore preferable in comparison to light collection only within the chip plane, or using fibre like waveguides where ultimately a significantly smaller collection angle would be likely.12 Measurements were taken with the excitation LED placed at approximately 2–3 mm distance from the microlens and the detector unit was placed orthogonal to the chip plane, as seen in Figure 3b. This setup was preferred as the excitation light from the LED was found to be restricted within the plane of the microfluidic chip.9 Placing the detection apparatus orthogonal to the microfluidic system reduces therefore the possibility of the excitation light rays paths terminating on the detector active area and potentially improves the detection sensitivity of the system.
Figure 3.
(a) (i) Photograph and (ii) schematic of the fluorescence detection apparatus used in conjunction with the microfluidic and wave-guiding devices. (b) Relative orientation of the excitation and detection apparatus with respect to the microfluidic device.
Fluoro-immunoassay reagents and procedure
The fluoro-immunoassay performed in this experiment was carried out using a sandwich style immunoassay configuration, whereby the antibody is immobilised on the surface of the microfluidic chip. The target antigen is bound to the antibody and a secondary labelled antibody binds to a remaining free epitope of the captured cTnI during the reaction phases. This configuration results in the captured antigen being effectively “sandwiched” between the two reaction antibodies. The end point reaction is determined by the detected fluorescence from the secondary labelled antibodies, which relate directly to the concentration of captured antigens.
Reaction reagents comprised monoclonal anti-cardiac cTnI capture antibodies (19C7), FITC conjugated monoclonal anti-cardiac cTnI detection antibodies (16A11) and human cTnI biomarkers, all of which were purchased from Hytest Ltd (Turku, Finland). PBS solution of pH 7.4 was used as the washing buffer (Sigma, UK). Serial dilutions of the cTnI antigen samples were prepared by the dilution of a stock solution with PBS buffer, such that samples of concentrations of 100, 50, 25, 5, 1, and 0.1 ng/ml were obtained. The capture antibodies were immobilised within the microfluidic channel forming the on-chip biosensing area. It has been demonstrated previously that antibodies can be immobilised passively onto the surface of the PMMA chips by direct incubation.26 Several techniques exist in the literature by which chemical modifications of the PMMA surface can lead to improved antibody immobilisation.27, 28 Such methodologies increase however design complexity and therefore a passive immobilisation methodology was adopted here owing to its simplicity. To functionalise the microfluidic channels, following microchip fabrication, the microchannel was loaded by capillary forces with 50 μg/ml solution of the capture antibodies. The openings of the filled chip was sealed with polyimide electrical tape containing a mild silicone adhesive (Pro Power PPC225, Farnell, UK) and the chip was incubated at 4 °C overnight. The polyimide tape prevents evaporation of the test solution during incubation and the silicone adhesive, in addition to being biocompatible, allows for the tape to be removed without any adhesive residue being left behind on the chip. Following incubation the capture antibody solution was removed and the chip washed three times with PBS buffer to remove any unbound antibodies. The buffer was then removed and the channel loaded with a 1 mg/ml solution of BSA, re-sealed with fresh polyimide tape and incubated at room temperature for 2 h. The BSA acts to block the microchannel so that during the sandwich immunoassay non specific binding of the reaction antigen or labelled antibody is minimised and binding to the bound capture antibodies becomes the most dynamically favoured outcome. Finally, the BSA solution was removed and the channel flushed three times with PBS buffer to remove any unbound/residual BSA. The channel was then loaded with PBS until testing.
EXPERIMENTAL RESULTS
Microlens validation
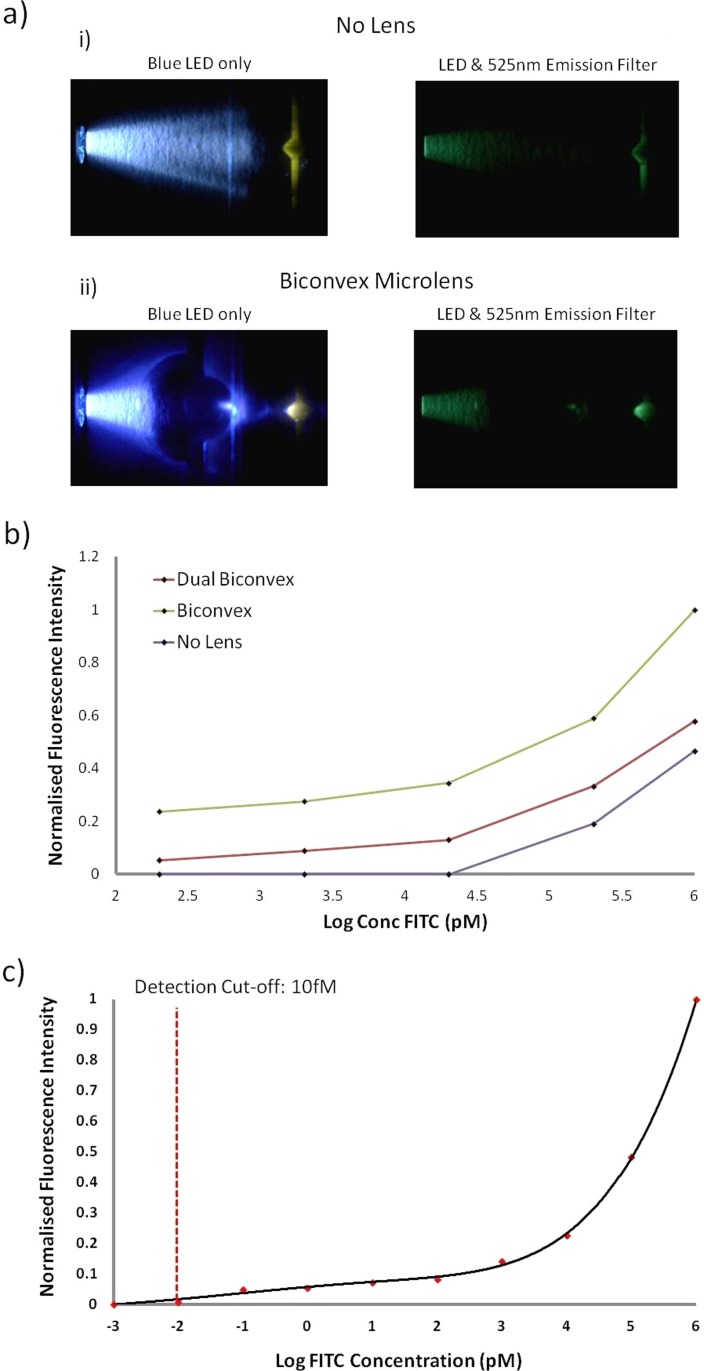
Figure 4 shows photographs of the laser cut PMMA microlens structures focusing the light from a blue LED onto a reaction area within the microfluidic channel containing FITC, with and without the presence of a suitable emission filter. The photographs provide a qualitative visual confirmation that lensing is indeed occurring.
Figure 4.
(a) Photographs of the fabricated lenses focusing light from an LED excitation source with and without a 525 nm emission filter for systems containing (i) no lenses and (ii) a biconvex lens. (b) Comparison of the detected fluorescence for various dilutions of FITC using systems containing no lens, biconvex and dual biconvex lens configurations. (c) Lower detection limit of the optimised developed system for the detected fluorescence of various samples of FITC contained within the microfluidic chamber of the polymeric chip using the biconvex lens design.
To test the focusing ability of the fabricated lenses, a complete system was devised, comprising a single polymeric chip containing a microfuidic channel and microlenses. Tests were performed investigating the lower detection limits of serial dilutions of a stock 1 μM FITC fluorophore solution when loaded into the microfluidic channels of the polymeric device. Both microlens configurations were examined and compared to a system containing no microlenses. In all tests, the excitation source and microfluidic channel retain the same spatial orientation. Sample dilutions examined comprised 1000, 200, 20, 2, and 0.2 nM of FITC. For each tested FITC sample, a new polymeric device was used to eliminate the possibility of cross contamination between samples. The blue LED was coupled to the outer most microlens and the detection rig mounted orthogonal to the detection zone of the fluidic channel. Results of these tests can be seen in Figure 4b, which shows a graph of the detected FITC intensity when interrogating each of the sample dilutions with the three optical configurations. It is found that the use of the planar lenses increased the detection sensitivity, potentially allowing for detection of samples below the examined 0.2 nM threshold. In comparison, a system with no lenses could not detect samples with a concentration of less than 20 nM. The results also demonstrate that the bioconvex configuration offers superior detection limits of approximately 2 to 4 times greater detected fluorescence signal than with the dual biconvex configuration. Based on these results the biconvex configuration was selected for the fluorescence immunoassay tests.
Prior to performing on-chip fluoro-immunoassays, the system was optimised to improve the dynamic range for fluorescence detection of the bound FITC labelled antibodies, post reaction. This primarily comprised adjustment of the photodetector fluorescence radiant sensitivity (as configurable on the photomultiplier module) and the driving voltage of the LED (2.1 V), such that the system is optimised for low level fluorescence detection. If this were not accommodated for, the system may not be sensitive enough to detect samples at lower concentrations, or conversely would reach saturation at higher sample concentrations. The results for the lower detection limits for FITC detection are illustrated in Figure 4, where the system was found to have a detection limit of approximately 10fM of FITC, which is comparable to the performance of larger and more expensive commercial fluorescence readers (e.g., 3fM/well FITC for Gemini XPS microplate reader, Molecular Devices, US and 1fM/well of FITC for FLx800 Fluorescence Microplate Reader, BioTek, US). Such detection capabilities make the current system ideal for performing other low level fluorescence bioassays such as real-time sybr green PCR, fluorescent lifetime measurements, etc., in a similar fashion to the multi use of a general fluorescent reading devices.
cTnI fluoro-immunoassay
A typical fluoro-immunoassay was performed by loading the functionalised chip first with the biomarker containing test solution into the microchannel, sealing with polyimide tape and incubating at room temperature for 30 min. Fluids are simply dispensed into the loading port of the device and migrate to the exit port through no external energy input of forced agitation of the device owing to the use of capillary forces to propel fluids in the microchannel. Samples are removed from the device by simply pipetting the solution at the exit port, which acts to propel the fluid out of the microfluidic channel due to the suction force of the pipette. It is also worth noting that gravitational forces can also be used to remove the test fluid by holding the chip vertically, with the loading port at a point higher than the exit port, thereby the gravitational forces becoming greater than the surface contact forces of the fluid within the device. With the chip in this orientation a simple tissue or fibrous membrane, which due to the porous nature of the materials, acts as a capillary pump drawing out the fluid while capturing the waste solution from the device. Both methodologies provide great simplicity of use for the microfluidic device.
Following the initial incubation of the biomarker, the solution was then removed and the channel washed three times with PBS buffer to remove any unbound biomarker. The channel was loaded afterwards with a 100 μg/ml solution of the FITC labelled detection antibody, sealed with fresh polyimide tape and incubated for 30 min at room temperature within a darkened box so as to prevent degradation of the FITC during incubation. The labelled antibody containing solution was then removed and the microchannel washed three times with PBS buffer to remove any unbound antibodies. The sample was then ready for fluorescence detection measurements.
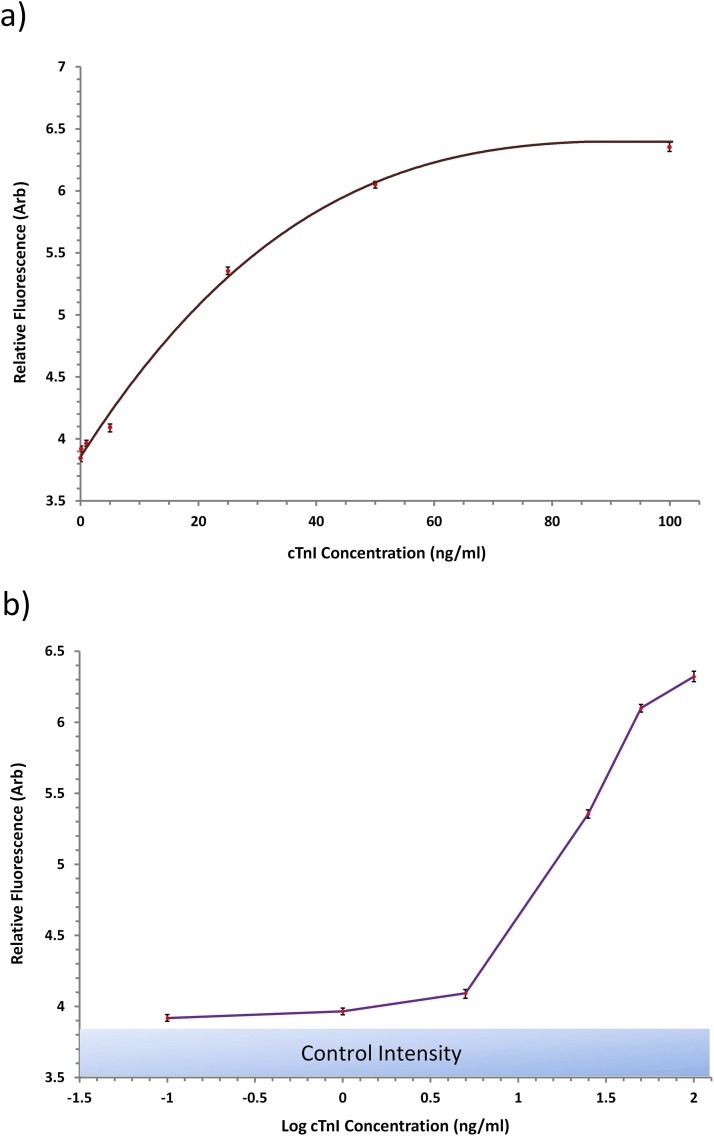
The test procedure was performed with independently fabricated devices for all the aforementioned concentrations of cTnI and also for a sample of PBS buffer containing no cTnI, which acted as a control sample. Results for the detected fluorescence for the examined samples can be seen in Figure 5, where the raw fluorescence data against concentration is illustrated. The fluorescence detected at each concentration was measured from three independent samples and the presented results represent the average fluorescence. From the measurements, a reasonable fit could be made of the data points to construct a calibration curve of the immune-detection system as seen in Figure 5a. The system exhibits a near linear response for detected concentration below 50 ng/ml and the dynamic range of the system is approximately 0–100 ng/ml of cTnI. The lowest concentration measured by the system was 0.1 ng/ml, which is within the threshold of clinically applicable concentration detection limits. Following standard methodologies to determine the lower limit of detection, measurements were recording of 15 blank samples, the average was determined, and the corresponding concentration determined of the average + 3 standard deviations. The system lower limit of detection was found to be approximately 0.08 ng/ml of cTnI. Although there are several alternative commercial techniques and research based biosensors capable of detection limits lower than the demonstrated platform,15, 29 the clinically relevant cut-off levels required for diagnostic use is in the range of 0.01–0.1 ng/ml of biomarker.15, 30, 31 Therefore, the detection capabilities of our presented detection platform, with a lower limit of detection (LLD) of 0.08 ng/ml is within the diagnostics threshold and highly applicable for clinical use. In contrast, comparing against a typical clinically implemented point of care device such as the Cobas h232 (Roche Diagnostics LTD), our lower detection limit surpass this benchmark, further reinforcing the point of care (POC) potential of the current platform.
Figure 5.
Results from the endpoint of the fluoro-immunoassay for the detection of cTnI. (a) Calibration curve and (b) log concentration graphs of the detected fluorescence.
If such a device was to be implemented at the POC for “real” operational scenarios, it would be required to operate using more challenging samples, where biomarkers would be contained in a patient's blood/serum samples. The work we have presented has focused on the proof of concept of the immuno-detection platform by which capillary systems operate with integrated optical components, and as such, has currently only been demonstrated using samples in buffer solutions. It is anticipated that the direct use of blood samples within the device would yield a higher LLD due to interferences of the constituents within blood. However, such challenges can be readily overcome by filtration of blood samples using porous membranes32 or by enhancement of the biosensing element of the device. Both techniques would be compatible with the presented biomicrofluidic platform and will be the focus of future research.
CONCLUSIONS
CO2 laser ablation techniques have been demonstrated to be applicable for the rapid manufacture of PMMA based substrates to create both embedded micro optical lenses and novel, new capillary action microfluidics channels. The use of backend processing and thermal annealing allows for the surface quality of the fabricated lenses to be improved upon in comparison to their native form following laser processing. The resulting manufactured device readily allows for the focusing of the light from a standard LED light source onto a finite area within the chip, offering a marked improvement in detection sensitivity (approximately 2 to 4 times) of FITC samples for both fabricated lens configurations, as compared to a system where no lens is present We found that the optimised system, comprised of a single biconvex lens, allowed for a lower FITC detection limit of 10fM, which is comparable to the performance of larger and more complex commercial instrumentation.
LEDs offer interesting alternative optical excitation sources as they are cheap, low power consuming, readily available over a large range of wavelengths, allow for direct excitation of all prominent fluorophores/quantum dots/fluorescent proteins and can be readily integrated into miniaturised platforms. The manufactured device, in conjunction with a rudimentary and portable fluorescence detection rig, was demonstrated to perform fluorescence based immunoassays with a high degree of sensitivity. The current system has been optimised for the use of FITC excitation and detection, but could readily be adapted for the use of alternative fluorophores or fluorescing substances (quantum dots, fluorescent proteins, etc) by adjustment of the LED source and the excitation and emission filters. Furthermore, the PMMA microchannel device platform can be readily applied to alternative biological assay formats, such as real-time PCR and liquid phase bioluminescent based assays, due to its excellent material properties and biocompatibility.
With the proposed platform, we have demonstrated a low cost and power detection system of a prominent cardiac biomarker, cTnI, using standard fluoro-immunoassay technology. To achieve this we have successfully brought together several complimentary technological features within the presented biomicrofluic device, which include capillary flow based microfluidics, on-chip optical components and embedded biosensing elements. The device has been designed to operate complimentary to a bespoke fluorescence excitation and detection rig to create a complete and portable system which addresses the address challenges of transferral of microfluidic/lab-on-a-chip technology out of the laboratory and into the realms of real work operational use. Despite the simplicity of the current platform, the device is capable of cTnI detection at clinically acceptable concentrations, with a lower detection limit of 0.08 ng/ml. We therefore believe that the microfabricated chip device and the fluorescence detection rig devised in this work offers considerable potential for use within a clinical environment as POC instrumentation and for alternative portable diagnostics based uses, such as within environmental sampling, on-site hazardous chemical detection, etc.
References
- Kuswandi B., Nuriman J., Huskens J., and Verboom W., “Optical sensing systems for microfluidic devices: A review,” Anal. Chim. Acta 601, 2, 141–155 (2007). 10.1016/j.aca.2007.08.046 [DOI] [PubMed] [Google Scholar]
- Myers F. B. and Lee L. P., “Innovations in optical microfluidic technologies for point-of-care diagnostics,” Lab Chip 8, 2015–2031 (2008). 10.1039/b812343h [DOI] [PubMed] [Google Scholar]
- Camou S., Fujita H., and Fujii T., “PDMS 2D optical lens integrated with microfluidic channels: Principle and characterization,” Lab Chip 3(1), 40–45 (2003). 10.1039/b211280a [DOI] [PubMed] [Google Scholar]
- Rosenauer M. and Vellekoop M. J., “A novel microfluidic system for fluorescent sample analysis fabricated by rapid prototyping,” IEEE SENSORS 2008 Conference (IEEE, 2008). 10.1109/ICSENS.2008.4716401 [DOI]
- Guo H., Zhao P., Xiao G., Zhang Z., and Yao J., “Optical manipulation of microparticles in an SU-8/PDMS hybrid microfluidic chip incorporating a monolithically integrated on-chip lens set,” IEEE J. Sel. Top. Quantum Electron. 16(4), 919–926 (2010). 10.1109/JSTQE.2009.2033212 [DOI] [Google Scholar]
- Hsieh J., Weng C. J., Yin H. L., and Chou H. Y., “Realisation and characterization of SU-8 micro cylindrical lenses for in-plane micro optical systems,” Microsyst. Technol. 11, 429–437 (2005). 10.1007/s00542-004-0463-7 [DOI] [Google Scholar]
- Rosenauer M., Bechugger W., Finoulst I., Verhaert P., and Vellekoop M., “Miniaturized flow cytometer with 3D hydrodynamic particle focusing and integrated optical elements applying silicon photodiodes,” Microfluid Nanofluid 10, 761–771 (2011). 10.1007/s10404-010-0707-z [DOI] [Google Scholar]
- Wang Z., El-Ali J., Engelund M., Gotsaed T., Perch-Nielsen I. R., Mogensen K. B., Snakenborg D., Kutter J. P., and Wolff A., “Measurements of scattered light on a microchip flow cytometer with integrated polymer based optical elements,” Lab Chip 4, 372–377 (2004). 10.1039/b400663a [DOI] [PubMed] [Google Scholar]
- Seo J. and Lee L. P., “Disposable integrated microfluidics with self-aligned planar microlenses,” Sens. Actuators B 99(2–3), 615–622 (2004). 10.1016/j.snb.2003.11.014 [DOI] [Google Scholar]
- Roulet J.-C., Voelkel R., Herzig H. P., Verpoorte S., de Rooij N. F., and Daendliker R., “Microlens systems for fluorescence detection in chemical microsystems,” Opt. Eng. 40(05), 814–821 (2001). 10.1117/1.1359522 [DOI] [Google Scholar]
- Naessens K., Ottevaereb H., Van Daele P., and Baets R., “Flexible fabrication of microlenses in polymer layers with excimer laser ablation,” Appl. Surf. Sci. 208–209, 159–164 (2003). 10.1016/S0169-4332(02)01359-4 [DOI] [Google Scholar]
- Watts B. R., Zhang Z., Xu C. Q., Cao X., and Lin M., “Scattering detection using a photonic-microfluidic integrated device with on-chip collection capabilities,” Electrophoresis (published online 2013). 10.1002/elps.201300195 [DOI] [PubMed]
- Bilenberg B., Nielsen T., Clausen B., and Kristensen A., “PMMA to SU-8 bonding for polymer based lab-on-a-chip systems with integrated optics,” J. Micromech. Microeng. 14, 814–818 (2004). 10.1088/0960-1317/14/6/008 [DOI] [Google Scholar]
- Fleger M. and Neyer A., “PDMS microfluidic chip with integrated waveguides for optical detection,” Microelectron. Eng. 83, 1291–1293 (2006). 10.1016/j.mee.2006.01.086 [DOI] [Google Scholar]
- Mohammed M. I. and Desmulliez M. P. Y., “Lab-on-a-chip based immunosensor principles and technologies for the detection of cardiac biomarkers: A review,” Lab Chip 11, 569–595 (2011). 10.1039/c0lc00204f [DOI] [PubMed] [Google Scholar]
- Mohammed M. I., Sills G. J., Brodie M. J., Ellis E., and Girkin J. M., “A complete miniaturised genotyping system for the detection of single nucleotide polymorphisms in human DNA samples,” Sens. Actuators B 139, 1, 83–90 (2009). 10.1016/j.snb.2008.09.015 [DOI] [Google Scholar]
- Girkin J. M., Mohammed M. I., and Ellis E. M., “A miniaturised integrated biophotonic point-of care genotyping system,” Faraday Discuss. 149, 115–123 (2011). 10.1039/c005271j [DOI] [PubMed] [Google Scholar]
- Dacres H., Dumancic M M., Horne I., and Trowell S. C., “Direct comparison of fluorescence- and bioluminescence-based resonance energy transfer methods for real-time monitoring of thrombin-catalysed proteolytic cleavage,” Biosens. Bioelectron. 24(5), 1164–1170 (2009). 10.1016/j.bios.2008.07.021 [DOI] [PubMed] [Google Scholar]
- Mohammed M. I., Abraham E., and Desmulliez M. P. Y., “Rapid laser prototyping of valves for microfluidic autonomous systems,” J. Micromech. Microeng. 23, 035034 (2013). 10.1088/0960-1317/23/3/035034 [DOI] [Google Scholar]
- Mohammed M. I. and Desmulliez M. P. Y., “The manufacturing of packaged capillary action microfluidic systems by means of CO2 laser processing,” J. Microsyst. Technol. 19(6), 809–818 (2013). 10.1007/s00542-013-1792-1 [DOI] [Google Scholar]
- Mohammed M. I. and Desmulliez M. P. Y., “CO2 laser machining of fully packaged autonomous microfluidic systems,” DTIP 2012 - Symposium on Design, Test, Integration and Packaging of MEMS/MOEMS, Art. No. 6235292, pp. 63–70 (EDA Publishing, 2012).
- Klank H., Kutter J. P., and Geschke O., “CO2-laser micromachining and back-end processing for rapid production of PMMA-based microfluidic systems,” Lab Chip 2, 242–246 (2002). 10.1039/b206409j [DOI] [PubMed] [Google Scholar]
- Malek C. G. K., “Laser processing for bio-microfluidics applications (part I),” Anal. Bioanal. Chem. 385(8), 1351–1361 (2006). 10.1007/s00216-006-0514-2 [DOI] [PubMed] [Google Scholar]
- Malek C. G. K., “Laser processing for bio-microfluidics applications (part II),” Anal. Bioanal. Chem. 385(8), 1362–1369 (2006). 10.1007/s00216-006-0517-z [DOI] [PubMed] [Google Scholar]
- Nayak N. C., Lam Y. C., Yue C. Y., and Sinha A. T., “CO2-laser micromachining of PMMA: The effect of polymer molecular weight,” J. Micromech. Microeng. 18, 095020 (2008). 10.1088/0960-1317/18/9/095020 [DOI] [Google Scholar]
- Irawan R., Tjin S. C., Fang X., and Fu C. Y., “Integration of optical fiber light guide, fluorescence detection system, and multichannel disposable microfluidic chip,” Biomed. Microdevices 9, 413–419 (2007). 10.1007/s10544-007-9052-8 [DOI] [PubMed] [Google Scholar]
- Wang H., Meng S., Guo K., Liu Y., Yang P., Zhong W., and Liu B., “Microfluidic immunosensor based on stable antibody-patterned surface in PMMA microchip,” Electrochem. Commun. 10, 447–450 (2008). 10.1016/j.elecom.2008.01.005 [DOI] [Google Scholar]
- Charles P. T., Adams A. A., P. B.Howell, Jr., Trammell S. A., Deschamps J. R., and Kusterbeck A. W., “Fluorescence-based sensing of 2,4,6-Trinitrotoluene (TNT) using a multi-channeled poly(methyl methacrylate) (PMMA) microimmunosensor,” Sensors 10, 876–889 (2010). 10.3390/s100100876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan V. S. and Jarolim P., “How to interpret elevated cardiac troponin levels,” Circulation 124, 2350–2354 (2011). 10.1161/CIRCULATIONAHA.111.023697 [DOI] [PubMed] [Google Scholar]
- McDonnell B., Hearty S., Leonard P., and O'Kennedy R., “Cardiac biomarkers and the case for point-of-care testing,” Clin. Biochem. 42, 549–561. (2009). 10.1016/j.clinbiochem.2009.01.019 [DOI] [PubMed] [Google Scholar]
- Morrow D. A., Cannon C. P., Jesse R. L., Newby L. K., Ravkilde J., Storrow A. B., Wu A. H. B., and Christenson R. H., “National academy of clinical biochemistry laboratory medicine practice guidelines: Clinical characteristics and utilization of biochemical markers in acute coronary syndromes,” Clin. Chem. 53(4), 552–574 (2007). 10.1373/clinchem.2006.084194 [DOI] [PubMed] [Google Scholar]
- Gervais L. and Delamarche E., “Toward one-step point-of-care immunodiagnostics using capillary-driven microfluidics and PDMS substrates,” Lab Chip 9, 3330–3337 (2009). 10.1039/b906523g [DOI] [PubMed] [Google Scholar]