Abstract
The colonization of the New World by the Palearctic species Drosophila subobscura was first detected in 1978 in South America and around 1982 in western North America. The ensuing dramatic expansion of the species, in territory as well as numbers, provides an opportunity for studying evolution in a scale rarely possible. We have used 10 restriction endonucleases to analyze the mitochondrial DNA (mtDNA) of individuals from 23 widely dispersed localities. Only two mtDNA composite morphs have been detected in the Americas. None of the two morphs has been found in Africa, and only one in the Atlantic islands; but both are widespread in Europe, which provides no clue of the precise geographic origin of the colonizers. The amount of nucleotide-substitution polymorphism detected in D. subobscura is typical for animals, but it is greater in the Old than in the New World, presumably due to the recent colonization by a limited number of colonizers. Assuming standard evolutionary rates of mtDNA base substitution, the mtDNA morphs found in D. subobscura can be traced to a single one that existed no less than one million years ago. We argue against the inference that the D. subobscura flies now living descend from only one or a few females that lived at that time. This type of inference, which we call the “Mother Eve hypothesis,” has been made to conclude that the human population went through a severe constriction about 200,000 years ago, so that all living humans descend from only one or a few women who lived at that time. The Mother Eve hypothesis is fallacious.
Keywords: population genetics, colonization, human population size, founder effects, Mother Eve hypothesis
Full text
PDF

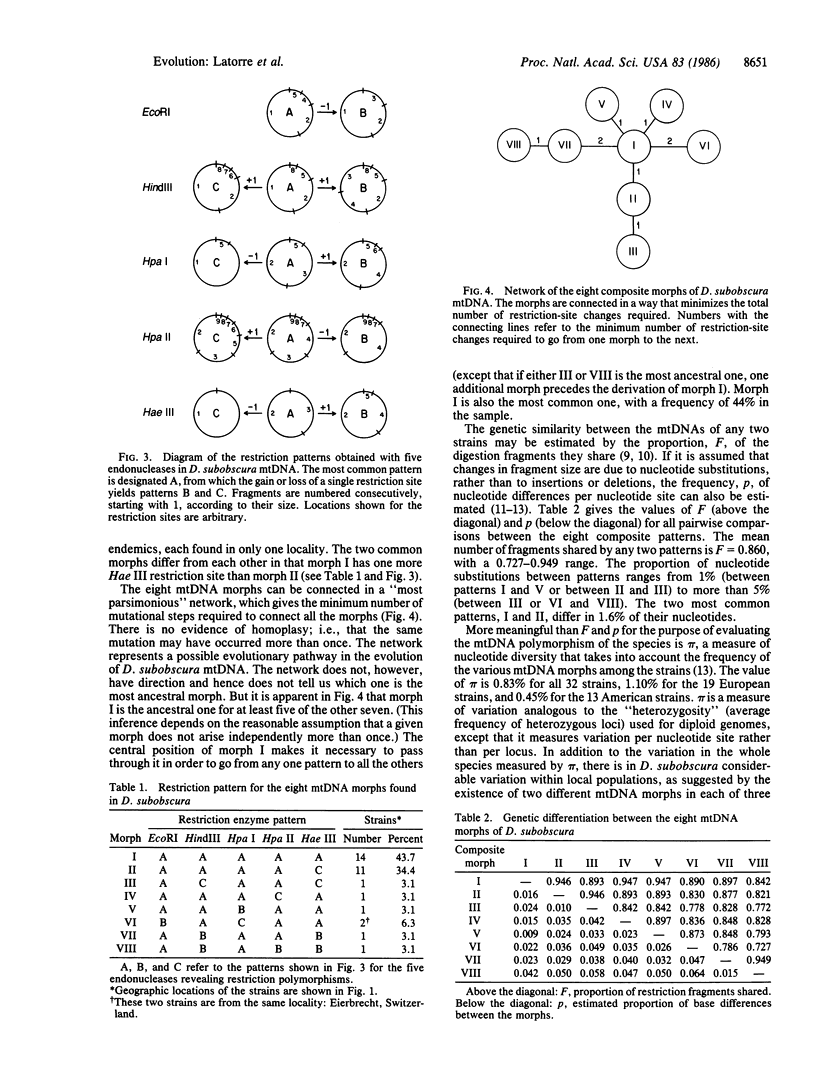
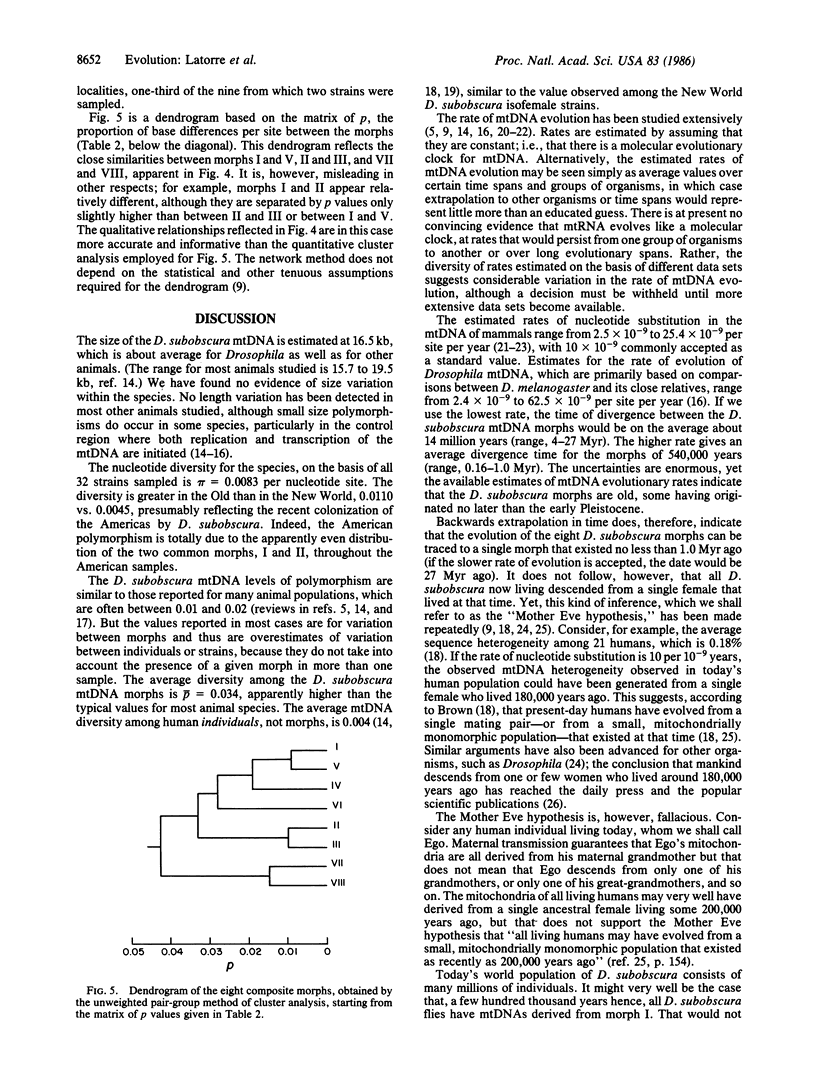

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avise J. C., Lansman R. A., Shade R. O. The use of restriction endonucleases to measure mitochondrial DNA sequence relatedness in natural populations. I. Population structure and evolution in the genus Peromyscus. Genetics. 1979 May;92(1):279–295. doi: 10.1093/genetics/92.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avise J. C., Neigel J. E., Arnold J. Demographic influences on mitochondrial DNA lineage survivorship in animal populations. J Mol Evol. 1984;20(2):99–105. doi: 10.1007/BF02257369. [DOI] [PubMed] [Google Scholar]
- Brown W. M., George M., Jr, Wilson A. C. Rapid evolution of animal mitochondrial DNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1967–1971. doi: 10.1073/pnas.76.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. M. Polymorphism in mitochondrial DNA of humans as revealed by restriction endonuclease analysis. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3605–3609. doi: 10.1073/pnas.77.6.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. M., Prager E. M., Wang A., Wilson A. C. Mitochondrial DNA sequences of primates: tempo and mode of evolution. J Mol Evol. 1982;18(4):225–239. doi: 10.1007/BF01734101. [DOI] [PubMed] [Google Scholar]
- Cann R. L., Brown W. M., Wilson A. C. Polymorphic sites and the mechanism of evolution in human mitochondrial DNA. Genetics. 1984 Mar;106(3):479–499. doi: 10.1093/genetics/106.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen E. S., Thoday J. M., Dover G. Rate of turnover of structural variants in the rDNA gene family of Drosophila melanogaster. Nature. 1982 Feb 18;295(5850):564–568. doi: 10.1038/295564a0. [DOI] [PubMed] [Google Scholar]
- Densmore L. D., Wright J. W., Brown W. M. Length variation and heteroplasmy are frequent in mitochondrial DNA from parthenogenetic and bisexual lizards (genus Cnemidophorus). Genetics. 1985 Aug;110(4):689–707. doi: 10.1093/genetics/110.4.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa M., Kishino H., Yano T. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J Mol Evol. 1985;22(2):160–174. doi: 10.1007/BF02101694. [DOI] [PubMed] [Google Scholar]
- Lansman R. A., Shade R. O., Shapira J. F., Avise J. C. The use of restriction endonucleases to measure mitochondrial DNA sequence relatedness in natural populations. III. Techniques and potential applications. J Mol Evol. 1981;17(4):214–226. doi: 10.1007/BF01732759. [DOI] [PubMed] [Google Scholar]
- Miyata T., Hayashida H., Kikuno R., Hasegawa M., Kobayashi M., Koike K. Molecular clock of silent substitution: at least six-fold preponderance of silent changes in mitochondrial genes over those in nuclear genes. J Mol Evol. 1982;19(1):28–35. doi: 10.1007/BF02100221. [DOI] [PubMed] [Google Scholar]
- Nei M., Li W. H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M., Tajima F. DNA polymorphism detectable by restriction endonucleases. Genetics. 1981 Jan;97(1):145–163. doi: 10.1093/genetics/97.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevosti A., Garcia M. P., Serra L., Aguade M., Ribo G., Sagarra E. Association between allelic isozyme alleles and chromosomal arrangements in European populations and Chilean colonizers of Drosophila subobscura. Isozymes Curr Top Biol Med Res. 1983;10:171–191. [PubMed] [Google Scholar]
- Solignac M., Monnerot M., Mounolou J. C. Mitochondrial DNA evolution in the melanogaster species subgroup of Drosophila. J Mol Evol. 1986;23(1):31–40. doi: 10.1007/BF02100996. [DOI] [PubMed] [Google Scholar]
- Upholt W. B. Estimation of DNA sequence divergence from comparison of restriction endonuclease digests. Nucleic Acids Res. 1977;4(5):1257–1265. doi: 10.1093/nar/4.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]