Abstract
Background:
Head and neck squamous cell carcinoma is often associated with human papillomavirus (HPV) infection. Positive HPV status has been associated with increased response to treatment and improved prognosis in terms of recurrence-free and overall survival. In certain instances, diagnosis is performed through fine-needle aspiration of lymph nodes with metastatic carcinoma, often demonstrating extensive tumor necrosis. We evaluated the effect of tumor necrosis on deoxyribonucleic acid (DNA) adequacy for HPV molecular testing.
Materials and Methods:
Retrospective review of the pathology files at our institution identified cases of squamous cell carcinoma (SCC) diagnosed by fine-needle aspiration (FNA) on which HPV DNA molecular testing was performed. The cases were classified according to percent tumor necrosis into three categories (<10% necrosis, 10-70% necrosis and >70% necrosis) and the percentage of cases with adequate HPV DNA for molecular testing in each of the categories was compared. When available, p16 immunohistochemistry performed on the cases was compared with HPV status by molecular testing.
Results:
A total of 70 cases from 67 patients were included in the study. Adequate DNA for molecular HPV testing was obtained from samples of 47 cases (67%) while samples from 23 cases (33%) were inadequate for molecular testing. Of the adequate samples, 36 (77%) were positive and 11 (23%) were negative for high-risk HPV. Adequate DNA for testing was obtained in 22 out of 33 cases showing no necrosis (67%), 10 out of 16 cases showing partial necrosis (63%) and in 13 out of 17 cases showing extensive necrosis (76%).
Conclusion:
Our study found that HPV molecular testing is not influenced by percent tumor necrosis or method by which FNA was performed. We believe that a portion of the FNA specimen obtained from head and neck lesions diagnosed as SCC during the rapid on-site evaluation should be sent for HPV DNA testing, independent of the amount of tumor necrosis, thus guaranteeing availability of specimen for HPV testing.
Keywords: Head and neck, human papillomavirus, molecular testing, p16, squamous cell carcinoma
INTRODUCTION
Squamous cell carcinoma (SCC) is the most common primary carcinoma of the head and neck region, with histology that varies from well-differentiated and keratinizing to undifferentiated and non-keratinizing types.[1] Its incidence has increased steadily over the last three decades and a total of 40,250 new cases of oral cavity and pharynx carcinoma were diagnosed in the US in 2012 with approximately 7,850 disease related deaths.[2]
Poor oral hygiene as well as tobacco and alcohol use are well-known risk factors for the development of head and neck squamous cell carcinoma (HNSCC).[3] However, up to 60% of the cases have detectable underlying infection by oncogenic serotypes of human papillomavirus (HPV), particularly serotype 16.[1,4,5] The oncogenic potential of high-risk (HR) HPV serotypes is related to its ability to introduce the E5, E6 and E7 genes into the host genome, resulting in loss of tumor suppressor function (p21, p53 and pRb pathways, respectively) and defects in apoptosis.[6]
It has been shown that the presence of HPV deoxyribonucleic acid (DNA) in HNSCC and p16 expression are markers of improved prognosis in terms of recurrence-free and overall survival.[7,8,9,10] Interestingly, recent publications have shown that p16 over-expression is associated with lower recurrence rates regardless of the main treatment modalities (i.e., surgical excision and/or radiotherapy).[11] This significant difference in the outcome between HPV-positive and HPV-negative HNSCC has made it a common practice to evaluate these lesions for the presence of underlying HPV infection, either by the use of surrogate markers such as p16 immunohistochemistry or by HPV DNA detection by molecular studies.
HPV-related HNSCC tend to occur in younger patients without history of tobacco or alcohol use.[5] Most of the patients have clinically identifiable lesions, located in the tonsils or base of tongue; in approximately 3-5% of cases, patients present with higher stage disease in the form of metastatic cystic cervical lymph nodes. Fine-needle aspiration (FNA) is the most commonly employed diagnostic modality for the initial evaluation of these nodes.[5,12,13] The diagnostic material obtained by FNA is also used for assessment of the HPV status of the tumor. In cytology preparations, SCC characteristically demonstrates large cells with abundant dense, orange or yellow-staining cytoplasm. The nuclei might be small and pyknotic in well-differentiated lesions or might be large and irregular with vesicular and clumped chromatin in higher grade tumors. Nucleus to cytoplasm ratio increases as the carcinoma becomes more poorly-differentiated. Ghost cells are not uncommon, as are keratin pearls in cases of keratinizing SCC. The background is commonly “dirty,” with necrotic debris, abundant neutrophils and dyskeratotic cells.
It is not uncommon to encounter an FNA specimen mostly comprising necrotic material and a few diagnostic viable tumor cells from the lymph node containing metastatic HNSCC; thus, limiting the cellularity of the cell-block preparation and p16 immunohistochemistry analysis [Figure 1]. The adequacy of such necrotic FNA specimens for the evaluation of HPV DNA by molecular methods has not been well-established. In this study, we present our institutional experience with the effect of extensive tumor necrosis in lymph nodes with metastatic HNSCC on HPV DNA evaluation and detection.
Figure 1.
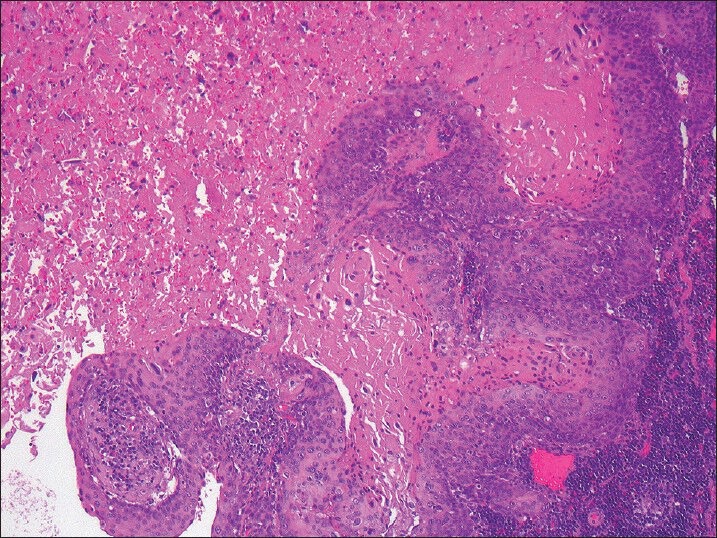
Metastatic squamous cell carcinoma to cervical lymph node with extensive tumor necrosis (H and E, ×100)
MATERIALS AND METHODS
The institutional pathology files of the Hospital of the University of Pennsylvania were retrospectively searched for cases of SCC diagnosed by FNA on which HPV DNA testing had been performed (2009 to 2012). The following data points were recorded on each case: Patient age, sex, prior history of SCC, site of FNA, method of localizing the lesion (manual vs. radiologic guidance), number of passes and diagnosis rendered at rapid on-site evaluation (ROSE). The procedure for ROSE included expressing a portion of the specimen onto a glass slide; this was divided between an air-dried slide for onsite Diff-Quick® staining and alcohol slide for later Papanicolau staining. The specimen in the needle was rinsed into Normosol® for cell block preparation and HR HPV analysis. The results of molecular testing for HPV DNA (performed on the needle rinse) and p16 immunohistochemistry (performed on cell blocks when available) were also recorded.
The cases with cytology slides available for review were examined to confirm the diagnosis of metastatic HNSCC and assess percent tumor necrosis. The percent necrosis in each case was semi-quantitatively assessed on a sliding scale as follows: No necrosis (none or less than 10% necrosis seen in the entire specimen), partial necrosis (10-70% of necrosis seen in the entire specimen) and extensive necrosis (more than 70% necrosis seen in the entire specimen) [Figures 2 and 3].
Figure 2.
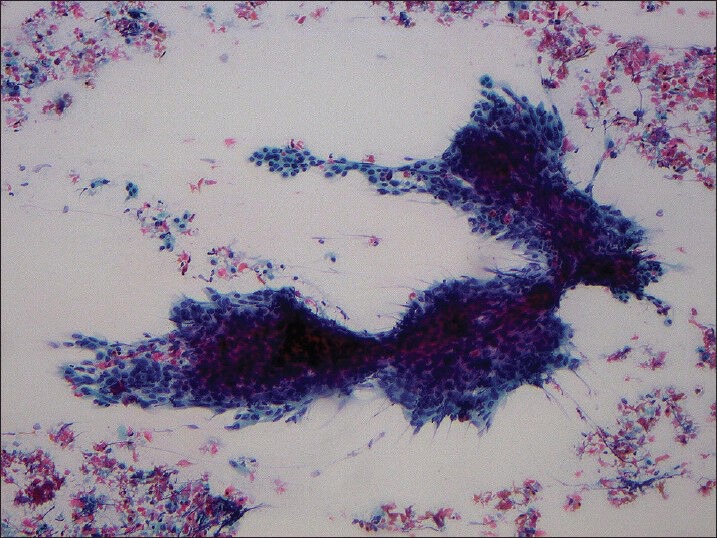
Smear obtained from squamous cell carcinoma metastatic to cervical lymph node showing less than 10% tumor necrosis (Papanicolaou stain, ×100)
Figure 3.
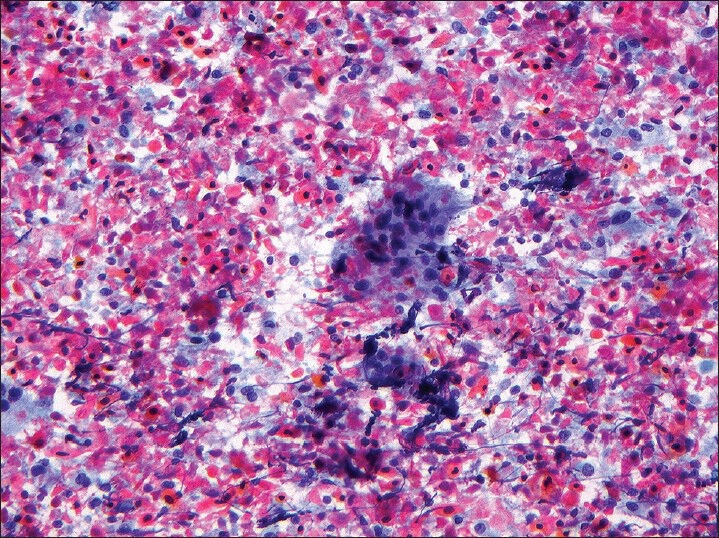
Smear obtained from squamous cell carcinoma metastatic to cervical lymph node showing more than 70% tumor necrosis (Papanicolaou stain, ×200)
Molecular testing for HPV
Molecular testing for HR HPV was performed on all specimens in the molecular laboratory of the Hospital of the University of Pennsylvania. DNA extraction from the FNA needle rinse performed utilizing spin column-based nucleic acid purification (Qiagen). HPV testing was then performed (Cervista® HPV HR from Hologic), according to manufacturer's instructions, allowing for detection of 14 serotypes of HPV designated as HR by the International Agency for Research on Cancer (serotypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68).
Immunohistochemistry for p16
When available, immunohistochemistry for p16 was performed on 4-μm sections cut from formalin-fixed, paraffin-embedded cell blocks and stained using monoclonal antibody to p16 (MTM Labs/Ventana®; clone E6H4; prediluted). Automated staining was performed on a Leica BOND-III® automated slide stainer (Leica Microsystems GmBH) according to standard protocols. Detection was performed utilizing Leica's refine detection kit (Leica Microsystems GmBH). Hematoxylin was utilized as a counterstain. Positive immunostaining was determined by diffuse nuclear or cytoplasmic staining in the tumor cells [Figure 4].
Figure 4.
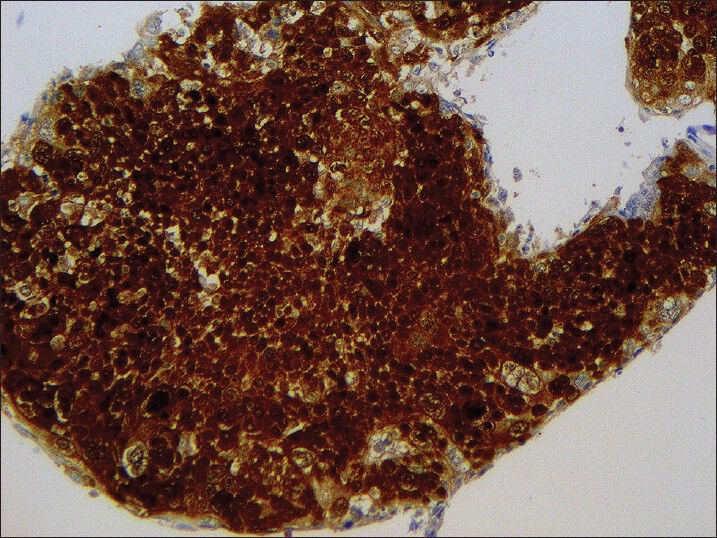
p16 immunohistochemical stain performed on cell block from metastatic squamous cell carcinoma to cervical lymph node, showing strong and diffuse nuclear and cytoplasmic positivity (Hematoxylin counterstain, ×200)
RESULTS
The results are illustrated in Tables 1 and 2. The case cohort included 70 cases (n = 70) from 67 patients (49 male and 18 female) ranging in age from 29 to 90 years (average age 62 years). Cytology slides were available for review in all but four cases. p16 immunohistochemistry was performed on cell blocks in 43 cases. A total of 37 patients (53%) had a prior diagnosis of HNSCC and 33 subjects (47%) had no history of HNSCC. Of the latter group, 13 patients (13/33; 19%) had known, but unbiopsied oropharyngeal lesions, 13 (13/33; 19%) had metastatic SCC of unknown origin, 3 (3/33; 4%) had a history of non-HNSCC (cervix – 1; skin – 2), 3 (3/33; 4%) had known but unbiopsied masses at sites other than oropharynx (parotid – 1; esophagus – 1; lung – 1) and 1 (1/33; 1%) had recently found bilateral cervical lymphadenopathy without prior tissue diagnosis at the time.
Table 1.
Molecular HPV DNA results according to method of obtaining specimens, specimen quality, specimen site and history of prior HNSCC (n=70)
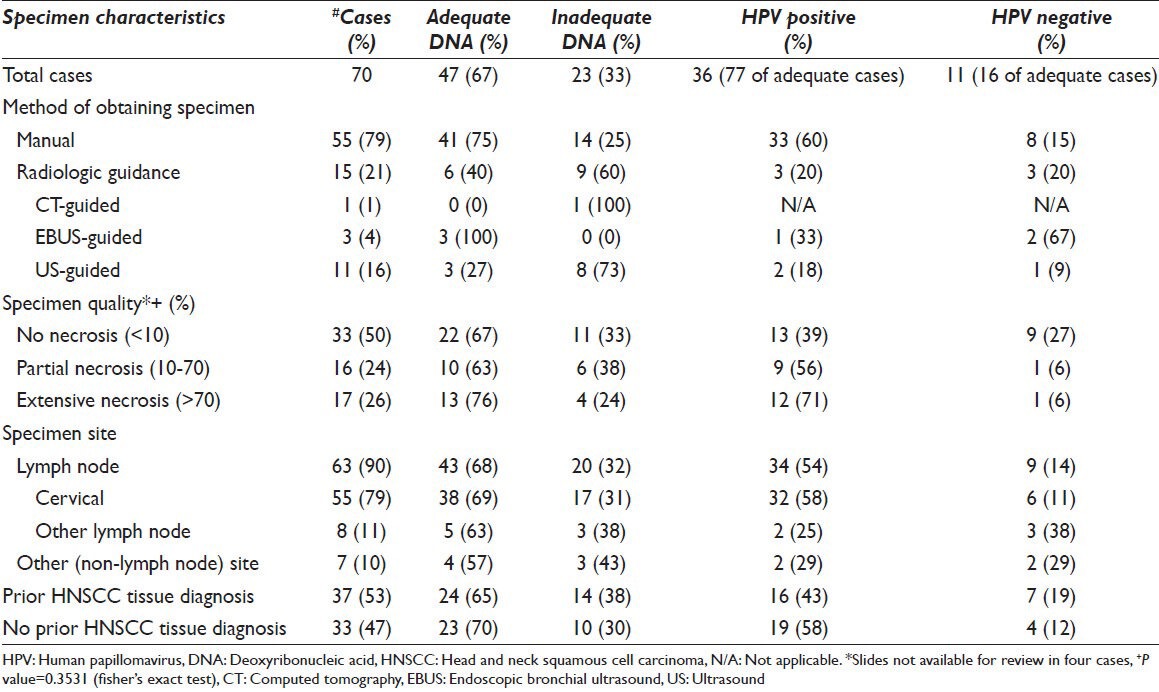
Table 2.
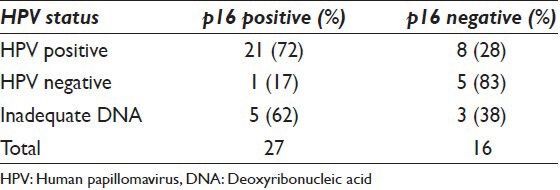
Molecular HPV DNA results and p16 immunohistochemistry correlation (n=43)
In 63 cases (63/70; 90%), the FNA procedure was performed on lymph nodes, anatomically distributed as follows: Cervical – 55 (55/63; 79%), submandibular – 4 (4/63; 6%), supraclavicular – 1 (1/63; 1%), thoracic level VII – 1 case (1/63; 1%) and thoracic level XI – 2 (2/63; 3%). The remaining seven cases (10%) were obtained from FNA procedures performed on parotid, tonsil, soft palate, jaw, lung, chest wall and temporal scalp.
In 55 (79%) cases, the FNA was procured by palpation; radiologic guidance was utilized in the remaining 15 (21%) cases (ultrasound – 11 cases; endoscopic bronchial ultrasound – 3 cases; computer tomography – 1 case).
The average number of FNA passes was 2.18/case. ROSE was performed in 69 (69/70; 99%) cases; of these, 54 (78%) were diagnostic for tumor, 8 (12%) were interpreted as atypical/suspicious and 7 (10%) were non-diagnostic.
With respect to percent tumor necrosis, of the 66 cases with slides available for review, 33 (33/66; 50%) cases showed no necrosis, 16 (16/66; 24%) cases showed partial necrosis and 17 (17/66; 26%) cases showed extensive necrosis.
Molecular testing for HPV
Adequate DNA for HPV DNA testing was obtained from 47 cases (67%), while 23 cases (33%) were considered inadequate. Of the 47 adequate cases, 36 cases (36/47; 77%) were positive and 11 cases (11/47; 23%) were negative for HR HPV.
When DNA adequacy was analyzed in relation to specimen quality, adequate DNA for testing was obtained in 22 out of 33 cases (67%) showing no necrosis (0-10%), 10 out of 16 cases (63%) showing partial necrosis (10-70%) and in 13 out of 17 cases (76%) showing extensive necrosis (>70%). This difference is not statistically significant (P = 0.3531). When analyzed in relation to the method of obtaining the specimen, manual FNA (by palpation) yielded 41 out of 55 cases with adequate DNA for molecular HPV testing (75%) while those specimens obtained with radiologic guidance yielded 6 out of 15 cases with adequate DNA for molecular HPV testing (40%) [Table 1].
HPV – p16 immunohistochemistry correlation
The immunohistochemical analysis for p16 was performed on cell blocks of 43 cases; 27 were positive (63%) and 16 were negative (37%) for p16. When compared with the HPV status, 21 HR HPV positive cases were also p16 positive (72% of HPV positive cases), while eight HR HPV positive cases were p16 negative (28% of HPV positive cases). Of the six HR HPV negative cases on which p16 was performed, five were negative (83% of HPV negative cases) and one was p16 positive (17% of HPV negative cases). In eight cases with inadequate DNA for HR HPV testing, five were p16 positive (62% of inadequate cases) and three were negative (38% of inadequate cases) [Table 2].
DISCUSSION
Incidence of HNSCC of has increased in recent years and despite significant advances in treatment modalities, the 5-year survival rate remains at 50%.[14] It is now well-known that HPV tumor status in HNSCC predicts response to therapy and a positive role in terms of recurrence-free and overall survival.[7,8,9,10] Multiple methods have been described to evaluate HPV status in these lesions, including p16 immunohistochemistry as a surrogate marker for HPV infection, HPV in situ hybridization, HPV DNA detection by polymerase chain reaction (PCR) and HPV16 E6 and E7 messenger ribonucleic acid detection.[14]
HNSCC usually presents as an evident mass or lesion in the upper aerodigestive tract, amenable to biopsy for histopathologic diagnosis and HPV testing. However, in up to 5% of cases the patients present with metastatic disease in regional lymph nodes, with occult primary.[12] In this scenario, the only tissue available for HPV testing is the one obtained via FNA of these lymph nodes. It is not uncommon for the metastatic deposits in lymph nodes from HNSCC to undergo necrosis leading to cystification; thus limiting the amount of viable tissue available for ancillary studies such as p16 immunohistochemistry. It has been shown that HR HPV testing can be successfully performed on FNA specimens from HNSCC of limited tumor cellularity.
Evaluation of HPV status in FNA samples of HNSCC is common practice, particularly when dealing with metastatic HNSCC to lymph nodes with occult primary tumors. HPV detection in this cytologic material can be performed indirectly through p16 immunohistochemistry on cell-blocks or with direct methods of identifying HR HPV DNA utilizing PCR analysis or in situ hybridization.[12,15,16,17] Until date, there is no consensus as to which of these is the best method for testing for HPV in cytology specimens.
In the current study, we confirmed that HPV DNA testing can successfully be performed on limited HNSCC samples obtained from metastatic sites via FNA.[18] Interestingly, the amount of tumor necrosis in these samples had no effect on the adequacy of DNA obtained for HR HPV DNA testing. This even held true for cases exhibiting extensive tumor necrosis (>70% necrosis); the percentage of cases with adequate DNA for HR HPV testing in this group was similar to cases without tumor necrosis (76% vs. 67%).
Cases that were positive for HR HPV DNA were also positive for p16 by immunohistochemistry (72%). Similarly, the majority of cases negative for HR HPV DNA lacked p16 expression (83%). Occasional cases did show discordant results between HR HPV DNA results and p16 expression, in accordance with the results reported by Liang et al.[19]
We also found that the method of obtaining the specimen, i.e. manual versus radiologic guidance, showed no significant differences in the adequacy of DNA obtained for HPV testing. However, in cases on which the specimen was obtained manually, adequate DNA for HR HPV testing was obtained in 75% of the cases as opposed to 40% in cases that were biopsied under radiologic guidance. Lesions aspirated under radiologic guidance are most often deep seated lesions as compared with superficially located and easily accessible lesions that do not require radiologic guidance. It is therefore easier to obtain a larger amount of tissue in from superficially located lesion as opposed to deep seated lesions. It is likely that this difference in the total amount of tissue (regardless of the amount of tumor necrosis) might explain this difference in DNA adequacy.
In this study, we show that HR HPV DNA testing is not influenced by the amount of necrosis in the tumor, the method by which the FNA was performed or the location of the lymph node with metastatic disease. Hence, we believe that FNA specimen obtained from head and neck lesions diagnosed as SCC during ROSE can be successfully triaged for HPV DNA testing, independent of the amount of necrosis. This would guarantee availability of tumor cells for HPV testing, even in cases in which p16 immunohistochemistry cannot be performed due to extensive necrosis, allowing for the most appropriate treatment options in the current era of personalized medicine.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this article declare that we qualify for authorship as defined by the ICJME. Each author has participated sufficiently in the work and takes public responsibility for appropriate portions of the content of the article.
RL, MP and MN are responsible for execution and analysis of the work and drafting the manuscript.
GW, KM and VL are responsible for planning and revising the article critically for important intellectual content.
ZB is responsible for conceiving and coordinating the whole work, execution and analysis of the work and final approval of the version to be published.
Each author acknowledges that the final version was read and approved.
ETHICS STATEMENT BY ALL AUTHORS
All authors take the responsibility of maintaining relevant documentation of records, slides and other data used in this study on archival material as per the Institutional policy. All patient identifiers were suppressed in the final analysis of the data.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model (authors are blinded for reviewers and vice versa) through automatic online system.
Footnotes
Available FREE in open access from: http://www.cytojournal.com/text.asp?2013/10/1/21/120789
Contributor Information
Ricardo R. Lastra, Email: ricardo.lastra@uphs.upenn.edu.
Michelle R. Pramick, Email: michelle.pramick@uphs.upenn.edu.
Megan O. Nakashima, Email: nakashm@ccf.org.
Gregory S. Weinstein, Email: gregory.weinstein@uphs.upenn.edu.
Kathleen T. Montone, Email: kathleen.montone@uphs.upenn.edu.
Virginia A. LiVolsi, Email: linus@mail.med.upenn.edu.
Zubair W. Baloch, Email: baloch@mail.med.upenn.edu.
REFERENCES
- 1.Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: A virus-related cancer epidemic. Lancet Oncol. 2010;11:781–9. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atlanta: American Cancer Society; 2012. American Cancer Society. Cancer Facts and Figures 2012. [Google Scholar]
- 3.Blot WJ, McLaughlin JK, Winn DM, Austin DF, Greenberg RS, Preston-Martin S, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48:3282–7. [PubMed] [Google Scholar]
- 4.Syrjänen K, Syrjänen S, Lamberg M, Pyrhönen S, Nuutinen J. Morphological and immunohistochemical evidence suggesting human papillomavirus (HPV) involvement in oral squamous cell carcinogenesis. Int J Oral Surg. 1983;12:418–24. doi: 10.1016/s0300-9785(83)80033-7. [DOI] [PubMed] [Google Scholar]
- 5.Lajer CB, von Buchwald C. The role of human papillomavirus in head and neck cancer. APMIS. 2010;118:510–9. doi: 10.1111/j.1600-0463.2010.02624.x. [DOI] [PubMed] [Google Scholar]
- 6.Termine N, Panzarella V, Falaschini S, Russo A, Matranga D, Lo Muzio L, et al. HPV in oral squamous cell carcinoma vs head and neck squamous cell carcinoma biopsies: A meta-analysis (1988-2007) Ann Oncol. 2008;19:1681–90. doi: 10.1093/annonc/mdn372. [DOI] [PubMed] [Google Scholar]
- 7.Sedaghat AR, Zhang Z, Begum S, Palermo R, Best S, Ulmer KM, et al. Prognostic significance of human papillomavirus in oropharyngeal squamous cell carcinomas. Laryngoscope. 2009;119:1542–9. doi: 10.1002/lary.20533. [DOI] [PubMed] [Google Scholar]
- 8.Wittekindt C, Gültekin E, Weissenborn SJ, Dienes HP, Pfister HJ, Klussmann JP. Expression of p16 protein is associated with human papillomavirus status in tonsillar carcinomas and has implications on survival. Adv Otorhinolaryngol. 2005;62:72–80. doi: 10.1159/000082474. [DOI] [PubMed] [Google Scholar]
- 9.Lassen P, Eriksen JG, Hamilton-Dutoit S, Tramm T, Alsner J, Overgaard J. Effect of HPV-associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27:1992–8. doi: 10.1200/JCO.2008.20.2853. [DOI] [PubMed] [Google Scholar]
- 10.Lewis JS, Jr, Thorstad WL, Chernock RD, Haughey BH, Yip JH, Zhang Q, et al. p16 positive oropharyngeal squamous cell carcinoma: An entity with a favorable prognosis regardless of tumor HPV status. Am J Surg Pathol. 2010;34:1088–96. doi: 10.1097/PAS.0b013e3181e84652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim TW, Choi SY, Ko YH, Baek CH, Son YI. The prognostic role of p16 expression in tonsil cancer treated by either surgery or radiation. Clin Exp Otorhinolaryngol. 2012;5:207–12. doi: 10.3342/ceo.2012.5.4.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang MQ, El-Mofty SK, Dávila RM. Detection of human papillomavirus-related squamous cell carcinoma cytologically and by in situ hybridization in fine-needle aspiration biopsies of cervical metastasis: A tool for identifying the site of an occult head and neck primary. Cancer. 2008;114:118–23. doi: 10.1002/cncr.23348. [DOI] [PubMed] [Google Scholar]
- 13.Schmalbach CE, Miller FR. Occult primary head and neck carcinoma. Curr Oncol Rep. 2007;9:139–46. doi: 10.1007/s11912-007-0012-5. [DOI] [PubMed] [Google Scholar]
- 14.Schlecht NF, Brandwein-Gensler M, Nuovo GJ, Li M, Dunne A, Kawachi N, et al. A comparison of clinically utilized human papillomavirus detection methods in head and neck cancer. Mod Pathol. 2011;24:1295–305. doi: 10.1038/modpathol.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krane JF. Role of cytology in the diagnosis and management of HPV-associated head and neck carcinoma. Acta Cytol. 2013;57:117–26. doi: 10.1159/000346715. [DOI] [PubMed] [Google Scholar]
- 16.Umudum H, Rezanko T, Dag F, Dogruluk T. Human papillomavirus genome detection by in situ hybridization in fine-needle aspirates of metastatic lesions from head and neck squamous cell carcinomas. Cancer. 2005;105:171–7. doi: 10.1002/cncr.21027. [DOI] [PubMed] [Google Scholar]
- 17.Solomides CC, Bibbo M, Wang ZX. Assessment of fine needle aspiration specimen adequacy for high-risk HPV detection and genotyping in oropharyngeal squamous cell carcinoma. Acta Cytol. 2012;56:196–8. doi: 10.1159/000335730. [DOI] [PubMed] [Google Scholar]
- 18.Jarboe EA, Hunt JP, Layfield LJ. Cytomorphologic diagnosis and HPV testing of metastatic and primary oropharyngeal squamous cell carcinomas: A review and summary of the literature. Diagn Cytopathol. 2012;40:491–7. doi: 10.1002/dc.22837. [DOI] [PubMed] [Google Scholar]
- 19.Liang C, Marsit CJ, McClean MD, Nelson HH, Christensen BC, Haddad RI, et al. Biomarkers of HPV in head and neck squamous cell carcinoma. Cancer Res. 2012;72:5004–13. doi: 10.1158/0008-5472.CAN-11-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]


