Abstract
Background and aim
Subjects infected with H. pylori containing cagA do not always induce serum CagA antibody. Our previous meta-analysis showed that serum CagA seropositivity was associated with gastric cancer even in East Asian countries. However, it remains unclear why serum CagA positive status is associated with gastric cancer. In this study, we aimed to examine the relationship between anti CagA antibody titer and the levels of pepsinogen, and histological score.
Methods
Eighty-eight H. pylori positive Japanese patients with gastritis were included. Serum CagA antibody titer, pepsinogen (PG) I and PG II were evaluated by enzyme-linked immunosorbent assay. Histological scores were evaluated according to Update Sydney System. CagA expression was examined by immunoblot.
Results
Seroprevalence of CagA antibody was found in 75.0%. Interestingly, serum CagA antibody titer was significantly correlated with PG I and PG II levels (P = 0.003 and 0.004, respectively). Serum CagA antibody titer was also significantly correlated with mucosal inflammation in the corpus (P = 0.04). On the other hand, bacterial density was not related with CagA antibody titer. CagA expression level of the strains was irrespective of the status of PG and serum CagA antibody.
Conclusions
Subjects with higher serum CagA antibody titer can be considered as high risk population for the development of gastric cancer from the point of strong gastric inflammatory response even in Japan. Host recognition rather than bacterial colonization might be associated with the difference of serum CagA antibody titer.
Keywords: Helicobacter pylori, CagA, serum antibody, pepsinogen
Introduction
Helicobacter pylori is a spiral Gram-negative bacterium that infects more than half of the world’s population 1. H. pylori infection is now accepted to be linked to severe gastritis-associated diseases, including peptic ulcer and gastric cancer 1. The infection remains latent in the majority of infected patients, only a minority of individuals with H. pylori infection ever develop it 2. Uemura et al. reported that gastric cancer developed in approximately 3% of H. pylori-infected patients, compared to none of the uninfected patients 3. In addition to host, environmental, and dietary factors, the differences in the virulence of H. pylori strains are related with the varying outcomes of H. pylori infection.
The best studied virulence factor of H. pylori is the CagA protein. CagA producing strains are reported to be associated with severe clinical outcomes, especially in Western countries 4–7. CagA is a highly immunogenic protein with a molecular weight between 120 and 140 kDa 8, 9. In 2003, Huang et al. performed meta-analysis of the association between CagA seropositivity and gastric cancer 10. They concluded that the infection of CagA positive strains increase the risk of gastric cancer. However, because they included studies from both Western and Asian countries, it was not clear whether an association between CagA seropositivity and gastric cancer really exists in East Asian countries. In East Asian countries, it is difficult to prove the importance of the cagA gene in clinical outcomes because almost all H. pylori strains possess the cagA gene. For example, we previously examined 491 Japanese strains from a region in the middle of Japan (Kyoto) and found that 96.3% of the strains were cagA gene-positive, irrespective of clinical outcomes 11; similar results have been published for different regions in Japan 12–14 and other countries in East Asia 15, 16.
Interestingly, subjects infected with cagA-positive H. pylori do not always induce serum CagA antibody even in East Asian countries. For example, although most Japanese H. pylori possess cagA, serum CagA antibody is detected in only 53.7 to 81.1% of infected subjects in Japan 17, 18. This suggests that serum CagA antibody rather than the presence of cagA may be a more useful marker to detect the high risk population for severe outcomes in East Asian countries. Intriguingly, we reported that CagA seropositivity was significantly associated with gastric cancer even in East Asian countries in meta-analysis 19. This suggests that anti-CagA antibody can be used as a biomarker for gastric cancer even in East Asian countries.
It remains unclear why not all subjects have serum CagA antibody in Japan. As described above, subjects with serum CagA antibody can be considered as a high risk group for gastric cancer. Several factors such as bacterial factors and/or host recognition of CagA, and environmental factors may affect the difference of serum CagA antibody titer. In addition, it is not clear why serum CagA positive is associated with gastric cancer. In this study, we aimed to examine the relationship between anti CagA antibody titer and the levels of pepsinogen (PG), and histological score.
Methods
Patients
Patients were considered to be H. pylori-infected when at least one of rapid urease test, culture, and microscopic examination showed positive results. Total of 88 H. pylori-positive Japanese patients with gastritis (29 males, 59 females, aged 22–87 years [mean, 58.4 years]) were recruited. Patients with drug allergies and those with serious complications, such as cardiac diseases, renal diseases, and hepatic diseases, were excluded from the study. Four biopsy samples (2 from the antrum and 2 from the corpus) were endoscopically obtained from each patient and used for H. pylori culture and histopathologic examination. Written informed consent was obtained from all participants, and the protocol was approved by the Ethics Committee of Oita University.
ELISA for serum CagA antibody titer and pepsinogen
Serum anti CagA IgG antibody was measured by using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Genesis Diagnostics Ltd, Cambridgeshire, UK). Equal and more than 6.25 U/mL was defined as positive based on the manufacturer’s instructions. The level of the serum PG I and PG II were measured by Pepsinogen ELISA kit (Eiken, Co. Ltd., Tokyo, Japan) according to the manufacturer’s instructions.
Histological analysis
All biopsy materials were fixed in 10% buffered formalin for 24 h, then embedded in paraffin. Serial sections were stained with hematoxylin and eosin and with May–Giemsa stain. State of the gastric mucosa was evaluated according to the updated Sydney system 20. The degree of inflammation, neutrophil activity, atrophy, intestinal metaplasia, and bacterial density were classified into four grades: 0, ‘normal’; 1, ‘mild’; 2, ‘moderate’; and 3, ‘marked’.
Isolation and genotyping of H. pylori
Antral biopsy specimens were obtained for isolation of H. pylori using standard culture methods 11. H. pylori DNA was extracted from confluent plate cultures using a commercially available kit (QIAGEN, Valencia, CA). The presence of cagA were determined by polymerase chain reaction (PCR) using primer pair cagTF; 5′-ACC CTA GTC GGT AAT GGG-3′ and cagTR; 5′-GCT TTA GCT TCT GAY ACY GC-3′ (Y = C or T) designed in the 3′ repeat region of cagA, as described previously 21. The PCR conditions were initial denaturation for 5 min at 95°C, 35 amplification steps (95°C for 30 s, 56°C for 30 s, and 72°C for 30 s), and a final extension cycle of 7 min at 72°C, using Blend Taq® DNA polymerase (TOYOBO, Osaka, Japan).
Immunoblot
Whole protein extracts from H. pylori isolates were obtained by suspending the bacteria in Laemmli sample buffer (Bio-Rad Laboratories, Inc., CA) and boiling this suspension at 100 °C for 10 minutes. Immunoblotting was performed using standard methods. Two type of anti-CagA antibody (Abcom, Hong Kong, and Santa Cruz Biotechnology, Inc., CA) was used as primary antibody at a 1:2000 dilution. Secondary anti-mouse or rabbit IgG was diluted 1:2000 (Jackson ImmunoResearch Lab, Inc., PA). Detection was performed using ECL Plus reagents (GE Healthcare, Buckinghamshire, UK). Protein concentrations were determined by the Lowry method and adjusted.
Statistical analysis
The univariate association was quantified by the chi-square test. Spearman rank coefficients (r) were determined to evaluate the association between anti CagA antibody titer and the levels of PG, and histological score. A P value of less than 0.05 was accepted as statistically significant. The SPSS statistical software package version 19.0 (SPSS, Inc., Chicago, IL) was used for all statistical analyses.
Results
Association between serum CagA antibody titer and PG
Total of 88 patients with gastritis were examined their serum CagA antibody titer. Serum CagA antibody titer was ranged from 0.3 to 137.1 U/mL and average titer was 32.1 ± 33.4 U/mL. When equal and more than 6.25 U/mL was defined as positive based on the manufacturer’s instructions, 66 (75.0%) patients were serum CagA antibody positive and remaining 22 were considered as negative. The average levels of PG I and II were 62.6 ± 37.0 (range; 8.7 to 259.0) and 21.6 ± 12.6 (range; 2.4 to 74.6) ng/mL, respectively. The PGI/II ratio was ranged from 1.1 to 13.6 and average was 3.3 ± 1.9.
The comparison of age, gender and PG level according to the status of CagA antibody was shown in Table 1. There was no difference of average age between serum CagA antibody positive and negative group (P = 0.49). The percentage of male was significantly higher in serum CagA antibody negative group than positive group (54.5 vs. 25.7%, P = 0.01). Among 59 female, 49 (83.0%) showed serum CagA antibody positive. On the other hand, serum CagA antibody positive rate was 58.6% (17/29) in male. In fact, serum CagA antibody titer was significantly higher in female than male (38.6 ± 35.7 vs. 18.6 ± 23.2 U/mL, P = 0.003). PG II level was significantly higher in serum CagA antibody positive group than negative group (P = 0.04). PG I level was also higher in serum CagA antibody positive than negative group; however, it was not statistical significance (P = 0.30). There was no difference of PG levels between male and female (data not shown).
Table 1.
The comparisons of age, gender and PG level according to the status of serum CagA antibody
| Serum CagA antibody positive |
Serum CagA antibody negative |
p | |
|---|---|---|---|
| n | 66 | 22 | |
| Age (y) | 57.9 ± 13.2 | 60.0 ± 11.6 | 0.49 |
| Male | 17 (25.7 %) | 12 (54.5%) | 0.01 |
| PG I | 64.9 ± 38.8 | 58.8 ± 30.9 | 0.30 |
| PG II | 23.1 ± 13.2 | 17.6 ± 8.8 | 0.04 |
| PG I/II | 5.6 ± 21.8 | 3.5 ± 1.7 | 0.12 |
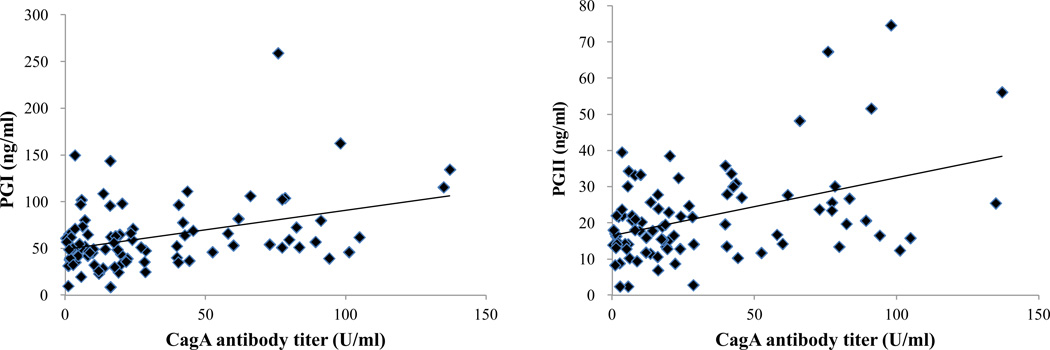
The correlation between serum CagA antibody titers and PG levels was also examined (Fig. 1). Serum CagA antibody titer was significantly correlated with PG I level (r = 0.30, P = 0.003). In addition, serum CagA antibody titer was also correlated with PG II level (r = 0.30, P = 0.004). There was no correlation between serum CagA antibody titer and PG I/II ratio (P = 0.77) Even when only serum CagA antibody positive group was selected, serum CagA antibody titer was significantly correlated with PG I and PG II (r = 0.40, P = 0.001 for PG I, r = 0.40, P = 0.001 for PG II, respectively).
Figure 1. The correlation between serum CagA antibody titer and PG I, PG II.
Association between serum CagA antibody titer and histological score
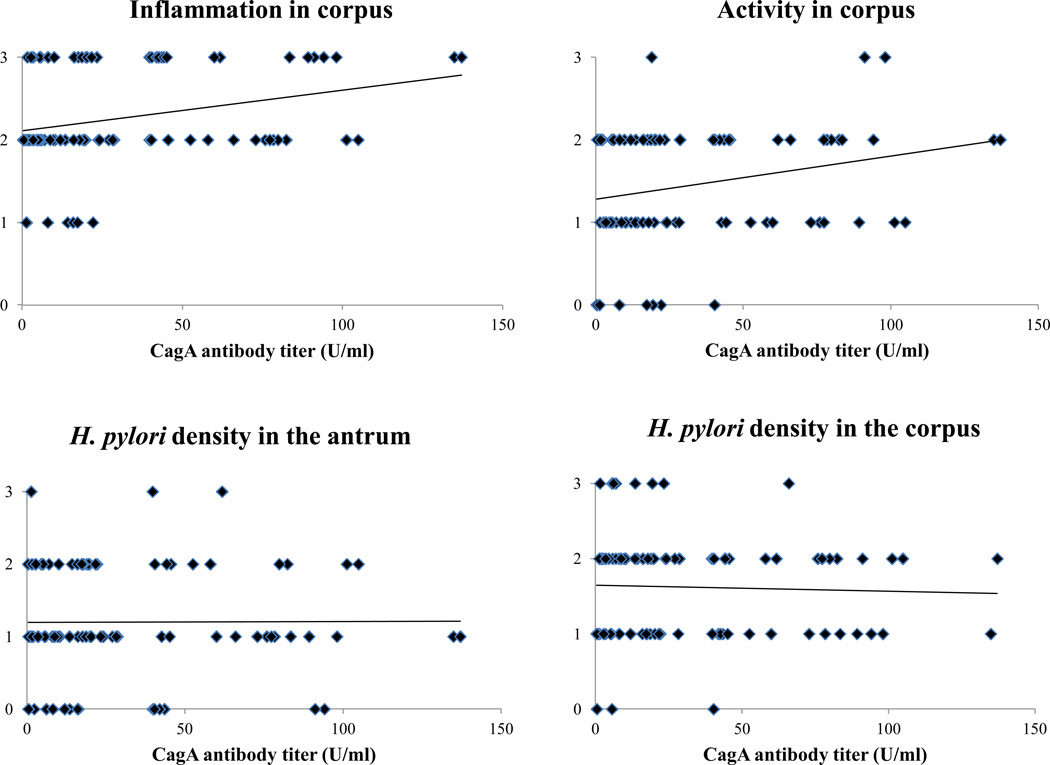
Next, the relationship between serum CagA antibody titer and histological score was examined. There were no significant differences of each score between serum CagA antibody positive and negative group (Table 2). However, the correlation between serum CagA antibody titer and histological score was examined, the inflammation in the corpus was significantly correlated with serum CagA antibody titer (r = 0.26, P = 0.01) (Fig. 2). Mucosal activity in the corpus was tended to be correlated with serum CagA antibody titer, however there was no statistical significance (P = 0.07). These correlations was not found in the antrum (P = 0.47 for the inflammation, P = 0.60 for the activity). On the other hand, there was no association between serum CagA antibody titer and bacterial density both in the antrum and corpus (P = 0.87 and 0.79, respectively) (Fig. 2). This suggests that low bacterial density cannot be a reason for low serum CagA antibody titer. Neither atrophy nor intestinal metaplasia both in the antrum and corpus was correlated with serum CagA antibody titer. PG II was significantly correlated with inflammation and activity in the corpus (P < 0.001, < 0.001, respectively). These correlations was not found in the antrum (P = 0.20 for the inflammation, P = 0.15 for the activity). Bacterial density in the antrum was significantly correlated with activity and inflammation in the antrum (P = 0.001 and P < 0.001, respectively), whereas bacterial density in the corpus was not correlated with any histological score. Even when only serum CagA antibody positive group was selected, serum CagA antibody titer was significantly correlated with inflammation and activity in the corpus (r = 0.26, P = 0.04 for inflammation, r = 0.24, P = 0.04 for activity, respectively).
Table 2.
The comparison of histological scores according to the status of serum CagA antibody
| Serum CagA antibody positive |
Serum CagA antibody negative |
p | |
|---|---|---|---|
| n | 66 | 22 | |
| Antrum | |||
| Inflammation | 2.38 ± 0.51 | 2.41 ± 0.59 | 0.73 |
| Activity | 1.29 ± 0.73 | 1.55 ± 0.91 | 0.22 |
| Atrophy | 1.41 ± 0.65 | 1.36 ± 0.79 | 0.86 |
| Intestinal metaplasia | 0.26 ± 0.68 | 0.55 ± 0.96 | 0.09 |
| Bacterial density | 1.20 ± 0.76 | 1.20 ± 0.76 | 0.75 |
| Corpus | |||
| Inflammation | 2.30 ± 0.60 | 2.14 ± 0.56 | 0.23 |
| Activity | 1.48 ± 0.70 | 1.36 ± 0.65 | 0.51 |
| Atrophy | 0.65 ± 0.85 | 0.55 ± 0.85 | 0.48 |
| Intestinal metaplasia | 0.09 ± 0.33 | 0.18 ± 0.50 | 0.38 |
| Bacterial density | 1.65 ± 0.64 | 1.64 ± 0.79 | 0.89 |
Figure 2. The correlation between serum CagA antibody titer and gastric mucosal inflammation, bacterial density.
Association between serum CagA antibody and bacterial CagA expression
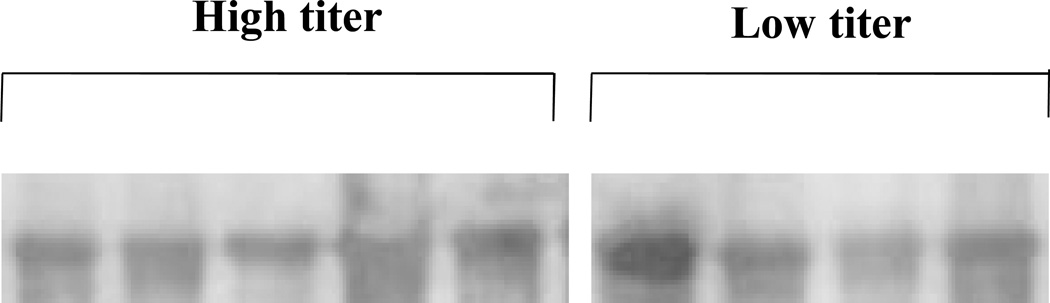
The presence of cagA in the strain was examined by PCR using randomly selected 28 patients including 19 serum CagA antibody positive and 9 negative cases. PCR showed that all 28 samples were cagA-positive. To examine whether the difference of serum CagA antibody titer is attribute to the bacterial CagA expression level, bacterial CagA expression levels were examined by immunoblot. We selected 4 samples from serum CagA antibody negative/low PG II level, and 5 samples from serum CagA antibody positive/high PG II level. As a result, there was no difference of CagA expression level (Fig. 3). Even in the strain isolated from patients with serum CagA antibody negative/low PG II level, the CagA expression was found and there was no significant difference compared with that of serum CagA antibody positive/high PG II level. This suggests that low CagA expression level in the bacteria does not contribute to the low serum CagA antibody titer.
Figure 3. CagA protein expression profile.
Bacterial protein was extracted in the strains from the patients with serum CagA antibody negative/low PG II and serum CagA antibody positive/high PG II, respectively. CagA protein expression was examined by Western blot.
Discussion
In East Asian countries, different CagA seropositivity has been reported despite almost all H. pylori possessing cagA. CagA seropositivity in gastritis ranged from 53.7 to 81.1%, even in Japan 17, 18. In our meta-analysis, CagA seropositivity was associated with gastric cancer even in East Asian countries, although the odds ratio in East Asian countries was smaller than in studies that included Western countries 19. Furthermore, even in the H. pylori-negative population, the presence of anti-CagA antibodies increases the risk of gastric cancer 19. This evidence confirms that CagA antibodies can potentially remain positive for a longer period of time than the anti-H. pylori antibody 22, 23. Accordingly, anti-CagA antibody was related to gastric cancer in both H. pylori-positive and -negative populations in East Asian countries.
Serum PG has been found to be a marker of gastric mucosal status including atrophy and inflammation 24. There are two forms of PG: PG I and PG II, and both are produced by the chief and mucus neck cells in the gastric fundus and corpus. PG II is also produced by the pyloric glands in the antrum and Brunner’s glands in the proximal duodenum. Although atrophy is usually diagnosed by endoscopic biopsy, there is a significant potential sampling errors in identifying atrophy by random biopsy because atrophy of gastric mucosa could be patchy. On the other hand, PG was reported to be used as a surrogate marker for gastric mucosal status 25. Serum PG I and PG II are known to increase by H. pylori infection. However, as PG II exhibits a greater raise relative to PG I, the PG I/II ratio decrease in the presence of H. pylori. After that, as the fundic gland mucosa reduces, PG I levels gradually decrease, whereas PG II levels remain fairly constant. As the result, a stepwise reduction of the PG I/II ratio is closely correlated with the progression from normal gastric mucosa to extensive atrophic gastritis. In the present study, serum CagA antibody was significantly correlated with the levels of PG I and II, but not PG I/II ratio. Consistent with our findings, Fukuda et al. reported that serum PG I and II level but not PG I/II ration were significantly higher in serum CagA antibody positive compared with negative children 26. Serum PG was reported to be correlated with gastric inflammatory score 27. In addition, the cagA status was reported to be associated with various kinds of cytokines including interleukin-8 and may cause severe inflammation in the stomach 28. It is also possible that gastritis increases permeability of the gastric epithelial surface, enabling back diffusion of PGs after secretion 27. These findings suggest that serum CagA antibody titer was associated with gastric inflammation, but not atrophy.
Shimoyama et al. reported that inflammation in the antrum and the corpus was more significant in serum CagA antibody positive when they examined the presence of serum CagA antibody by immunoblot 29. In the present study, although there were no significant differences of each histological score between serum CagA antibody positive and negative group, the mucosal inflammation in the corpus was significantly correlated with serum CagA antibody titer. This finding also supported that different level of antibody production from lymphocytes induced by H. pylori infection can contribute to the various serum CagA antibody level. Interestingly, positive correlation between the inflammatory score and serum CagA antibody titer was found only in the corpus but not in the antrum. Corpus dominant gastritis rather than antrum dominant gastritis was a risk factor to develop gastric ulcer and gastric cancer 3, 30. In addition, even when only serum CagA antibody positive group was selected, serum CagA antibody titer was significantly correlated with inflammation and activity in the corpus. Therefore, antibody titer rather than the presence of antibody can be a useful marker for advanced inflammation in the stomach in Japan. This suggests that serum CagA antibody titer might be available marker to predict a gastric cancer in Japan. It has also been reported that measurement of serum levels of C-reactive protein (CRP) using a high-sensitivity assay (hs-CRP) can reveal subclinical inflammatory states which may reflect vascular inflammation 31. Recent report showed that the mean serum level of hs-CRP was significantly higher in H. pylori positive group than H. pylori negative group although the level of hs-CRP was not different between CagA antibody positive and negative group in Iran 32. It is better to examine the association between serum CagA antibody and hs-CRP in Japan in the further study.
In our study, in spite of cagA positive by PCR, the prevalence of serum CagA antibody was 75.0%, which was consistent with previous studies from Japan 17, 33. The cagA gene is located at one end of the cag pathogenicity island (PAI), an approximately 40-kbp-region that is thought to have been incorporated into the H. pylori genome by horizontal transfer from an unknown source 34. The cag PAI encodes a type IV secretion system, through which CagA is delivered into host cells 35, 36. CagA has been reported to interact with various target molecules in host cells; the best studied is the cytoplasmic Src homology 2 domain of Src homology 2 phosphatase (SHP-2). Mutations of SHP-2 have been found in various human malignancies and mice that lacked the SHP-2-binding site developed hyperplastic antral tumors 37, indicating that SHP-2 plays an important role in gastric cancer. Therefore, other gene(s) except for cagA in cag PAI can contribute to the difference of serum CagA antibody titer. However, almost of case was cag PAI positive in Japan 29. Therefore, it is unlikely that diversity of cag PAI can contribute to the difference of serum CagA antibody titer. Furthermore, CagA expression pattern was not associated with the serum CagA antibody titer. In addition, there was no association between serum CagA antibody titer and bacterial density in the antrum and corpus by histological examination. This suggests that low serum CagA antibody titer cannot attribute to the low bacterial density.
Therefore, our findings suggest that host and environmental factors can affect the difference of serum CagA antibody titer. For example, even when healthy volunteers were infected with same strains, they showed different histological score 38. Therefore, host recognition can be associated with the difference of serum CagA antibody titer. We found that serum CagA antibody positive rate was significantly higher in female than male irrespective of the status of PG. In general, estrogen stimulates immune responses and testosterone is immunosuppressive 39. H. pylori infected female mice showed the higher IgG2c levels than male mice 40. In addition, a previous study showed that a better vaccine efficiency of H. pylori infection was obtained in females than male 41. This suggests that immune responses differ between the genders. Host genetic polymorphisms can determine the susceptibility to and severity of infection 2. Especially, inflammatory cytokine gene polymorphisms (interleukin (IL)-1 gene cluster, TNF-α, IL-10, and IL-8) have been reported to be correlated with gastric cancer 42–47. In addition, environmental factors such as diet (e.g., salt intake) can also affect the gastric cancer incidence 48. Loh et al. reported that increased expression of cagA in response to high salt conditions 49. Furthermore, they showed that co-culture of gastric epithelial cells with H. pylori in high salt conditions resulted in the increased tyrosine-phosphorylated CagA and increased secretion of IL-8 by the epithelial cells compared with low salt conditions. These findings provide important insights into mechanisms through which high-salt diets increase the risk for gastric cancer among subjects infected with cagA-positive H. pylori. Further studies using host and environmental information are necessary to elucidate the contributing factors for serum CagA antibody titer.
However, we should keep a caution for the difference of serum CagA antibody titer examined by ELISA. We found a significant heterogeneity in a meta-analysis 19. This heterogeneity appeared to result from the use of different populations or different methods, or from differences in the antigens used to detect anti-CagA antibodies. We previously examined the relationship between serum CagA antibody and gastric cancer in a Japanese population using two different recombinant CagA antigens 18. CagA seropositivity was 82% by OraVax antigen and 72% by Chiron antigen, irrespective of the existence of gastric cancer, when determining the cutoff value by the population living in the same region (Kyoto, Japan). This suggests that numerical results from studies using different antigens and different protocols may not be comparable 50, 51. Because many recombinant CagA as coating antigen in ELISA system were derived from European strain, recombinant CagA derived from East Asian strain may be proper in East Asian countries. The CagA can be of 2 types: East-Asian-type CagA and Western-type CagA according to the difference of amino acid sequences of the C-terminal of CagA 52. Individuals infected with East-Asian-type CagA strains reportedly have an increased risk of peptic ulcer or gastric cancer compared with individuals with Western-type CagA strains 53, 54. East Asian-type CagA or Western-type CagA status may also affect the serum CagA antibody titer and/or different sensitivity of assay. At present, there are no reports that examine the prevalence of East Asian-type CagA-specific antibody in sera. Yasuda et al. reported the development of monoclonal antibody against East Asian-type CagA for developing a sandwich-ELISA system 55. However, this is the system for detecting East Asian type-CagA strains but not serum antibody. To detect serum East Asian-type CagA-specific antibody, the development of an ELISA assay using East Asian-type CagA-specific antigen will be required.
In conclusion, our study revealed that high serum CagA antibody titer was significantly correlated with PG I, PG II and inflammation in the corpus. Therefore, subjects with higher serum CagA antibody titer can be considered as high risk population for the development of gastric cancer from the point of strong gastric inflammatory response even in Japan.
Acknowledgement
This report is based on work supported in part by grants from the National Institutes of Health (DK62813) (YY), and grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (22390085, 22659087, 24406015 and 24659200) (YY), (23790798) (SS) and Special Coordination Funds for Promoting Science and Technology from the Japan Science and Technology Agency (JST).
We thank Ms. Ayaka Takahashi, Ms. Miyuki Matsuda and Ms. Yoko Kudo for excellent technical assistance.
Footnotes
Potential competing interests: The authors declare that they have no competing interests.
References
- 1.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 2.Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 2006;19:449–490. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 4.Blaser M, Perez-Perez G, Kleanthous H, et al. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–2115. [PubMed] [Google Scholar]
- 5.Kuipers E, Pérez-Pérez G, Meuwissen S, Blaser M. Helicobacter pylori and atrophic gastritis: importance of the cagA status. J Natl Cancer Inst. 1995;87:1777–1780. doi: 10.1093/jnci/87.23.1777. [DOI] [PubMed] [Google Scholar]
- 6.Nomura A, Lee J, Stemmermann G, Nomura R, Perez-Perez G, Blaser M. Helicobacter pylori CagA seropositivity and gastric carcinoma risk in a Japanese American population. J Infect Dis. 2002;186:1138–1144. doi: 10.1086/343808. [DOI] [PubMed] [Google Scholar]
- 7.Parsonnet J, Friedman G, Orentreich N, Vogelman H. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut. 1997;40:297–301. doi: 10.1136/gut.40.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Covacci A, Censini S, Bugnoli M, et al. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci U S A. 1993;90:5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tummuru M, Cover T, Blaser M. Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect Immun. 1993;61:1799–1809. doi: 10.1128/iai.61.5.1799-1809.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang J, Zheng G, Sumanac K, Irvine E, Hunt R. Meta-analysis of the relationship between cagA seropositivity and gastric cancer. Gastroenterology. 2003;125:1636–1644. doi: 10.1053/j.gastro.2003.08.033. [DOI] [PubMed] [Google Scholar]
- 11.Yamaoka Y, Kodama T, Kita M, Imanishi J, Kashima K, Graham D. Relationship of vacA genotypes of Helicobacter pylori to cagA status, cytotoxin production, and clinical outcome. Helicobacter. 1998;3:241–253. doi: 10.1046/j.1523-5378.1998.08056.x. [DOI] [PubMed] [Google Scholar]
- 12.Ito Y, Azuma T, Ito S, et al. Analysis and typing of the cagA gene from cagA -positive strains of Helicobacter pylori isolated in Japan. J Clin Microbiol. 1997;35:1710–1714. doi: 10.1128/jcm.35.7.1710-1714.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimoyama T, Fukuda S, Tanaka M, Mikami T, Saito Y, Munakata A. High prevalence of the CagA-positive Helicobacter pylori strains in Japanese asymptomatic patients and gastric cancer patients. Scand J Gastroenterol. 1997;32:465–468. doi: 10.3109/00365529709025082. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen L, Uchida T, Tsukamoto Y, et al. Helicobacter pylori dupA gene is not associated with clinical outcomes in the Japanese population. Clin Microbiol Infect. 2010;16:1264–1269. doi: 10.1111/j.1469-0691.2009.03081.x. [DOI] [PubMed] [Google Scholar]
- 15.Miehlke S, Kibler K, Kim J, et al. Allelic variation in the cagA gene of Helicobacter pylori obtained from Korea compared to the United States. Am J Gastroenterol. 1996;91:1322–1325. [PubMed] [Google Scholar]
- 16.Pan Z, van der Hulst R, Feller M, et al. Equally high prevalences of infection with cagA-positive Helicobacter pylori in Chinese patients with peptic ulcer disease and those with chronic gastritis-associated dyspepsia. J Clin Microbiol. 1997;35:1344–1347. doi: 10.1128/jcm.35.6.1344-1347.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimoyama T, Fukuda S, Tanaka M, Mikami T, Munakata A, Crabtree J. CagA seropositivity associated with development of gastric cancer in a Japanese population. J Clin Pathol. 1998;51:225–228. doi: 10.1136/jcp.51.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamaoka Y, Kodama T, Kashima K, Graham D. Antibody against Helicobacter pylori CagA and VacA and the risk for gastric cancer. J Clin Pathol. 1999;52:215–218. doi: 10.1136/jcp.52.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiota S, Matsunari O, Watada M, Yamaoka Y. Serum Helicobacter pylori CagA antibody as a biomarker for gastric cancer in east-Asian countries. Future Microbiol. 2010;5:1885–1893. doi: 10.2217/fmb.10.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dixon M, Genta R, Yardley J, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Yamaoka Y, Osato M, Sepulveda A, et al. Molecular epidemiology of Helicobacter pylori: separation of H. pylori from East Asian and non-Asian countries. Epidemiol Infect. 2000;124:91–96. doi: 10.1017/s0950268899003209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sörberg M, Engstrand L, Ström M, Jönsson K, Jörbeck H, Granström M. The diagnostic value of enzyme immunoassay and immunoblot in monitoring eradication of Helicobacter pylori. Scand J Infect Dis. 1997;29:147–151. doi: 10.3109/00365549709035875. [DOI] [PubMed] [Google Scholar]
- 23.Klaamas K, Held M, Wadström T, Lipping A, Kurtenkov O. IgG immune response to Helicobacter pylori antigens in patients with gastric cancer as defined by ELISA and immunoblotting. Int J Cancer. 1996;67:1–5. doi: 10.1002/(SICI)1097-0215(19960703)67:1<1::AID-IJC1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 24.Miki K. Gastric cancer screening by combined assay for serum anti- Helicobacter pylori IgG antibody and serum pepsinogen levels - "ABC method". Proc Jpn Acad Ser B Phys Biol Sci. 2011;87:405–414. doi: 10.2183/pjab.87.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim N, Jung HC. The role of serum pepsinogen in the detection of gastric cancer. Gut Liver. 2010;4:307–319. doi: 10.5009/gnl.2010.4.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukuda Y, Isomoto H, Ohnita K, et al. Impact of CagA status on serum gastrin and pepsinogen I and II concentrations in Japanese children with Helicobacter pylori infection. J Int Med Res. 2003;31:247–252. doi: 10.1177/147323000303100401. [DOI] [PubMed] [Google Scholar]
- 27.Wagner S, Haruma K, Gladziwa U, et al. Helicobacter pylori infection and serum pepsinogen A, pepsinogen, C, and gastrin in gastritis and peptic ulcer: significance of inflammation and effect of bacterial eradication. Am J Gastroenterol. 1994;89:1211–1218. [PubMed] [Google Scholar]
- 28.Yamaoka Y, Kita M, Kodama T, Sawai N, Kashima K, Imanishi J. Induction of various cytokines and development of severe mucosal inflammation by cagA gene positive Helicobacter pylori strains. Gut. 1997;41:442–451. doi: 10.1136/gut.41.4.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimoyama T, Fukuda S, Nakasato F, Yoshimura T, Mikami T, Munakata A. Relation of CagA seropositivity to cag PAI phenotype and histological grade of gastritis in patients with Helicobacter pylori infection. World J Gastroenterol. 2005;11:3751–3755. doi: 10.3748/wjg.v11.i24.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansson LE, Nyrén O, Hsing AW, et al. The risk of stomach cancer in patients with gastric or duodenal ulcer disease. N Engl J Med. 1996;335:242–249. doi: 10.1056/NEJM199607253350404. [DOI] [PubMed] [Google Scholar]
- 31.Singh SK, Suresh MV, Voleti B, Agrawal A. The connection between C-reactive protein and atherosclerosis. Ann Med. 2008;40:110–120. doi: 10.1080/07853890701749225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jafarzadeh A, Hassanshahi GH, Nemati M. Serum levels of high-sensitivity C-reactive protein (hs-CRP)in Helicobacter pylori-infected peptic ulcer patients and its association with bacterial CagA virulence factor. Dig Dis Sci. 2009;54:2612–2616. doi: 10.1007/s10620-008-0686-z. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki G, Cullings H, Fujiwara S, et al. Low-positive antibody titer against Helicobacter pylori cytotoxin-associated gene A (CagA) may predict future gastric cancer better than simple seropositivity against H. pylori CagA or against H. pylori. Cancer Epidemiol Biomarkers Prev. 2007;16:1224–1228. doi: 10.1158/1055-9965.EPI-06-1048. [DOI] [PubMed] [Google Scholar]
- 34.Censini S, Lange C, Xiang Z, et al. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci U S A. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asahi M, Azuma T, Ito S, et al. Helicobacter pylori CagA protein can be tyrosine phosphorylated in gastric epithelial cells. J Exp Med. 2000;191:593–602. doi: 10.1084/jem.191.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Backert S, Selbach M. Role of type IV secretion in Helicobacter pylori pathogenesis. Cell Microbiol. 2008;10:1573–1581. doi: 10.1111/j.1462-5822.2008.01156.x. [DOI] [PubMed] [Google Scholar]
- 37.Judd LM, Alderman BM, Howlett M, et al. Gastric cancer development in mice lacking the SHP2 binding site on the IL-6 family co-receptor gp130. Gastroenterology. 2004;126:196–207. doi: 10.1053/j.gastro.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 38.Graham DY, Opekun AR, Osato MS, et al. Challenge model for Helicobacter pylori infection in human volunteers. Gut. 2004;53:1235–1243. doi: 10.1136/gut.2003.037499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morell V. Zeroing in on how hormones affect the immune system. Science. 1995;269:773–775. doi: 10.1126/science.7638587. [DOI] [PubMed] [Google Scholar]
- 40.Sheh A, Lee CW, Masumura K, et al. Mutagenic potency of Helicobacter pylori in the gastric mucosa of mice is determined by sex and duration of infection. Proc Natl Acad Sci U S A. 2010;107:15217–15222. doi: 10.1073/pnas.1009017107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aebischer T, Laforsch S, Hurwitz R, Brombacher F, Meyer TF. Immunity against Helicobacter pylori: significance of interleukin-4 receptor alpha chain status and gender of infected mice. Infect Immun. 2001;69:556–558. doi: 10.1128/IAI.69.1.556-558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El-Omar E, Carrington M, Chow W, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 43.Machado JC, Pharoah P, Sousa S, et al. Interleukin 1B and interleukin 1RN polymorphisms are associated with increased risk of gastric carcinoma. Gastroenterology. 2001;121:823–829. doi: 10.1053/gast.2001.28000. [DOI] [PubMed] [Google Scholar]
- 44.El-Omar EM, Rabkin CS, Gammon MD, et al. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003;124:1193–1201. doi: 10.1016/s0016-5085(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 45.Machado JC, Figueiredo C, Canedo P, et al. A proinflammatory genetic profile increases the risk for chronic atrophic gastritis and gastric carcinoma. Gastroenterology. 2003;125:364–371. doi: 10.1016/s0016-5085(03)00899-0. [DOI] [PubMed] [Google Scholar]
- 46.Sugimoto M, Furuta T, Shirai N, et al. Different effects of polymorphisms of tumor necrosis factor-alpha and interleukin-1 beta on development of peptic ulcer and gastric cancer. J Gastroenterol Hepatol. 2007;22:51–59. doi: 10.1111/j.1440-1746.2006.04442.x. [DOI] [PubMed] [Google Scholar]
- 47.Sugimoto M, Furuta T, Shirai N, et al. Effects of interleukin-10 gene polymorphism on the development of gastric cancer and peptic ulcer in Japanese subjects. J Gastroenterol Hepatol. 2007;22:1443–1449. doi: 10.1111/j.1440-1746.2006.04613.x. [DOI] [PubMed] [Google Scholar]
- 48.Wroblewski LE, Peek RM, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23:713–739. doi: 10.1128/CMR.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loh JT, Torres VJ, Cover TL. Regulation of Helicobacter pylori cagA expression in response to salt. Cancer Res. 2007;67:4709–4715. doi: 10.1158/0008-5472.CAN-06-4746. [DOI] [PubMed] [Google Scholar]
- 50.Yamaoka Y, Kodama T, Graham D, Kashima K. Comparison of four serological tests to determine the CagA or VacA status of Helicobacter pylori strains. J Clin Microbiol. 1998;36:3433–3434. doi: 10.1128/jcm.36.11.3433-3434.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamaoka Y, Graham D. CagA status and gastric cancer unreliable serological tests produce unreliable data. Gastroenterology. 1999;117:745. doi: 10.1016/s0016-5085(99)70476-2. [DOI] [PubMed] [Google Scholar]
- 52.Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat Rev Gastroenterol Hepatol. 2010;7:629–641. doi: 10.1038/nrgastro.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vilaichone RK, Mahachai V, Tumwasorn S, Wu JY, Graham DY, Yamaoka Y. Molecular epidemiology and outcome of Helicobacter pylori infection in Thailand: a cultural cross roads. Helicobacter. 2004;9:453–459. doi: 10.1111/j.1083-4389.2004.00260.x. [DOI] [PubMed] [Google Scholar]
- 54.Matsunari O, Shiota S, Suzuki R, et al. Association between Helicobacter pylori virulence factors and gastroduodenal diseases in Okinawa, Japan. J Clin Microbiol. 2012;50:876–883. doi: 10.1128/JCM.05562-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yasuda A, Uchida T, Nguyen L, et al. A novel diagnostic monoclonal antibody specific for Helicobacter pylori CagA of East Asian type. APMIS. 2009;117:893–899. doi: 10.1111/j.1600-0463.2009.02548.x. [DOI] [PubMed] [Google Scholar]