ABSTRACT
Phosphoethanolamine (PEA) on Neisseria gonorrhoeae lipid A influences gonococcal inflammatory signaling and susceptibility to innate host defenses in in vitro models. Here, we evaluated the role of PEA-decorated gonococcal lipid A in competitive infections in female mice and in male volunteers. We inoculated mice and men with mixtures of wild-type N. gonorrhoeae and an isogenic mutant that lacks the PEA transferase, LptA. LptA production conferred a marked survival advantage for wild-type gonococci in the murine female genital tract and in the human male urethra. Our studies translate results from test tube to animal model and into the human host and demonstrate the utility of the mouse model for studies of virulence factors of the human-specific pathogen N. gonorrhoeae that interact with non-host-restricted elements of innate immunity. These results validate the use of gonococcal LptA as a potential target for development of novel immunoprophylactic strategies or antimicrobial treatments.
IMPORTANCE Gonorrhea is one of the most common bacterial sexually transmitted infections, and increasing antibiotic resistance threatens the use of currently available antimicrobial therapies. In this work, encompassing in vitro studies and in vivo studies of animal and human models of experimental genital tract infection, we document the importance of lipid A’s structure, mediated by a single bacterial enzyme, LptA, in enhancing the fitness of Neisseria gonorrhoeae. The results of these studies suggest that novel agents targeting LptA may offer urgently needed prevention or treatment strategies for gonorrhea.
IMPORTANCE
Gonorrhea is one of the most common bacterial sexually transmitted infections, and increasing antibiotic resistance threatens the use of currently available antimicrobial therapies. In this work, encompassing in vitro studies and in vivo studies of animal and human models of experimental genital tract infection, we document the importance of lipid A’s structure, mediated by a single bacterial enzyme, LptA, in enhancing the fitness of Neisseria gonorrhoeae. The results of these studies suggest that novel agents targeting LptA may offer urgently needed prevention or treatment strategies for gonorrhea.
OBSERVATION
Gonorrhea remains a global health problem. The worldwide incidence of Neisseria gonorrhoeae infection equaled or surpassed that of Chlamydia trachomatis as the most common bacterial sexually transmitted infection for the first time in 2008 (1). Increasing bacterial resistance to antibiotics used to treat gonorrhea, coupled with a dearth of new antimicrobial therapies in development, raises the specter of incurable N. gonorrhoeae infections (2). The potential of adverse reproductive health consequences of untreatable gonococcal infections as well as increased HIV transmission in areas where both infections are prevalent is alarming (3). Identification of new therapeutic and vaccine targets for gonorrhea may be more important now than ever before.
The most abundant lipid constituent of the N. gonorrhoeae outer membrane is lipooligosaccharide (LOS), a glycolipid comprised of an antigenically variable oligosaccharide core (4) attached to lipid A, which is frequently decorated by phosphoethanolamine (PEA) at the 4′ position (5, 6). The presence or absence of PEA-decorated lipid A (PEA-lipid A) profoundly influences inflammatory signaling (7, 8) and bacterial susceptibility to innate host defenses, including the bactericidal activities of normal human serum, complement, and cationic antimicrobial peptides (CAMPs) (5, 9). To assess the importance of this structure during human infection, we constructed PEA transferase (lptA) deletion mutants of N. gonorrhoeae strain FA1090, which cannot add PEA to lipid A, and tested FA1090 ΔlptA in competitive infections with isogenic lptA+ gonococci in the female murine lower genital tract and the human male urethra.
Strains.
N. gonorrhoeae FA1090 is a porin serotype PIB-3, streptomycin (Sm)-resistant strain that has been used extensively in experimental human infection studies (10). Bacteria were cultured on gonococcal agar (GC agar) supplemented with Kellogg’s supplement I and ferric nitrate or in GC broth as described previously (11) with or without antibiotics as appropriate. Cultures were incubated at 35 to 37°C with 5 to 7% CO2.
FA1090 ΔlptA was constructed without altering the antibiotic susceptibility of the wild-type strain, as previously described (12). Briefly, a two-gene cassette containing both a selectable marker (chloramphenicol [Cm] acetyltransferase [CAT] conferring Cm resistance) and a counterselectable marker (rpsL, conferring Sm sensitivity on the naturally resistant strain FA1090) was cloned into the lptA gene and used to replace the wild-type gene in the FA1090 chromosome by allelic exchange; transformants were selected on GC agar with 1 µg Cm/ml. The resulting intermediate strain was Cm resistant and Sm sensitive. A second transformation replaced the CAT rpsL cassette with an unmarked deletion encompassing approximately 80% of the lptA coding sequence; transformants were selected on GC agar with 100 µg Sm/ml. The resulting strain, FA1090 ΔlptA, was Cm sensitive and Sm resistant, as is wild-type strain FA1090. PCR amplification of the lptA locus and analysis of the FA1090 ΔlptA genome sequence confirmed deletion of the gene in the mutant. The wild-type lptA gene from strain FA19 (identical to that of FA1090) was introduced into the FA1090 deletion mutant using the pGCC4 lptA+ vector as previously described (5). The wild-type lptA gene is under the control of a lac promoter and is positioned between lctP and aspC in C′FA1090 ΔlptA.
Electrophoretic characterization of gonococcal LOS and lipid A biochemical analyses.
Whole-cell proteinase K-digested lysates of gonococci were resolved by Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and LOS bands were identified by silver staining and Western blotting with monoclonal antibody 3F11 as previously described (9). Lipooligosaccharides were purified from N. gonorrhoeae strains grown in GC broth, and lipid A was isolated by mild acid hydrolysis as previously described (9). Lipid A was purified by extraction with chloroform and methanol; purified material was used for compositional analysis and determination of mass by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) using a model 4700 proteomics analyzer instrument (Applied Biosystems). Oligosaccharides were purified by size exclusion column chromatography; linkage analysis was performed by gas chromatography-mass spectrometry (GC-MS) using an Alltech AT-1 fused-silica capillary column on a Hewlett-Packard HP5890 gas chromatograph equipped with mass selective detector 5970 MSD.
PMB MIC determination.
MICs of polymyxin B (PMB) were determined by spotting approximately 105 CFU of N. gonorrhoeae suspensions onto GC agar containing 2-fold differences in PMB, from 0.19 to 200 µg PMB/ml; the MIC was defined as the highest concentration of PMB at which bacterial growth was observed after a 24-h incubation.
Experimental murine infection.
All animal experiments were conducted at the Uniformed Services University of the Health Sciences according to the guidelines of the Association for the Assessment and Accreditation of Laboratory Animal Care under a protocol that was approved by the University’s Institutional Animal Care and Use Committee. Female BALB/c mice (6 to 8 weeks old; National Cancer Institute) were treated with water-soluble 17β-estradiol and antibiotics to increase susceptibility to N. gonorrhoeae as described previously (13). Groups of mice were inoculated intravaginally with wild-type FA1090 combined with similar numbers of FA1090 ΔlptA or C′FA1090 ΔlptA CFU (total dose, 106 CFU N. gonorrhoeae; 7 mice/group). Vaginal swabs were collected every other day for 6 days starting on day 2 postinoculation and suspended in 100 µl GC broth. Vaginal swab suspensions and inocula were cultured quantitatively on GC agar with 100 µg Sm/ml (total number of CFU) and GC agar with Sm plus 10 µg PMB/ml (FA1090 or C′FA1090 ΔlptA CFU).
Experimental human infection.
Procedures for participant recruitment, informed consent, intraurethral inoculation, and antibiotic treatment were as previously described (11). Experimental human infections were conducted in the Clinical and Translational Research Center of the North Carolina Translational and Clinical Sciences Institute at the University of North Carolina at Chapel Hill according to the guidelines of the U.S. Department of Health and Human Services and the University’s Institutional Review Board under a protocol that was approved as a U.S. investigational new device (IND). All study participants provided written informed consent. In two separate trials, volunteers received approximately equal numbers of N. gonorrhoeae FA1090 and FA1090 ΔlptA CFU (total dose, 105 to 106 CFU N. gonorrhoeae; 3 men/trial). Gonococci in the inoculum suspensions were predominantly opacity-associated adhesin (Opa) negative and piliated and expressed the same PilE sequence as previously characterized FA1090 variants used in experimental human infection studies (14). First-void urine specimens were obtained daily after inoculation. Urine sediment was cultured quantitatively on GC agar with 3 µg vancomycin, 12.5 units nystatin, and 5 µg trimethoprim lactate/ml, which permits the growth of FA1090 and FA1090 ΔlptA. Up to 96 colonies per subject per culture were replica plated on GC agar with and without 7.5 µg colistin/ml to distinguish the 2 strains; only wild-type FA1090 grows in the presence of the polycationic antibiotic colistin.
Competitive index calculations and statistics.
For experimental murine and human infections, results were expressed as the competitive index (CI) for infected individuals using the equation CI = [mutant CFU (output)/wild-type CFU (output)]/[mutant CFU (input)/wild-type CFU (input)]. For murine infections, in which total CFU and wild-type CFU were enumerated directly from vaginal specimen cultures, the limit of detection of 1 CFU was assigned for a strain that was not recovered from an infected mouse. For human infections, in which total CFU were enumerated directly from urine sediment cultures and up to 96 CFU per specimen was subcultured to identify wild-type or mutant colonies, the limit of detection (1/96 CFU) was assigned for the output proportion of a strain that was not detected from an infected man. A CI of <1 indicates that the mutant is less fit than the wild-type strain. For experimental human infections, we also compared the proportion of wild-type FA1090 among recovered N. gonorrhoeae isolates on the final day of participation for each infected subject to the proportion in the inoculum using a single-sample t test, with the proportion for the null hypothesis equal to 0.54 (the mean proportion of strain FA1090 in the inoculum). With a clearly directional hypothesis that the lptA+ strain would predominate, we used SigmaStat for Windows, v3.5 (Systat Software, Inc.), to calculate one-tailed statistics, with the type I error rate controlled at 0.05.
Lack of LptA-mediated PEA attachment to lipid A renders N. gonorrhoeae susceptible to a model CAMP in vitro.
PEA addition at the 4′ position of FA1090 lipid A was abrogated in FA1090 ΔlptA and restored in the complemented strain C′FA1090 ΔlptA (see Fig. S1A to D in the supplemental material). All strains produced 4.2- and 4.5-kDa LOS species; the former reacted with monoclonal antibody 3F11 (data not shown), confirming the presence of the lacto-N-neotetraose epitope associated with naturally acquired gonococcal urethritis (15). As expected, deletion of lptA rendered FA1090 hypersusceptible to the model CAMP PMB (MICs, 100 µg PMB/ml for FA1090 versus 0.78 µg PMB/ml for FA1090 ΔlptA), which was reversed by 32-fold in the complemented strain (data not shown). Importantly, in vitro growth rates of FA1090 ΔlptA and wild-type FA1090 were indistinguishable (data not shown), and the ΔlptA mutant did not show a fitness difference from wild-type FA1090 when cocultured in vitro through stationary phase in the absence of selective pressure (data not shown). From mixtures containing similar numbers of ΔlptA mutant and wild-type FA1090 cells exposed to 5 µg PMB/ml for 45 min in vitro, only wild-type N. gonorrhoeae with PEA-lipid A survived (data not shown), confirming the importance of this structure in CAMP resistance.
LptA-mediated PEA attachment to lipid A confers a survival advantage to N. gonorrhoeae during competitive infection in mice.
Experimental infection of female BALB/c mice has been used to study innate host defenses against N. gonorrhoeae and the mechanisms by which gonococci evade these defenses (16–18). Groups of female BALB/c mice were inoculated intravaginally with mixtures containing similar numbers of wild-type FA1090 and either FA1090 ΔlptA or C′FA1090 ΔlptA CFU (total dose, 5 × 106 to 7 × 106 CFU). The relative number of colonies of each strain recovered from vaginal swabs from infected mice was determined for up to 6 days after inoculation; output ratios of the two strains were normalized to the ratio of strains in the inoculum. We observed a 10- to 10,000-fold decrease in recovery of the lptA mutant relative to the wild-type parent strain FA1090 on days 2 to 6 of infection (Fig. 1A). No mutant bacteria were recovered from several mice on multiple days postinoculation, whereas wild-type gonococci were recovered from these mice at levels of >103 to >106 CFU/ml vaginal swab suspension (Fig. 1A, open circles). Complementation of lptA restored recovery of the mutant by day 6 postinoculation (Fig. 1B). Packiam et al. recently showed similar results with gonococcal strain FA19 during competitive infection in mice (19).
FIG 1 .
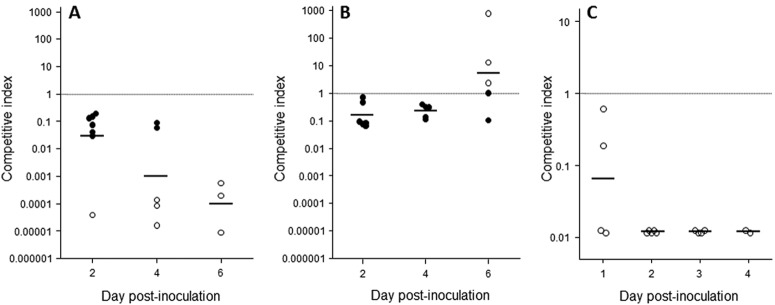
PEA decoration of N. gonorrhoeae lipid A confers a competitive advantage during genital tract infection. The competitive index (CI) was equal to [FA1090 ΔlptA or C′FA1090 ΔlptA/FA1090 (output)]/[FA1090 ΔlptA or C′FA1090 ΔlptA/FA1090 (input)]. (A) Competitive infections in female BALB/c mice (n = 7) with FA1090 ΔlptA and FA1090; (B) female BALB/c mice (n = 7) with C′FA1090 ΔlptA and FA1090; (C) male volunteers (n = 6) with FA1090 ΔlptA and FA1090. Note the different y axis scale in panel C, imposed by the limit of detection of 100-fold-decreased fitness in experimental human infections. (A and C) A CI of <1 indicates that the mutant is less fit than the wild type. Solid circles indicate mice from which both wild-type N. gonorrhoeae FA1090 and FA1090 ΔlptA were recovered (A) and both wild-type N. gonorrhoeae FA1090 and C′FA1090 ΔlptA/FA1090 were recovered (B). Open circles indicate infected mice or men from whom only wild-type N. gonorrhoeae FA1090 was recovered (A and C) or only C′FA1090 ΔlptA was recovered (B). Horizontal bars indicate geometric mean CIs.
LptA-mediated PEA attachment to lipid A confers a survival advantage to N. gonorrhoeae in the male urethra.
To determine potential influences of host restriction differences in mice and humans (as well as differences in female and male genital tracts) on the contribution of PEA-lipid A to in vivo gonococcal survival, we performed competitive infections with wild-type N. gonorrhoeae FA1090 and the lptA mutant in the human challenge model of gonococcal urethritis (10). Six subjects were inoculated intraurethrally with approximately equal mixtures of FA1090 and FA1090 ΔlptA (total dose, 105 to 106 CFU). Five subjects (83%) developed gonococcal urethritis 2 to 4 days after inoculation; one remained uninfected for 5 days after inoculation (Table 1). The relative number of each strain recovered was determined by subculturing up to 96 colonies from each positive urine sediment culture on selective media; output ratios of the two strains were normalized to the ratio of strains in the inoculum. Among colonies tested, only wild-type FA1090 was recovered (Table 1). We observed the maximum possible 100-fold decrease in the recovery of the lptA mutant relative to that of wild-type FA1090 on days 2 to 4 of infection (Fig. 1C).
TABLE 1 .
Competitive infection of male volunteers with N. gonorrhoeae FA1090 and FA1090 ΔlptAh
| Trial | Subject identifier | Inoculum sizea |
Day of treatmentb |
Urethral swab culture |
Bacteriuriac | Pyuriad | Urethritise | % wild-type gonococci |
||
|---|---|---|---|---|---|---|---|---|---|---|
| Inoculated | Recoveredf | Pg | ||||||||
| 1 | 100 | 5.8 | 3 | + | 5.2 | 6.9 | + | 55 | ≥99 | |
| 101 | 5.1 | 4 | + | 5.6 | 7.4 | + | 55 | ≥99 | ||
| 104 | 5.5 | 5 | − | ND | 4.0 | − | 55 | ND | ||
| <0.0001 | ||||||||||
| 2 | 110 | 5.7 | 2 | + | 5.5 | 6.8 | + | 53 | ≥99 | |
| 111 | 5.6 | 3 | + | 4.1 | 5.9 | + | 53 | ≥99 | ||
| 112 | 5.4 | 4 | + | 5.1 | 6.3 | + | 53 | ≥99 | ||
Log10 CFU N. gonorrhoeae inoculated intraurethrally.
Day postinoculation.
Log10 CFU N. gonorrhoeae/ml urine sediment on day of treatment.
Log10 white blood cells (WBC)/ml urine sediment on day of treatment.
Greater than 5.8 log10 WBC/ml urine sediment (equivalent to >4 WBC/high-power field) on day of treatment.
The limit of detection for recovered FA1090 ΔlptA was 1/96 colonies characterized.
Single-sample t test for the percentage of wild-type FA1090 lptA+ recovered versus the mean percentage inoculated.
+, positive; −, negative; ND, none detected.
In our competitive infection studies, PEA-lipid A clearly provided a substantial fitness benefit to gonococci during infection, both in the human male urethra and in the murine female genital tract, compared to N. gonorrhoeae lacking PEA attached to lipid A. The mouse model likely underestimates the importance of PEA-lipid A during infection due to differences in human and murine complement-binding proteins. However, some factors are apparently not host restricted (e.g., CAMP-mediated killing of the lptA mutant), confirming that the murine model can provide important information regarding the pathogenesis of gonococcal infection that is translatable to humans. In the mouse model, reduced infectivity resulting from lptA deletion in N. gonorrhoeae strain FA19 occurs only during competitive infections; noncompetitive infections with wild-type FA19, the FA19 ΔlptA mutant, or the complemented FA19 lptA mutant produce similar kinetics of vaginal colonization and bacterial recovery (19). However, only wild-type N. gonorrhoeae FA19 and the complemented FA19 lptA mutant induce proinflammatory host responses to gonococcal infection in female mice. John and colleagues previously demonstrated reduced inflammatory stimulation of human THP-1 cells by an lptA null mutant of the related pathogen N. meningitidis and by commensal Neisseria species that lack lptA (20). Thus, PEA decoration of gonococcal lipid A not only increases inflammatory responses in vitro and during murine infection but also protects N. gonorrhoeae from the consequences of inflammation, including increased CAMP production. Experimental human infection studies with inocula containing only N. gonorrhoeae FA1090 or FA1090 ΔlptA are needed to confirm whether PEA-decorated lipid A plays dual immunostimulatory and protective roles during male urethral infection. The demonstrated impaired fitness of N. gonorrhoeae mutants lacking PEA in men and female mice validates gonococcal LptA as an important target for development of novel immunoprophylactic strategies or antimicrobial treatments for gonorrhea in males and females in the face of diminishing treatment options.
SUPPLEMENTAL MATERIAL
N. gonorrhoeae FA1090 lipid A structures. Negative-ion MALDI-TOF mass spectra (showing [M-H]− monoisotopic ions) of lipid A from wild-type FA1090 (A), FA1090 ΔlptA (B), and C′FA1090 ΔlptA (C). (D) Putative structures, with their calculated monoisotopic masses (Mmi), for lipid A species 1 to 4 identified in the spectra. Species 3 and 4 with PEA residues at the 4′ position (circled) are absent in FA1090 ΔlptA. Ions due to the loss of water (−18 Da), a change in fatty acid length (e.g., C14 to C12, resulting in −28 Da), and sodiated species (+22 Da) were also observed. Ion 1860.60 was present in both wild-type FA1090 and C′FA1090 ΔlptA lipid A; its structure remains to be identified. Download
ACKNOWLEDGMENTS
This work was supported by the National Institute of Allergy and Infectious Diseases through grant U19AI031496, the National Center for Research Resources and the National Center for Advancing Translational Sciences through grant UL1TR000083, and a VA Merit Award to W.M.S. from the Medical Research Service of the Department of Veterans Affairs. W.M.S. was supported in part by a Senior Research Career Scientist Award from the Medical Research Service of the Department of Veterans Affairs. J.A.D. was supported in part by the Burroughs Wellcome Fund Career Award for Medical Scientists. The isolation and analysis of lipid A was supported in part by a Department of Energy grant, DE-FG-02-93ER20097, to the Complex Carbohydrate Research Center.
The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the Department of Veterans Affairs, the Department of Energy, or the Department of Defense.
We thank JoAnn Kuruc, Amanda Crooks, and the staff of the North Carolina Translational and Clinical Sciences Institute for regulatory and clinical support of experimental human infection studies.
The authors declare no competing financial interests or other relationships relevant to the study.
Footnotes
Citation Hobbs MM, Anderson JE, Balthazar JT, Kandler JL, Carlson RW, Ganguly J, Begum AA, Duncan JA, Lin JT, Sparling PF, Jerse AE, Shafer WM. 2013. Lipid A’s structure mediates Neisseria gonorrhoeae fitness during experimental infection of mice and men. mBio 4(6):e00892-13. doi:10.1128/mBio.00892-13.
REFERENCES
- 1. World Health Organization 2012. Global incidence and prevalence of selected curable sexually transmitted infections —2008. WHO, Geneva, Switzerland [Google Scholar]
- 2. Unemo M, Shafer WM. 2011. Antibiotic resistance in Neisseria gonorrhoeae: origin, evolution, and lessons learned for the future. Ann. N. Y. Acad. Sci. 1230:E19–E28. 10.1111/j.1749-6632.2011.06215.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lewis DA. 2010. The gonococcus fights back: is this time a knock out? Sex. Transm. Infect. 86:415–421 [DOI] [PubMed] [Google Scholar]
- 4. Shafer WM, Datta A, Kolli VS, Rahman MM, Balthazar JT, Martin LE, Veal WL, Stephens DS, Carlson R. 2002. Phase variable changes in genes lgtA and lgtC within the lgtABCDE operon of Neisseria gonorrhoeae can modulate gonococcal susceptibility to normal human serum. J. Endotoxin Res. 8:47–58 [PubMed] [Google Scholar]
- 5. Lewis LA, Choudhury B, Balthazar JT, Martin LE, Ram S, Rice PA, Stephens DS, Carlson R, Shafer WM. 2009. Phosphoethanolamine substitution of lipid A and resistance of Neisseria gonorrhoeae to cationic antimicrobial peptides and complement-mediated killing by normal human serum. Infect. Immun. 77:1112–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lewis LA, Shafer WM, Dutta Ray T, Ram S, Rice PA. 2013. Phosphoethanolamine residues on the lipid A moiety of Neisseria gonorrhoeae lipooligosaccharide modulate binding of complement inhibitors and resistance to complement killing. Infect. Immun. 81:33–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. John CM, Liu M, Jarvis GA. 2009. Natural phosphoryl and acyl variants of lipid A from Neisseria meningitidis strain 89I differentially induce tumor necrosis factor-alpha in human monocytes. J. Biol. Chem. 284:21515–21525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu M, John CM, Jarvis GA. 2010. Phosphoryl moieties of lipid A from Neisseria meningitidis and N. gonorrhoeae lipooligosaccharides play an important role in activation of both MyD88- and TRIF-dependent TLR4-MD-2 signaling pathways. J. Immunol. 185:6974–6984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Balthazar JT, Gusa A, Martin LE, Chouhury B, Carlson R, Shafer WM. 2011. Lipooligosaccharide structure is an important determinant in the resistance of Neisseria gonorrhoeae to antimicrobial agents of innate host defense. Front. Microbiol. 2:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hobbs MM, Sparling PF, Cohen MS, Shafer WM, Deal CD, Jerse AE. 2011. Experimental gonococcal infection in male volunteers: cumulative experience with Neisseria gonorrhoeae strains FA1090 and MS11mkC. Front. Microbiol. 2:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cohen MS, Cannon JG, Jerse AE, Charniga LM, Isbey SF, Whicker LG. 1994. Human experimentation with Neisseria gonorrhoeae: rationale, methods, and implications for the biology of infection and vaccine development. J. Infect. Dis. 169:532–537 [DOI] [PubMed] [Google Scholar]
- 12. Johnston DM, Cannon JG. 1999. Construction of mutant strains of Neisseria gonorrhoeae lacking new antibiotic resistance markers using a two gene cassette with positive and negative selection. Gene 236:179–184 [DOI] [PubMed] [Google Scholar]
- 13. Song W, Condron S, Mocca BT, Veit SJ, Hill D, Abbas A, Jerse AE. August 2008. Local and humoral immune responses against primary and repeat Neisseria gonorrhoeae genital tract infections of 17beta-estradiol-treated mice. Vaccine 26:5741–5751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Seifert HS, Wright CJ, Jerse AE, Cohen MS, Cannon JG. 1994. Multiple gonococcal pilin antigenic variants are produced during experimental human infections. J. Clin. Invest. 93:2744–2749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schneider H, Griffiss JM, Boslego JW, Hitchcock PJ, Zahos KM, Apicella MA. 1991. Expression of paragloboside-like lipooligosaccharides may be a necessary component of gonococcal pathogenesis in men. J. Exp. Med. 174:1601–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Feinen B, Jerse AE, Gaffen SL, Russell MW. 2010. Critical role of Th17 responses in a murine model of Neisseria gonorrhoeae genital infection. Mucosal Immunol. 3:312–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jerse AE, Wu H, Packiam M, Vonck RA, Begum AA, Garvin LE. 2011. Estradiol-treated female mice as surrogate hosts for Neisseria gonorrhoeae genital tract infections. Front. Microbiol. 2:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Packiam M, Veit SJ, Anderson DJ, Ingalls RR, Jerse AE. 2010. Mouse strain-dependent differences in susceptibility to Neisseria gonorrhoeae infection and induction of innate immune responses. Infect. Immun. 78:433–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Packiam M, Begum A, Sempowski G, Carlson R, Shafer W, Jerse A. 2012. Phosphoethanolamine modification of gonococcal lipid A confers an in vivo survival advantage and modulates induction of proinflammatory cytokines by differential binding to cationic antimicrobial peptides, poster P 219, p 365. Prog. XVIIIth Int. Pathog. Neisseria Conf. (IPNC). University of Würzburg, Wurzburg, Germany: http://neisseria.org/ipnc/2012/IPNC_2012_abstracts.pdf [Google Scholar]
- 20. John CM, Liu M, Phillips NJ, Yang Z, Funk CR, Zimmerman LI, Griffiss JM, Stein DC, Jarvis GA. 2012. Lack of lipid A pyrophosphorylation and functional lptA reduces inflammation by Neisseria commensals. Infect. Immun. 80:4014–4026 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
N. gonorrhoeae FA1090 lipid A structures. Negative-ion MALDI-TOF mass spectra (showing [M-H]− monoisotopic ions) of lipid A from wild-type FA1090 (A), FA1090 ΔlptA (B), and C′FA1090 ΔlptA (C). (D) Putative structures, with their calculated monoisotopic masses (Mmi), for lipid A species 1 to 4 identified in the spectra. Species 3 and 4 with PEA residues at the 4′ position (circled) are absent in FA1090 ΔlptA. Ions due to the loss of water (−18 Da), a change in fatty acid length (e.g., C14 to C12, resulting in −28 Da), and sodiated species (+22 Da) were also observed. Ion 1860.60 was present in both wild-type FA1090 and C′FA1090 ΔlptA lipid A; its structure remains to be identified. Download


