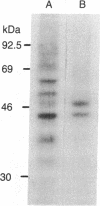
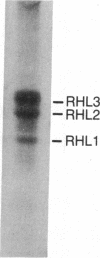
Abstract
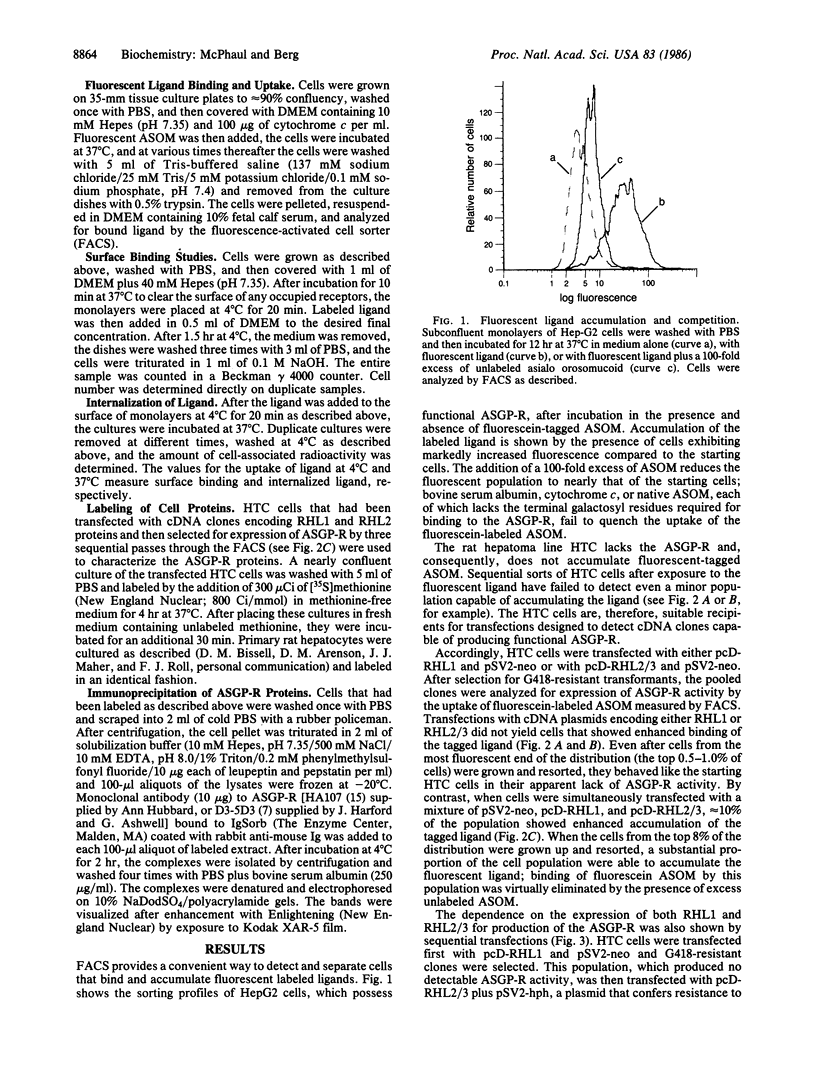
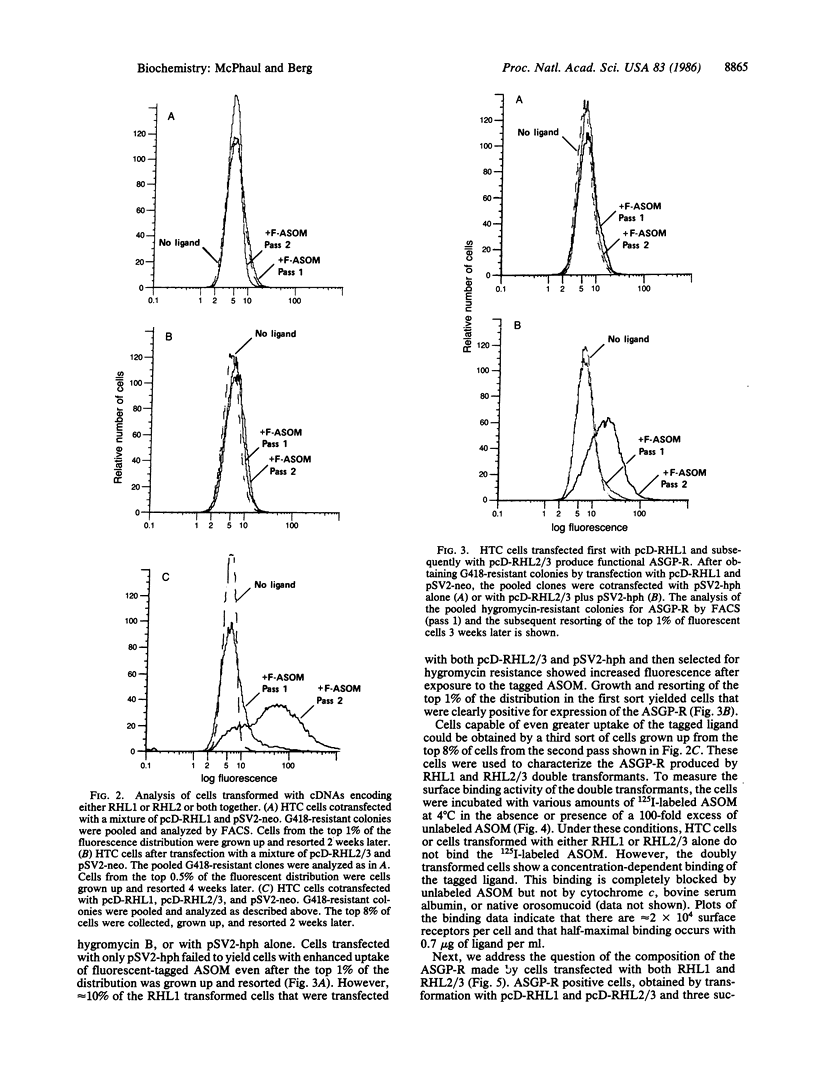
The rat asialoglycoprotein receptor (ASGP-R) has been expressed in cultured rat hepatoma cells (HTC cells) after transfection with cloned cDNAs. Fluorescence-activated cell sorting of transfected cells was used to identify the functional cDNA clones and to isolate cells expressing the ASGP-R. Simultaneous or sequential transfections with two cloned cDNAs that encode related but distinctive polypeptide chains were needed to obtain ASGP-R activity; transfection with either cDNA alone failed to produce detectable ASGP-R. The affinity of transduced ASGP-R for asialo orosomucoid is less than that of the native rat ASGP-R, and the number of surface receptors in clones expressing ASGP-R is about one-fifth that found on rat hepatocytes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwell G., Harford J. Carbohydrate-specific receptors of the liver. Annu Rev Biochem. 1982;51:531–554. doi: 10.1146/annurev.bi.51.070182.002531. [DOI] [PubMed] [Google Scholar]
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- Bartles J. R., Braiterman L. T., Hubbard A. L. Biochemical characterization of domain-specific glycoproteins of the rat hepatocyte plasma membrane. J Biol Chem. 1985 Oct 15;260(23):12792–12802. [PubMed] [Google Scholar]
- Drickamer K., Mamon J. F., Binns G., Leung J. O. Primary structure of the rat liver asialoglycoprotein receptor. Structural evidence for multiple polypeptide species. J Biol Chem. 1984 Jan 25;259(2):770–778. [PubMed] [Google Scholar]
- Frost E., Williams J. Mapping temperature-sensitive and host-range mutations of adenovirus type 5 by marker rescue. Virology. 1978 Nov;91(1):39–50. doi: 10.1016/0042-6822(78)90353-7. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Harford J., Lowe M., Tsunoo H., Ashwell G. Immunological approaches to the study of membrane receptors. A monoclonal antibody that inhibits the binding of asialoglycoproteins to the rat liver receptor. J Biol Chem. 1982 Nov 10;257(21):12685–12690. [PubMed] [Google Scholar]
- Kawasaki T., Ashwell G. Chemical and physical properties of an hepatic membrane protein that specifically binds asialoglycoproteins. J Biol Chem. 1976 Mar 10;251(5):1296–1302. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Markwell M. A. A new solid-state reagent to iodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal Biochem. 1982 Sep 15;125(2):427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Spiess M., Lodish H. F. Sequence of a second human asialoglycoprotein receptor: conservation of two receptor genes during evolution. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6465–6469. doi: 10.1073/pnas.82.19.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer C. J., Ashwell G. Studies on a mammalian hepatic binding protein specific for asialoglycoproteins. Evidence for receptor recycling in isolated rat hepatocytes. J Biol Chem. 1980 Apr 10;255(7):3008–3013. [PubMed] [Google Scholar]
- Zeitlin P. L., Hubbard A. L. Cell surface distribution and intracellular fate of asialoglycoproteins: a morphological and biochemical study of isolated rat hepatocytes and monolayer cultures. J Cell Biol. 1982 Mar;92(3):634–647. doi: 10.1083/jcb.92.3.634. [DOI] [PMC free article] [PubMed] [Google Scholar]