Abstract
Cu/Zn superoxide dismutase (Cu/Zn SOD) is a key enzyme in the metabolism of oxygen free radicals. The gene resides on chromosome 21 and is overexpressed in patients with Down syndrome. Cultured neurons of transgenic Cu/Zn SOD (Tg-Cu/Zn SOD) mice with elevated activity of Cu/Zn SOD were used to determine whether constitutive overexpression of Cu/Zn SOD creates an indigenous oxidative stress that predisposes the Tg-Cu/Zn SOD neurons to added insults. Neurons from three independently derived Tg-Cu/Zn SOD strains showed higher susceptibility than nontransgenic neurons to kainic acid (KA)-mediated excitotoxicity, reflected by an earlier onset and enhanced apoptotic cell death. This higher susceptibility of transgenic neurons to KA-mediated apoptosis was associated with a chronic prooxidant state that was manifested by reduced levels of cellular glutathione and altered [Ca2+]i homeostasis. The data are compatible with the thesis that overexpression of Cu/Zn SOD creates chronic oxidative stress in the transgenic neurons, which exacerbates their susceptibility to additional insults such as KA-mediated excitotoxicity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Shigenaga M. K., Hagen T. M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amstad P., Moret R., Cerutti P. Glutathione peroxidase compensates for the hypersensitivity of Cu,Zn-superoxide dismutase overproducers to oxidant stress. J Biol Chem. 1994 Jan 21;269(3):1606–1609. [PubMed] [Google Scholar]
- Amstad P., Peskin A., Shah G., Mirault M. E., Moret R., Zbinden I., Cerutti P. The balance between Cu,Zn-superoxide dismutase and catalase affects the sensitivity of mouse epidermal cells to oxidative stress. Biochemistry. 1991 Sep 24;30(38):9305–9313. doi: 10.1021/bi00102a024. [DOI] [PubMed] [Google Scholar]
- Avraham K. B., Schickler M., Sapoznikov D., Yarom R., Groner Y. Down's syndrome: abnormal neuromuscular junction in tongue of transgenic mice with elevated levels of human Cu/Zn-superoxide dismutase. Cell. 1988 Sep 9;54(6):823–829. doi: 10.1016/s0092-8674(88)91153-1. [DOI] [PubMed] [Google Scholar]
- Avraham K. B., Sugarman H., Rotshenker S., Groner Y. Down's syndrome: morphological remodelling and increased complexity in the neuromuscular junction of transgenic CuZn-superoxide dismutase mice. J Neurocytol. 1991 Mar;20(3):208–215. doi: 10.1007/BF01186993. [DOI] [PubMed] [Google Scholar]
- Batistatou A., Greene L. A. Internucleosomal DNA cleavage and neuronal cell survival/death. J Cell Biol. 1993 Aug;122(3):523–532. doi: 10.1083/jcb.122.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman J. S., Carson M., Smith C. D., Koppenol W. H. ALS, SOD and peroxynitrite. Nature. 1993 Aug 12;364(6438):584–584. doi: 10.1038/364584a0. [DOI] [PubMed] [Google Scholar]
- Behl C., Davis J. B., Lesley R., Schubert D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell. 1994 Jun 17;77(6):817–827. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Brown R. H., Jr Amyotrophic lateral sclerosis: recent insights from genetics and transgenic mice. Cell. 1995 Mar 10;80(5):687–692. doi: 10.1016/0092-8674(95)90346-1. [DOI] [PubMed] [Google Scholar]
- Busciglio J., Yankner B. A. Apoptosis and increased generation of reactive oxygen species in Down's syndrome neurons in vitro. Nature. 1995 Dec 21;378(6559):776–779. doi: 10.1038/378776a0. [DOI] [PubMed] [Google Scholar]
- Cadet J. L., Sheng P., Ali S., Rothman R., Carlson E., Epstein C. Attenuation of methamphetamine-induced neurotoxicity in copper/zinc superoxide dismutase transgenic mice. J Neurochem. 1994 Jan;62(1):380–383. doi: 10.1046/j.1471-4159.1994.62010380.x. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Excitotoxic cell death. J Neurobiol. 1992 Nov;23(9):1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- Church S. L., Grant J. W., Ridnour L. A., Oberley L. W., Swanson P. E., Meltzer P. S., Trent J. M. Increased manganese superoxide dismutase expression suppresses the malignant phenotype of human melanoma cells. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):3113–3117. doi: 10.1073/pnas.90.7.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle J. T., Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993 Oct 29;262(5134):689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Elroy-Stein O., Bernstein Y., Groner Y. Overproduction of human Cu/Zn-superoxide dismutase in transfected cells: extenuation of paraquat-mediated cytotoxicity and enhancement of lipid peroxidation. EMBO J. 1986 Mar;5(3):615–622. doi: 10.1002/j.1460-2075.1986.tb04255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elroy-Stein O., Groner Y. Impaired neurotransmitter uptake in PC12 cells overexpressing human Cu/Zn-superoxide dismutase--implication for gene dosage effects in Down syndrome. Cell. 1988 Jan 29;52(2):259–267. doi: 10.1016/0092-8674(88)90515-6. [DOI] [PubMed] [Google Scholar]
- Epstein C. J., Avraham K. B., Lovett M., Smith S., Elroy-Stein O., Rotman G., Bry C., Groner Y. Transgenic mice with increased Cu/Zn-superoxide dismutase activity: animal model of dosage effects in Down syndrome. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8044–8048. doi: 10.1073/pnas.84.22.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
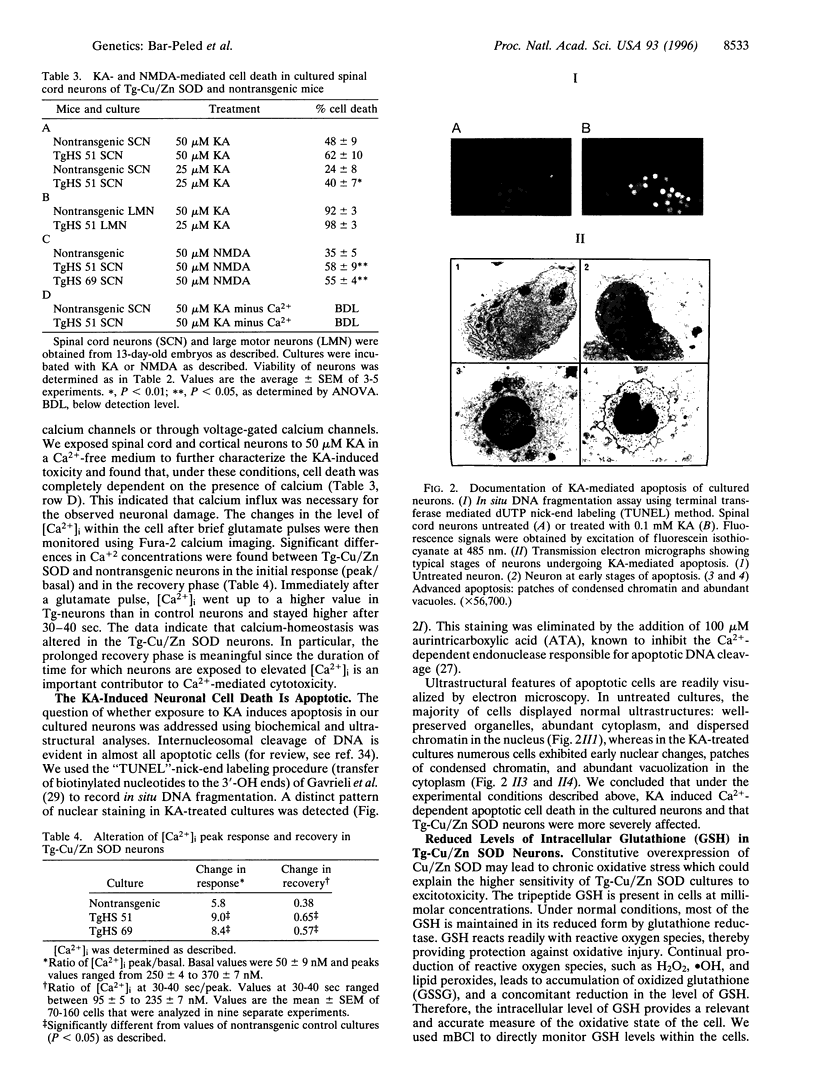
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagay Z. J., Weiss Y., Zusman I., Peled-Kamar M., Reece E. A., Eriksson U. J., Groner Y. Prevention of diabetes-associated embryopathy by overexpression of the free radical scavenger copper zinc superoxide dismutase in transgenic mouse embryos. Am J Obstet Gynecol. 1995 Oct;173(4):1036–1041. doi: 10.1016/0002-9378(95)91323-8. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Reactive oxygen species and the central nervous system. J Neurochem. 1992 Nov;59(5):1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- Hockenbery D. M., Oltvai Z. N., Yin X. M., Milliman C. L., Korsmeyer S. J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993 Oct 22;75(2):241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- Kelner M. J., Bagnell R., Montoya M., Estes L., Uglik S. F., Cerutti P. Transfection with human copper-zinc superoxide dismutase induces bidirectional alterations in other antioxidant enzymes, proteins, growth factor response, and paraquat resistance. Free Radic Biol Med. 1995 Mar;18(3):497–506. doi: 10.1016/0891-5849(94)00167-i. [DOI] [PubMed] [Google Scholar]
- Kubisch H. M., Wang J., Luche R., Carlson E., Bray T. M., Epstein C. J., Phillips J. P. Transgenic copper/zinc superoxide dismutase modulates susceptibility to type I diabetes. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9956–9959. doi: 10.1073/pnas.91.21.9956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levanon D., Lieman-Hurwitz J., Dafni N., Wigderson M., Sherman L., Bernstein Y., Laver-Rudich Z., Danciger E., Stein O., Groner Y. Architecture and anatomy of the chromosomal locus in human chromosome 21 encoding the Cu/Zn superoxide dismutase. EMBO J. 1985 Jan;4(1):77–84. doi: 10.1002/j.1460-2075.1985.tb02320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. J., Oberley L. W., St Clair D. K., Ridnour L. A., Oberley T. D. Phenotypic changes induced in human breast cancer cells by overexpression of manganese-containing superoxide dismutase. Oncogene. 1995 May 18;10(10):1989–2000. [PubMed] [Google Scholar]
- Mattson M. P., Barger S. W., Cheng B., Lieberburg I., Smith-Swintosky V. L., Rydel R. E. beta-Amyloid precursor protein metabolites and loss of neuronal Ca2+ homeostasis in Alzheimer's disease. Trends Neurosci. 1993 Oct;16(10):409–414. doi: 10.1016/0166-2236(93)90009-b. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Mutant mice, Cu,Zn superoxide dismutase, and motor neuron degeneration. Science. 1994 Dec 2;266(5190):1586–1587. [PubMed] [Google Scholar]
- Morrison K. E. Mechanisms in motor neurone disease: clues from genetic studies. Mol Med Today. 1995 Jul;1(4):195–201. doi: 10.1016/s1357-4310(95)91930-9. [DOI] [PubMed] [Google Scholar]
- Nakao N., Frodl E. M., Widner H., Carlson E., Eggerding F. A., Epstein C. J., Brundin P. Overexpressing Cu/Zn superoxide dismutase enhances survival of transplanted neurons in a rat model of Parkinson's disease. Nat Med. 1995 Mar;1(3):226–231. doi: 10.1038/nm0395-226. [DOI] [PubMed] [Google Scholar]
- Nelson S. K., Bose S. K., McCord J. M. The toxicity of high-dose superoxide dismutase suggests that superoxide can both initiate and terminate lipid peroxidation in the reperfused heart. Free Radic Biol Med. 1994 Feb;16(2):195–200. doi: 10.1016/0891-5849(94)90143-0. [DOI] [PubMed] [Google Scholar]
- Norris K. H., Hornsby P. J. Cytotoxic effects of expression of human superoxide dismutase in bovine adrenocortical cells. Mutat Res. 1990 Mar;237(2):95–106. doi: 10.1016/0921-8734(90)90015-j. [DOI] [PubMed] [Google Scholar]
- Olanow C. W. A radical hypothesis for neurodegeneration. Trends Neurosci. 1993 Nov;16(11):439–444. doi: 10.1016/0166-2236(93)90070-3. [DOI] [PubMed] [Google Scholar]
- Oyama F., Cairns N. J., Shimada H., Oyama R., Titani K., Ihara Y. Down's syndrome: up-regulation of beta-amyloid protein precursor and tau mRNAs and their defective coordination. J Neurochem. 1994 Mar;62(3):1062–1066. doi: 10.1046/j.1471-4159.1994.62031062.x. [DOI] [PubMed] [Google Scholar]
- Peled-Kamar M., Lotem J., Okon E., Sachs L., Groner Y. Thymic abnormalities and enhanced apoptosis of thymocytes and bone marrow cells in transgenic mice overexpressing Cu/Zn-superoxide dismutase: implications for Down syndrome. EMBO J. 1995 Oct 16;14(20):4985–4993. doi: 10.1002/j.1460-2075.1995.tb00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike C. J., Walencewicz-Wasserman A. J., Kosmoski J., Cribbs D. H., Glabe C. G., Cotman C. W. Structure-activity analyses of beta-amyloid peptides: contributions of the beta 25-35 region to aggregation and neurotoxicity. J Neurochem. 1995 Jan;64(1):253–265. doi: 10.1046/j.1471-4159.1995.64010253.x. [DOI] [PubMed] [Google Scholar]
- Regan R. F., Choi D. W. Glutamate neurotoxicity in spinal cord cell culture. Neuroscience. 1991;43(2-3):585–591. doi: 10.1016/0306-4522(91)90317-h. [DOI] [PubMed] [Google Scholar]
- Sakashita A., Epstein C. J., Carlson E., Koeffler H. P. Hematopoietic progenitor cells of transgenic mice with increased copper/zinc-superoxide dismutase activity are resistant to tumor necrosis factor. J Cell Physiol. 1994 Aug;160(2):233–238. doi: 10.1002/jcp.1041600204. [DOI] [PubMed] [Google Scholar]
- Schickler M., Knobler H., Avraham K. B., Elroy-Stein O., Groner Y. Diminished serotonin uptake in platelets of transgenic mice with increased Cu/Zn-superoxide dismutase activity. EMBO J. 1989 May;8(5):1385–1392. doi: 10.1002/j.1460-2075.1989.tb03519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M., Manor D. Confocal microscopic imaging of [Ca2+]i in cultured rat hippocampal neurons following exposure to N-methyl-D-aspartate. J Physiol. 1992 Mar;448:655–676. doi: 10.1113/jphysiol.1992.sp019063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe D. J. Normal and abnormal biology of the beta-amyloid precursor protein. Annu Rev Neurosci. 1994;17:489–517. doi: 10.1146/annurev.ne.17.030194.002421. [DOI] [PubMed] [Google Scholar]
- Shrieve D. C., Bump E. A., Rice G. C. Heterogeneity of cellular glutathione among cells derived from a murine fibrosarcoma or a human renal cell carcinoma detected by flow cytometric analysis. J Biol Chem. 1988 Oct 5;263(28):14107–14114. [PubMed] [Google Scholar]
- Smith R. G., Appel S. H. Molecular approaches to amyotrophic lateral sclerosis. Annu Rev Med. 1995;46:133–145. doi: 10.1146/annurev.med.46.1.133. [DOI] [PubMed] [Google Scholar]
- Steinman H. M. The Bcl-2 oncoprotein functions as a pro-oxidant. J Biol Chem. 1995 Feb 24;270(8):3487–3490. [PubMed] [Google Scholar]
- White C. W., Avraham K. B., Shanley P. F., Groner Y. Transgenic mice with expression of elevated levels of copper-zinc superoxide dismutase in the lungs are resistant to pulmonary oxygen toxicity. J Clin Invest. 1991 Jun;87(6):2162–2168. doi: 10.1172/JCI115249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarom R., Sapoznikov D., Havivi Y., Avraham K. B., Schickler M., Groner Y. Premature aging changes in neuromuscular junctions of transgenic mice with an extra human CuZnSOD gene: a model for tongue pathology in Down's syndrome. J Neurol Sci. 1988 Dec;88(1-3):41–53. doi: 10.1016/0022-510x(88)90204-3. [DOI] [PubMed] [Google Scholar]
- Yim M. B., Chock P. B., Stadtman E. R. Copper, zinc superoxide dismutase catalyzes hydroxyl radical production from hydrogen peroxide. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5006–5010. doi: 10.1073/pnas.87.13.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim M. B., Chock P. B., Stadtman E. R. Enzyme function of copper, zinc superoxide dismutase as a free radical generator. J Biol Chem. 1993 Feb 25;268(6):4099–4105. [PubMed] [Google Scholar]
- de Vos S., Epstein C. J., Carlson E., Cho S. K., Koeffler H. P. Transgenic mice overexpressing human copper/zinc-superoxide dismutase (Cu/Zn SOD) are not resistant to endotoxic shock. Biochem Biophys Res Commun. 1995 Mar 17;208(2):523–531. doi: 10.1006/bbrc.1995.1370. [DOI] [PubMed] [Google Scholar]