Abstract
Bone marrow mesenchymal stem cells (MSCs) have the potential to migrate to the site of injury and regulate the repair process. Aquaporin 1 (Aqp1) is a water channel molecule and a regulator of endothelial cell migration. To study the role of Apq1 in MSC migration, we manipulated the expression of the Aqp1 gene in MSCs and explored its effects on MSC migration both in vitro and in vivo. Overexpression of Aqp1 promoted MSC migration, while depletion of Aqp1 impaired MSC migration in vitro. When the green fluorescent protein (GFP) labeled Aqp1 overexpressing MSCs were systemically injected into rats with a femoral fracture, there were significantly more GFP-MSCs found at the fracture gap in the Aqp1-GFP-MSC-treated group compared to the GFP-MSC group. To elucidate the underlying mechanism, we screened several migration-related regulators. The results showed that β-catenin and focal adhesion kinase (FAK) were upregulated in the Aqp1-MSCs and downregulated in the Aqp1-depleted MSCs, while C-X-C chemokine receptor type 4 had no change. Furthermore, β-catenin and FAK were co-immunoprecipitated with Aqp1, and depletion of FAK abolished the Aqp1 effects on MSC migration. This study demonstrates that Aqp1 enhances MSC migration ability mainly through the FAK pathway and partially through the β-catenin pathway. Our finding suggests a novel function of Aqp1 in governing MSC migration, and this may aid MSC therapeutic applications.
Introduction
Mesenchymal stem cells (MSCs) have been characterized for decades [1], and utilized extensively for improving and treating many disease conditions, such as bone defects [2], myocardial infarction [3,4], and diabetes [5]. MSCs have low immunogenicity, and are relatively easy to obtain and culture [6]. However, the systemic administration of MSCs through circulation has some limitations; this is because only a few transplanting MSCs could reach the injured tissue sites, most of which are trapped and dead within a short duration in small blood vessels in the lung and other tissues [7]. Therefore, enhancing the MSC migration capacity may allow them to migrate to the injurious tissues more efficiently.
Cell migration is a complex process that orchestrates signal transduction, cytoskeleton rearrangement, and morphogenesis. In the canonical migration cycle, it mainly comprised three distinctive steps, including cell polarization, protrusion and adhesion formation, and rear retraction [8]. Multiple regulators play roles in cell migration, among which focal adhesion kinase (FAK) mainly conveys signals from the extracellular matrix to the cell in the adhesion complex [9]. FAK is a ubiquitously nonreceptor cytoplasmic protein tyrosine kinase [10], which plays key roles in embryonic development and many human diseases, like cancer and cardiovascular disease [11]. In addition, FAK is crucial for MSCs migrating to injured sites, in response to stromal cell-derived factor (SDF)-1 [12,13]. Another important pathway in the regulation of MSC migration is SDF-1α and its receptor C-X-C chemokine receptor type 4 (CXCR4) axis. Previous studies have shown that overexpression of CXCR4 accelerates MSC mobilization and angiogenesis in the infarcted myocardium [14].
The β-catenin is an important regulator in the Wnt pathway, which regulates many aspects of cell behavior, like fate decision, migration, and cell differentiation [15,16]. At the cell surface, β-catenin binds α-catenin, which links the complex to the actin cytoskeleton and recruits actin-remodeling agents. After stimulation, β-catenin dissociates from the E-cadherin/catenin cell membrane complex and translocates into cell nuclei, where it acts as a transcriptional coactivator binding with the members of the T cell factor/lymphoid enhancer factor (TCF/LEF) transcription factor family [17]. The target genes of TCF/LEF include matrix metalloproteinases, chemokines, or cytoskeletal proteins, which regulate cell migration and cancer invasion [18–20].
As one of the water channel molecules, aquaporin 1 (Aqp1) transports water across cell membranes. The structure, distribution, and biochemical properties of the Aquaporin family have been studied extensively over recent decades [21]. Recent evidence has shown that Aqp1 promotes tumor angiogenesis and growth by modulating endothelial cell migration through Lin-7 and β-catenin [22,23]. However, there has been no study of the role of Aqp1 in MSC migration. In this study, we demonstrate that Aqp1 could promote MSC migration through β-catenin and FAK pathways; overexpression of Aqp1 in MSCs could enable MSCs to migrate more efficiently to the fracture gap in a rat fracture model.
Materials and Methods
Isolation and characterization of MSCs
Male Sprague-Dawley (SD) rats were used under the animal license issued by the Hong Kong SAR Government and the local ethics committee. Briefly, bone marrow was harvested from femurs and layered onto Lymphoprep™ (1,077 g/mL; Axis-Shield) for centrifugation at 400 g for 25 min. The isolated mononuclear cells were suspended in an Alpha Essential Eagle's medium containing 10% fetal bovine serum (FBS), 1% penicillin–streptomycin–neomycin (Life Technologies), seeded into T75 flasks (Corning) at a density of 1×105 cells/cm2, and incubated at 37°C in a humidified atmosphere with 5% CO2/95% air. MSCs from passage 3 or 4 were used for flow cytometry analysis. The antibodies were as follows: PerCP-cy5.5-CD45, FITC-CD31, FITC-CD34, FITC-CD73, FITC-CD44, and PE-CD90 (BD Bioscience). Expanded cultures of the MSC were analyzed for chondrogenic, osteogenic, and adipogenic differentiation in vitro to determine multipotency in accordance with standard conditions, as described previously [24].
Plasmid construction and lentiviral transfection
Rat Aqp1 gene (GenBank number: NM_012778.1) was amplified from rat bone marrow MSC cDNA, cloned into pLenti-MCS-DsRed vector between EcoRI and SalI sites. For Aqp1 and FAK depleted experiments, Aqp1-shRNA, FAK-shRNA, and scramble control were designed following a previous report [25] and cloned into the plentiLox3.7 vector. The corresponding sequences were Aqp1-shRNA 5′-CTTCTCAAACCACTGGATT-3′, Scramble-shRNA 5′-ACTTGCCATACATGACTCT-3′, β catenin-shRNA 5′-GCCAGGTGGTCGTTAATAAA-3′, and FAK-shRNA 5′-CTGTAGCATAGAGTCAGA-3′. Lentivirus carrying the above vectors was produced in the 293FT cell line by transient transfection with the calcium phosphate transfection method, as described previously [7]. Cell supernatants containing lentiviral particles were collected after transfection for 48 and 72 h, filtered through a 0.45 membrane (Millipore), and subjected to further concentration by using a PEG-it Virus Precipitation kit (System Biosciences) following the manufacturer's instructions. MSCs were immortalized by lentiviral transduction of Simian virus 40 plasmid and a single colony was selected and amplified for the following experiments. For transduction, 1×105 MSCs were seeded into a six-well plate and incubated with lentiviruses together with 8 μg/mL polybrene in the incubator for 24 h. Stable transfected MSC lines were then established in the presence of blasticidin (10 μg/mL) for 2 weeks. The expression of Aqp1 in transfected stable cell lines was examined by using quantitative polymerase chain reaction (PCR) and western blot. To trace their fates in vivo, Aqp1-transfected MSCs and -DsRed MSCs were further labeled with the green fluorescent protein (GFP) gene by lentiviral transfection, as described above.
Quantitative PCR assays
RNA was extracted by using the PureLink RNA Mini Kit (Ambion), and contaminated genomic DNA was eliminated with DNAaseI treatment (Invitrogen). The first-strand cDNA was synthesized by M-MLV reverse transcriptase (Promega). Quantitative PCR of Aqp1 was performed by using SYBR green PCR master mix (Applied Biosystems), and the primers were as follows: Aqp1-forward: 5′-GCTCACCCGCAACTTCTCA-3′ and Aqp1-reverse: 5′-CCTCTATTTGGGCTTCATCTCC-3′. β catenin-forward: 5′-CGAGGACTCAATACCATTC C-3′ and β catenin-reverse: 5′-AGCCGTTTCTTGTAGTCCTG-3′.The correct size of the amplified products was checked by electrophoresis.
Western blot
Western blot examination was carried out, as described previously [7]. In brief, cells at the confluence were scraped and lysed in the RIPA buffer (Invitrogen). The total protein concentration was quantified by the BCA method (Pierce). Equal amount of proteins were run on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were then transferred from the gel into the PVDF membrane. After blocking in 5% milk, the membranes were incubated with primary antibodies overnight at 4°C. After being washed three times with the TBST buffer, the membranes were then incubated with secondary antibodies conjugated with horseradish peroxidase for 1 h at room temperature. Proteins were visualized with enhanced chemiluminescence (Pierce). The following primary antibodies were used: Aqp1 (1:1,000; Santa Cruz), β-catenin (1:3,000; BD Biosciences), GAPDH (1:1,000; Santa Cruz), β-actin (1:1,000; Santa Cruz), p-FAK (Y397; Cell Signaling Technology), and FAK (1:1,000; Santa Cruz).
Co-immunoprecipitation
Cells at the confluence were washed in ice-cold phosphate-buffered saline (PBS), scraped, and resuspended with 1 mL of lysis buffer containing 20 mM Tris-HCl (pH 8), 137 mM NaCl, 10% glycerol, 1% Nonidet P-40, and 2 mM EDTA. Cell lysate was precleared by incubation with 50 μL protein G-agorose slurry (Invitrogen) for 1 h at 4°C. After a brief centrifuge (2 min at 14,000 g at 4°C), the supernatant was incubated with a 1-μg Aqp1 (Santa Cruz) antibody and IgG (Epitomics, Inc.) overnight at 4°C, and then 50 μL of protein G–agarose slurry for 4 h. The immunoprecipitated samples were recovered after centrifugation, washed 3× for 2 min at 14,000 g at 4°C, each with a lysing buffer. The immunoprecipitated samples were boiled for 5 min and resuspended with 2× Laemmli buffer (4% SDS, 10% 2-mercaptoehtanol, 20% glycerol, 0.004% bromophenol blue, and 0.125 M Tris-HCl) for SDS-PAGE examination, as described previously.
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde for 20 min at room temperature. After washing with PBS, the cells were permeated with 0.1% Triton X-100 for 10 min and blocked with 5% bovine serum albumin (BSA) for 30 min at room temperature. Then, the cells were incubated with primary antibodies Aqp1 (1:100; Santa Cruz) and FAK (1:100; Santa Cruz) overnight at 4°C. After washing with PBS, the cells were incubated with Fluorescein anti-mouse IgG (1:200; Vector Laboratories) and Texas Anti-goat IgG (1:200; Vector Laboratories) for 1 h at room temperature. Nuclear counterstaining was performed with a DAPI vectashield mounting medium (Vector Laboratories). Immunostaining was observed under an Olympus FV1000 confocal microscope.
Rat tibia fracture model and MSC administration
The rat tibia fracture model was established as previously reported [26]. SD rats (n=10) were used in this study and placed randomly into two groups. Under general anesthesia, soft tissues were carefully dissected and a transverse osteotomy was performed with a ring cutter type saw at the midshaft of the tibia, which was fixed internally with a 21G needle. Fracture quality and position were assessed and confirmed by a digital X-ray machine (Faxitron Bioptics). Two days following fracture, 1×106 cells (Aqp1-MSCs and DsRed-MSCs) in 1 mL saline were injected through the tail vein into the fractured rats. All animals were terminated 5 days after the cell administration and the fractured tibia samples as well as other internal organs were harvested, prepared, and embedded in paraffin for further examinations.
Immunohistochemistry
Rat tibiae were fixed in 4% paraformaldehyde and decalcified in 9% formic acid for 4 weeks. After gradient dehydration in ethanol, the samples were embedded in paraffin. Sections were selected randomly for GFP staining from both groups. Briefly, sections were deparaffinized and antigen retrieval was done by immersing sections into 10 mM of citrate buffer at 70°C for 20 min. Blocked in 5% goat serum and 1% BSA for 30 min, sections were incubated with the rabbit anti-GFP antibody (1:100; Santa Cruz) overnight at 4°C. After being washed with PBS for three times, samples were incubated with the goat anti-rabbit antibody (1:100; Santa Cruz) for 30 min. Then, a signal was developed by incubating with DAB (Dako) for 1 min and observed under a microscope.
Cell migration assay
For transwell assay, cells (4×104 cells/cm2) were inoculated into the upper layer of a transwell insert (BD Falcon) in a serum-free medium with 10% FBS containing the medium at the bottom layer. After incubating for 10 h at 37°C, MSCs at the upper layer of the membrane were scraped and MSCs at the lower layer were stained with 0.05% crystal violet and photographed under a microscope. A number of cells were quantified in the randomly selected fields. For wound healing assay, confluent MSCs were scraped by a yellow plastic pipette tip. The width of the wound area was measured in triplicate wells under an inverse phase-contrast microscope.
Statistical analysis
Values are presented for each group as mean±SEM. The Student's t-test and one-way ANOVA were used for comparison of the mean values between different groups. P-value was calculated with SPSS16.0, and P<0.05 was considered to be statistically significant.
Results
Establishment of stable Aqp1 depleted and overexpressing MSCs
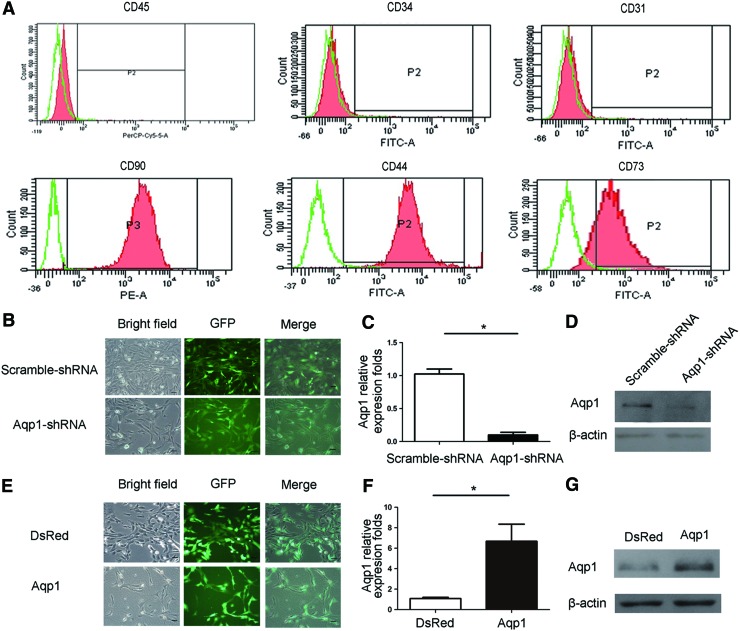
MSCs were isolated from rat femur marrow and characterized by using flow cytometry analyses of CD45 (2%), CD31 (1.5%), CD34 (2%), CD73 (80%), Cd44 (95%), and CD90 (95%) (Fig. 1A), which were further verified by trilineage differentiation assay. Considering the limited passage number of normal MSCs and the variation of different batches of MSCs, we chose to use the immortalized MSCs by transfection of SV40, as described previously [27,28]. These immortalized MSCs had a similar cell surface marker expression pattern and a trilineage differentiation potential. All the following experiments were carried out by using immortalized rat MSCs. We delivered Aqp1-specific shRNA and Aqp1 coding sequence by lentiviral transfection into MSCs and developed stable Aqp1-depleted MSCs and Aqp1 overexpressing cell lines designed as Aqp1-shRNA MSCs with Scramble-shRNA MSCs as control and Aqp1-MSCs with DsRed-MSCs as control, respectively. The transfection efficiency of transduction was high without any apparent effects on cell death and morphology change (Fig. 1B, E). The cell vitality of MSCs was not affected by transfection treatment (Table 1). Aqp1 shRNA transduction depleted the expression of Aqp1 both in the mRNA and protein levels (Fig. 1C, D). Similarly, Aqp1 transduction highly regulated the expression of Aqp1 both in the mRNA and protein levels (Fig. 1F, G). The expression status of Aqp1 did not change after successive passages. We also found that depletion or overexpression of Aqp1 had no effect on MSC trilineage differentiation (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/scd).
FIG. 1.
CD markers expression of mesenchymal stem cells (MSCs) and establishment of the stable aquaporin 1 (Aqp1) overexpressed and depleted MSCs cell line. MSCs from passage 3 or 4 were stained with PerCP-cy5.5-CD45, FITC-CD31, FITC-CD34, FITC-CD73, FITC-CD44, and PE-CD90 antibodies and detected by flow cytometry (A, red). Isotype IgG was used as a negative control (green) to gate positive part of CD45 (2%), CD31(1.5%), CD34 (2%), CD73 (80%), Cd44 (95%), and CD90 (95%) (P2). The immortalized MSCs were transduced with green fluorescent protein (GFP) labeling Aqp1-shRNA and control scramble-shRNA lentiviral vector (B), showing that the stable cell lines were established with high transfection efficiency. The expression of Aqp1 after transduction was quantified with real-time polymerase chain reaction (PCR) (C, n=3) and western blot (D) confirming the results. Aqp1 overexpressing MSC lines were established by lentiviral transfection carrying Aqp1 coding sequence (E). The expression of Aqp1 after transduction was quantified with real-time PCR (F, n=3) and western blot (G). Scale bar: 100 μm. All error bars represent SEM (*P<0.05). Color images available online at www.liebertpub.com/scd
Table 1.
Trypan Blue Staining Results of Various MSCs
| |
Trypan blue |
|
|
|---|---|---|---|
| Positive | Negative | Percentage (%) | |
| Aqp1-MSCs |
0 |
248 |
0.2 |
| |
0 |
232 |
|
| |
2 |
277 |
|
| |
1 |
251 |
|
| DsRed-MSCs |
0 |
186 |
0.3 |
| |
0 |
165 |
|
| |
1 |
172 |
|
| |
1 |
157 |
|
| Aqp1-sh MSCs |
0 |
23 |
0 |
| |
0 |
24 |
|
| |
0 |
13 |
|
| |
0 |
29 |
|
| Scramble MSCs |
0 |
34 |
0 |
| |
0 |
49 |
|
| |
0 |
29 |
|
| 0 | 27 | ||
MSC, mesenchymal stem cell; Aqp1, aquaporin 1.
Aqp1 augments MSC migration in vitro
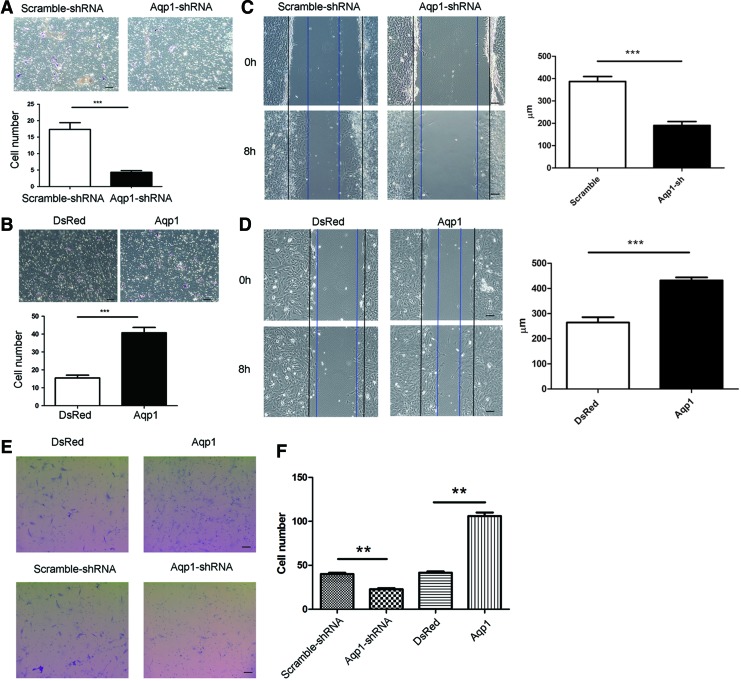
To assess the role of Aqp1 in MSC migration, we examined the effect of Aqp1 depletion on MSC migration in vitro. To exclude the effect of proliferation of MSCs on migration, we detected the proliferation rate of Aqp1-MSCs and Aqp1-sh MSCs and did not found any significant differences (Supplementary Fig. S2). MSC migration was evaluated by transwell assay. Migrated MSCs from a lower layer of membrane were fixed and stained. MSCs from randomly selected fields were calculated, and the number of Aqp1-shRNA MSCs in the lower layers of membrane was significantly less compared with the control group (Fig. 2A). In addition, wound healing assay was also performed by scraping the confluent cell layer. After 8 h, the distances between the wound edges in the Aqp1-shRNA MSCs were significantly wider than those of the control cells (Fig. 2C). Transwell assay indicated that overexpression of Aqp1 increased the number of transmembrane MSCs compared to the control group (Fig. 2B). Consistent with the transwell results, the distances between the wound edges in the Aqp1-MSCs were significantly smaller than those of the control group (Fig. 2D). Furthermore, we examined the migration ability of MSCs from primary culture after overexpression and knockdown of Aqp1 by transwell assay. The number of transmembrane MSCs was quantified, as described above. As expected, a higher number of MSCs migrated across the membrane after overexpressing Aqp1, whereas a lower number of MSCs migrated across the membrane after silencing Aqp1 (Fig. 2E, F). Taken together, the data revealed that Aqp1 enhanced the MSC migration ability in vitro.
FIG. 2.
Migration assays of Aqp1 depleted and overexpressed MSCs. Migrated Aqp1 depleted (A) and overexpressed (B) MSCs from transwell membrane were shown by crystal violet staining (purple) and quantified (n=5), there was significantly more Aqp1 MSCs that migrated across the membrane. Wound healing of Aqp1 depleted (C) and overexpressed (D) MSCs were observed and quantified under contrast microscope. Black and blue lines indicated start and end (8 h) positions of MSCs after scraping. Transwell migration assays were also performed by using the rat MSCs from primary culture after transfection of Aqp1 and Aqp1-shRNA (E). Migrating MSCs from bottom lower of the membrane were stained with crystal violet (purple) and quantified (F). Overexpressing Aqp1 in MSCs has significantly enhanced their cell migration ability in vitro. Scale bar: 100 μm. All error bars represent SEM (**P<0.01, ***P<0.001). Color images available online at www.liebertpub.com/scd
Aqp1 facilitates MSCs migrating to bone fracture site
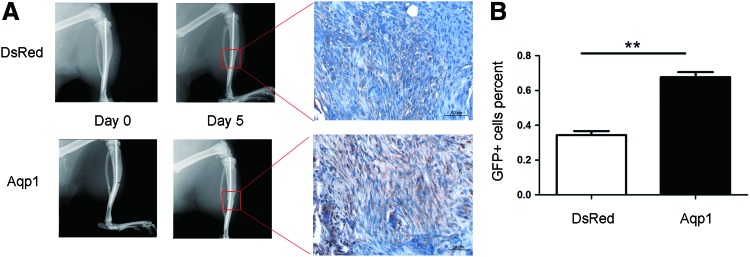
To test the effect of Aqp1 on MSC migration in vivo, we administrated GFP-labeled Aqp1-MSCs into rats with a tibial fracture and observed the fate of the MSCs. The established tibial fracture model was confirmed by an X-ray image (Fig. 3A). MSCs were administered into fractured rats at 2 days following fracture (osteotomy surgery) through their tail veins. After 5 days, all animals were terminated and the fractured tibiae harvested and prepared for paraffin histology. The GFP-positive cells at the fracture site were detected by using immunohistochemistry and quantified by using imaging software. We found that most of MSCs resided in the lungs, and GFP-positive MSCs were not seen in the contralateral tibia (Supplementary Fig. S3). Nevertheless, a large number of MSCs were found at the cartilaginous tissues at the fracture site (Fig. 3A). The percentage of GFP-positive cells at the fracture site was calculated and compared between the Aqp1-MSC and DsRed-MSC groups, and there were significantly higher GFP-positive cells in the Aqp1-MSC-treated group than in the DsRed-MSC-treated group (Fig. 3B).
FIG. 3.
Immunohistochemistry of administrated MSCs at the fracture site. X-ray images depicted tibial fracture after surgery (left, A) and 5 days later (right, A). There was only soft callus in the fracture gap at 5 days after fracture and GFP-positive cells were seen at the boxed areas, showing more GFP cells in the Aqp1-MSC group (A). The percentage of GFP-positive cells was quantified by the ratio of brown cells (GFP positive) to whole cells (blue cells plus brown cells) autoselected by software Image-Pro Plus 6.0 in the randomly selected fields, showing that there was significantly more GFP-MSCs in the Aqp1 MSC group (B, n=5). Scale bar: 50 μm. All error bars represent SEM (**P<0.01). Color images available online at www.liebertpub.com/scd
β-Catenin is involved in Aqp1-mediated migration of MSCs
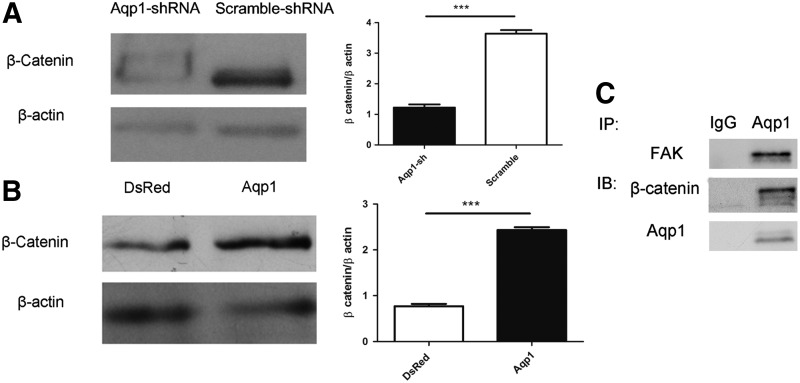
To explore the underlying mechanism of Aqp1 in MSC migration, we examined the expression of β-catenin both in the Aqp1-shRNA MSCs and the Aqp1-MSCs. Depletion of Aqp1 in MSCs led to the degradation of β-catenin (Fig. 4A). To address whether Aqp1 could contribute to the stability of β-catenin, we overexpressed Aqp1 in MSCs and found that the overexpression of Aqp1 could upregulate the expression of β-catenin (Fig. 4B). This regulation was restricted at the protein level, because the overexpression of Aqp1 had no effects on the mRNA level of β-catenin (Supplementary Fig. S4). Furthermore, we demonstrated that Aqp1 was co-immunoprecipitated with β-catenin, which implied that their physical interaction is likely beneficial for β-catenin stability (Fig. 4C and Supplementary Fig. S5A). The migration ability was similar in both Aqp1-MSCs and DsRed-MSCs when depleted β-catenin, indicating that other compensative pathways may exist (Supplementary Fig. S6).
FIG. 4.
Aqp1 affects expression of β-catenin. Western blot showed the expression of β-catenin of in Aqp1 depleted (A) and overexpressing (B) MSCs. The level of β-catenin was quantified by software ImageJ. Cell lysate was immunoprecipitated with the Aqp1 antibody and blotted with β-catenin, focal adhesion kinase (FAK), and Aqp1 antibodies (C). All error bars represent SEM (***P<0.001).
Aqp1 regulates migration of MSCs through FAK not CXCR4
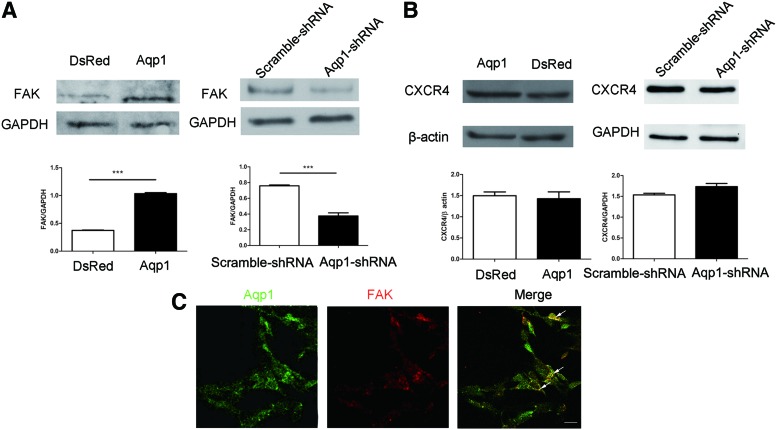
In addition to β-catenin, we also tested other pivotal regulators of cell migration such as FAK and CXCR4. The FAK level instead of CXCR4 increased when Aqp1 was overexpressed, while the phosphorylation of FAK was undetectable (Supplementary Fig. S7). The depletion of Aqp1 led to the degradation of FAK rather than CXCR4 (Fig. 5A, B). This meant that Aqp1 regulated the expression of FAK instead of CXCR4. To further explore the relationship between Aqp1 and FAK, we traced their location in cells by labeling with a fluorescent antibody and we demonstrated that Aqp1 was colocalized with FAK (Fig. 5C), which was further confirmed by co-immunoprecipitation assay (Fig. 4C and Supplementary Fig. S5B).
FIG. 5.
FAK level was regulated by Aqp1. Western blot showed the expression FAK (A) and C-X-C chemokine receptor type 4 (CXCR4) (B) in Aqp1 depleted and overexpressing MSCs and quantified by software ImageJ. The localization of Aqp1 (green) and FAK (red) labeled with fluorescent antibodies were displayed by confocal microscope (C). Colocalization area of Aqp1 and FAK was highlighted (arrow) and analyzed by software Image-Pro Plus 6.0 with Pearson's correlation Rr=0.814934. Scale bar: 100 μm. All error bars represent SEM (***P<0.001). Color images available online at www.liebertpub.com/scd
Depletion of FAK abolished Aqp1 effects on MSC migration
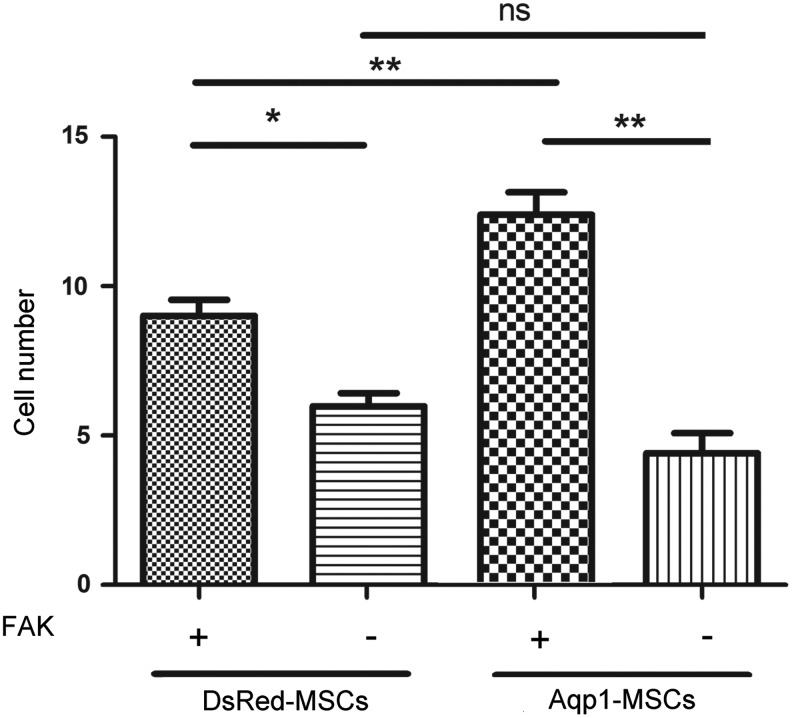
To discover whether FAK was indispensable for Aqp1 mediating MSC migration, we silenced FAK expression in the Aqp1-MSCs by introducing lentiviral FAK-shRNA. The migration of MSCs was examined by the transwell method. The overexpression of Aqp1 enhanced MSC migration, whereas the depletion of FAK dramatically reduced the migration ability of DsRed-MSCs and Aqp1-MSCs, which implies that the depletion of FAK in Aqp1-MSCs counteracted Aqp1 enhancing effects on MSC migration. The migration ability had no significant difference between DsRed-MSCs and Aqp1-MSCs after silencing the expression of FAK, which further demonstrated that FAK was downstream of Aqp1 (Fig. 6).
FIG. 6.
Transwell analysis of Aqp1-MSCs after depletion of FAK. Migrating MSCs were assessed by transwell and quantified, the data showed that the deletion of FAK expression in MSCs diminished cell migration ability. FAK gene expression was silenced in Aqp1-MSCs by lentiviral transfection. All error bars represent SEM (n=5, *P<0.05, **P<0.01, ns, none significance).
Discussion
This study reports that Aqp1 promotes MSC migration by upregulating the expression of β-catenin and FAK. After administrating it to rats, the Aqp1-MSCs migrated much more efficiently into the fracture sites. Furthermore, our study also reveals that Aqp1 interacts with FAK and β-catenin directly.
As one of the water channel molecules, Aqp1 is involved in endothelial cell migration and cancer invasion [29]. In our study, we reported that Apq1 contributed to MSC migration. The overexpression of Aqp1 accelerated MSC movement, whereas silencing Aqp1 reduced its migration ability. Moreover, we designed to test the migration capacity in vivo by administrating the Apq1-MSCs into tibia fracture rats. Previous studies have shown that MSCs were recruited systemically in bone fracture healing [30] and that both systemic and local MSC administration enhanced fracture healing [31,32]. Based on these studies, we evaluated the migration ability of MSCs by comparing the quantity of exogenous MSCs at the fracture site. The results showed significantly more MSCs at the fracture site in the Aqp1-MSC-treated group, thus suggesting that Aqp1-MSCs have enhanced tropism toward wounded tissue areas in vivo.
Utilizing MSC transplantation to enhance fracture healing has been reported previously, and the MSC retention ability at the fracture site is essential for enhancing the healing process [33]. In this study, we chose only one time point, which was 5 days after fracture (3 days after MSC administration) to examine the cell migration ability differences between the Aqp1 MSCs and the control MSCs, and we confirmed that more MSCs migrated to the fracture gap in the Aqp1 MSC group. One can assume that the fracture healing may be superior in the Aqp1 MSC group as more MSCs were reached in this group. Because the primary aim of the current study was to test the migration ability of Aqp1 MSCs in vivo, we did not purposely compare the fracture healing outcome, and this will be a subject for our future studies. Multiple factors have been identified as regulating MSC migration, such as CCR1, mi335, HIF-1α, and SDF/CXCR4 [34–37]. Among these factors, SDF/CXCR4 was an indispensable universal pathway to regulate the migration of many cell types, as has been studied and reported previously in MSCs [38–40]. In this study, we demonstrated that Aqp1 could regulate MSC migration independently of the SDF-1/CXCR4 pathway, indicating that Aqp1 may be a new and previously unknown factor controlling the MSC migration capacity.
The role of Aqp1 on cell migration is poorly understood. One study led by Verkman proposed that aquaporins might provide the major pathway for water entry into lamellipodia driven by the local osmotic gradient [22]. The alternative mechanism may be that Aqp1 promotes cell migration through lin7 and β-catenin, which decreases when there is depletion of Aqp1 in endothelial cells. The treatment of Aqp1 silenced cells with the proteasome inhibitor MG132 enhanced the recovery of β-catenin, which implies that a lack of Aqp1 targets the Lin-7/β-catenin complex to proteolytic degradation [23]. In this study, we found that β-catenin (at protein levels) was downregulated after silencing Aqp1 and upregulated after overexpressing Aqp1 in MSCs, thus implying that Aqp1 partially regulates the post-translational level of β-catenin, because overexpression of Aqp1 did not affect the β-catenin mRNA level. However, the precise relationship between Aqp1 and β-catenin still needs further study.
We found that Aqp1 also regulated the expression of FAK that was not known before. FAK binds directly to the intracellular domain of the β1-integrin subunit [41]. The mechanism of FAK-dependent cell migration is that FAK regulates the disassembly of focal adhesions as well as versatile regulators such as Rac, Rho, and N-WASP [42,43]. We showed that FAK physically interacted and colocalized with Aqp1, indicating there may be functional crosstalk of FAK and Aqp1 in the cell migration process. Our data show that the overexpression of Aqp1 increased the expression of FAK, whereas silencing Aqp1 decreased the expression of FAK. The regulation was only seen at the translational level because the overexpression of Aqp1 did not alter the mRNA level of FAK, suggesting that Aqp1 may enhance the stability of FAK. Anchoring at the focal adhesion site, FAK communicates with many other signal events such as Ras, Src, and the extracellular signal-regulated kinase-2/mitogen-activated protein kinase cascade [44]; however, how Aqp1 interacts and activates FAK to elicit downstream signal events still remains largely unknown and warrants future studies.
Conclusions
To summarize, we demonstrated that Aqp1 has a role in promoting MSC migration. The systemic administration of Aqp1-MSCs into a rat fracture model showed that significantly more Aqp1-MSCs migrated to the fracture site, suggesting the potential therapeutic use of genetically modified Aqp1-MSCs in cell therapy. We showed that Aqp1 regulates the expression of β-catenin, probably by maintaining the post-translational level of β-catenin. We demonstrated that Aqp1 exerts its migration enhancer function through modulating the expression of FAK. In conclusion, our studies discovered a novel function of Aqp1 in enhancing MSC migration ability without affecting other MSC functions. The alteration of Aqp1 expression may have implications for enhancing MSC migration in MSC clinical applications.
Supplementary Material
Acknowledgments
We are thankful for the financial support from the Hong Kong Government Research Grant Council, General Research Fund (grant no: CUHK471110) to G.L. for this research work. We also acknowledge the support from the National Basic Science and Development Programme of the People's Republic of China (973 programme, 2012CB518105).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Friedenstein AJ, Gorskaja JF. and Kulagina NN. (1976). Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol 4:267–274 [PubMed] [Google Scholar]
- 2.Yoshioka T, Mishima H, Ohyabu Y, Sakai S, Akaogi H, Ishii T, Kojima H, Tanaka J, Ochiai N. and Uemura T. (2007). Repair of large osteochondral defects with allogeneic cartilaginous aggregates formed from bone marrow-derived cells using RWV bioreactor. J Orthop Res 25:1291–1298 [DOI] [PubMed] [Google Scholar]
- 3.Ranganath Sudhir H, Levy O, Inamdar Maneesha S. and Karp Jeffrey M. (2012). Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell 10:244–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, Mu H, Pachori A. and Dzau V. (2007). Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci USA 104:1643–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ezquer FE, Ezquer ME, Parrau DB, Carpio D, Yañez AJ. and Conget PA. (2008). Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transplant 14:631–640 [DOI] [PubMed] [Google Scholar]
- 6.Phinney DG. and Prockop DJ. (2007). Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells 25:2896–2902 [DOI] [PubMed] [Google Scholar]
- 7.Song C. and Li G. (2011). CXCR4 and matrix metalloproteinase-2 are involved in mesenchymal stromal cell homing and engraftment to tumors. Cytotherapy 13:549–561 [DOI] [PubMed] [Google Scholar]
- 8.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT. and Horwitz AR. (2003). Cell migration: integrating signals from front to back. Science 302:1704–1709 [DOI] [PubMed] [Google Scholar]
- 9.Provenzano PP. and Keely PJ. (2009). The role of focal adhesion kinase in tumor initiation and progression. Cell Adh Migr 3:347–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lietha D, Cai X, Ceccarelli DFJ, Li Y, Schaller MD. and Eck MJ. (2007). Structural basis for the autoinhibition of focal adhesion kinase. Cell 129:1177–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schaller MD. (2010). Cellular functions of FAK kinases: insight into molecular mechanisms and novel functions. J Cell Sci 123:1007–1013 [DOI] [PubMed] [Google Scholar]
- 12.Song H, Cha M-J, Song B-W, Kim I-K, Chang W, Lim S, Choi EJ, Ham O, Lee S-Y, et al. (2010). Reactive oxygen species inhibit adhesion of mesenchymal stem cells implanted into ischemic myocardium via interference of focal adhesion complex. Stem Cells 28:555–563 [DOI] [PubMed] [Google Scholar]
- 13.Gao H, Priebe W, Glod J. and Banerjee D. (2009). Activation of signal transducers and activators of transcription 3 and focal adhesion kinase by stromal cell-derived factor 1 is required for migration of human mesenchymal stem cells in response to tumor cell-conditioned medium. Stem Cells 27:857–865 [DOI] [PubMed] [Google Scholar]
- 14.Zhang D, Fan G-C, Zhou X, Zhao T, Pasha Z, Xu M, Zhu Y, Ashraf M. and Wang Y. (2008). Over-expression of CXCR4 on mesenchymal stem cells augments myoangiogenesis in the infarcted myocardium. J Mol Cell Cardiol 44:281–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Logan CY. and Nusse R. (2004). The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20:781–810 [DOI] [PubMed] [Google Scholar]
- 16.Davidson KC, Adams AM, Goodson JM, McDonald CE, Potter JC, Berndt JD, Biechele TL, Taylor RJ. and Moon RT. (2012). Wnt/β-catenin signaling promotes differentiation, not self-renewal, of human embryonic stem cells and is repressed by Oct4. Proc Natl Acad Sci USA 109:4485–4490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polette M, Mestdagt M, Bindels S, Nawrocki-Raby B, Hunziker W, Foidart JM, Birembaut P. and Gilles C. (2007). β-catenin and ZO-1: shuttle molecules involved in tumor invasion-associated epithelial-mesenchymal transition processes. Cells Tissues Organs 185:61–65 [DOI] [PubMed] [Google Scholar]
- 18.Brabletz T, Jung A, Dag S, Hlubek F. and Kirchner T. (1999). β-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am J Pathol 155:1033–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hatsell S, Rowlands T, Hiremath M. and Cowin P. (2003). β-catenin and Tcfs in mammary development and cancer. J Mammary Gland Biol Neoplasia 8:145–158 [DOI] [PubMed] [Google Scholar]
- 20.Gilles C, Polette M, Mestdagt M, Nawrocki-Raby B, Ruggeri P, Birembaut P. and Foidart J-M. (2003). Transactivation of vimentin by β-catenin in human breast cancer cells. Cancer Res 63:2658–2664 [PubMed] [Google Scholar]
- 21.Borgnia M, Nielsen S, Engel A. and Agre P. (1999). Cellular and molecular biology of the aquaporin water channels. Annu Rev Biochem 68:425–458 [DOI] [PubMed] [Google Scholar]
- 22.Saadoun S, Papadopoulos MC, Hara-Chikuma M. and Verkman AS. (2005). Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature 434:786–792 [DOI] [PubMed] [Google Scholar]
- 23.Monzani E, Bazzotti R, Perego C. and La Porta CAM. (2009). AQP1 is not only a water channel: it contributes to cell migration through lin7/beta-catenin. PLoS One 4:e6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X, McClurg A, Zhou G-Q, McCaigue M, Armstrong MA. and Li G. (2007). Chondrogenic differentiation alters the immunosuppressive property of bone marrow-derived mesenchymal stem cells, and the effect is partially due to the upregulated expression of B7 molecules. Stem Cells 25:364–370 [DOI] [PubMed] [Google Scholar]
- 25.Splinter PL, Masyuk AI. and LaRusso NF. (2003). Specific inhibition of AQP1 water channels in isolated rat intrahepatic bile duct units by small interfering RNAs. J Biol Chem 278:6268–6274 [DOI] [PubMed] [Google Scholar]
- 26.Ozturan KE, Demir B, Yucel I, Cakıcı H, Yilmaz F. and Haberal A. (2011). Effect of strontium ranelate on fracture healing in the osteoporotic rats. J Orthop Res 29:138–142 [DOI] [PubMed] [Google Scholar]
- 27.Singer J, Charbord P, Keating A, Nemunaitis J, Raugi G, Wight T, Lopez J, Roth G, Dow L. and Fialkow P. (1987). Simian virus 40-transformed adherent cells from human long-term marrow cultures: cloned cell lines produce cells with stromal and hematopoietic characteristics. Blood 70:464–474 [PubMed] [Google Scholar]
- 28.Hamada H, Kobune M, Nakamura K, Kawano Y, Kato K, Honmou O, Houkin K, Matsunaga T. and Niitsu Y. (2005). Mesenchymal stem cells (MSC) as therapeutic cytoreagents for gene therapy. Cancer Sci 96:149–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verkman AS, Hara-Chikuma M. and Papadopoulos MC. (2008). Aquaporins—new players in cancer biology. J Mol Med (Berl) 86:523–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shirley D, Marsh D, Jordan G, McQuaid S. and Li G. (2005). Systemic recruitment of osteoblastic cells in fracture healing. J Orthop Res 23:1013–1021 [DOI] [PubMed] [Google Scholar]
- 31.Granero-Moltó F, Weis JA, Miga MI, Landis B, Myers TJ, O'Rear L, Longobardi L, Jansen ED, Mortlock DP. and Spagnoli A. (2009). Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells 27:1887–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quarto R, Mastrogiacomo M, Cancedda R, Kutepov SM, Mukhachev V, Lavroukov A, Kon E. and Marcacci M. (2001). Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med 344:385–386 [DOI] [PubMed] [Google Scholar]
- 33.Kumar S. and Ponnazhagan S. (2012). Mobilization of bone marrow mesenchymal stem cells in vivo augments bone healing in a mouse model of segmental bone defect. Bone 50:1012–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang J, Zhang Z, Guo J, Ni A, Deb A, Zhang L, Mirotsou M, Pratt RE. and Dzau VJ. (2010). Genetic modification of mesenchymal stem cells overexpressing CCR1 increases cell viability, migration, engraftment, and capillary density in the injured myocardium. Circ Res 106:1753–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tome M, Lopez-Romero P, Albo C, Sepulveda JC, Fernandez-Gutierrez B, Dopazo A, Bernad A. and Gonzalez MA. (2011). miR-335 orchestrates cell proliferation, migration and differentiation in human mesenchymal stem cells. Cell Death Differ 18:985–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raheja LF, Genetos DC, Wong A. and Yellowley CE. (2011). Hypoxic regulation of mesenchymal stem cell migration: the role of RhoA and HIF-1alpha. Cell Biol Int 35:981–989 [DOI] [PubMed] [Google Scholar]
- 37.Liu H, Liu S, Li Y, Wang X, Xue W, Ge G. and Luo X. (2012). The role of SDF-1-CXCR4/CXCR7 axis in the therapeutic effects of hypoxia-preconditioned mesenchymal stem cells for renal ischemia/reperfusion injury. PLoS One 7:e34608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghosh MC, Makena PS, Gorantla V, Sinclair SE. and Waters CM. (2012). CXCR4 regulates migration of lung alveolar epithelial cells through activation of Rac1 and matrix metalloproteinase-2. Am J Physiol Lung Cell Mol Physiol 302:L846–L856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wendel C, Hemping-Bovenkerk A, Krasnyanska J, Mees ST, Kochetkova M, Stoeppeler S. and Haier J. (2012). CXCR4/CXCL12 participate in extravasation of metastasizing breast cancer cells within the liver in a rat model. PLoS One 7:e30046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu X, Chen D, Zhang Y, Wu X, Huang Z, Zhou H. and Zhang Z. (2012). Overexpression of CXCR4 in mesenchymal stem cells promotes migration, neuroprotection and angiogenesis in a rat model of stroke. J Neurol Sci 316:141–149 [DOI] [PubMed] [Google Scholar]
- 41.Lechertier T. and Hodivala-Dilke K. (2012). Focal adhesion kinase and tumour angiogenesis. J Pathol 226:404–412 [DOI] [PubMed] [Google Scholar]
- 42.Wu X, Suetsugu S, Cooper LA, Takenawa T. and Guan J-L. (2004). Focal adhesion kinase regulation of N-WASP subcellular localization and function. J Biol Chem 279:9565–9576 [DOI] [PubMed] [Google Scholar]
- 43.Yang S. and Kim H-M. (2012). The RhoA-ROCK-PTEN pathway as a molecular switch for anchorage dependent cell behavior. Biomaterials 33:2902–2915 [DOI] [PubMed] [Google Scholar]
- 44.Mitra SK, Hanson DA. and Schlaepfer DD. (2005). Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol 6:56–68 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.