Significance
The search for a viable male contraceptive target has been a medical challenge for many years. Most strategies have focused on hormonal or germ-line strategies to produce dysfunctional sperm that are incapable of fertilization. The problem with such approaches is that they have intolerable side effects such as affecting male sexual activity or causing long-term irreversible effects on fertility. In addition, some strategies may transmit detrimental changes to future offspring. This manuscript describes a male contraceptive target within the autonomic nervous system, which would not affect the long-term viability of sperm nor the sexual or general health of males. In addition, due to the nature of the target, the contraceptive has the potential to be orally administered.
Keywords: sympathetic neurotransmission, noradrenaline, ATP
Abstract
Therapeutic targets for male contraception are associated with numerous problems due to their focus on disrupting spermatogenesis or hormonal mechanisms to produce dysfunctional sperm. Here we describe the dual genetic deletion of α1A-adrenergic G protein-coupled receptors (adrenoceptors) and P2X1-purinoceptor ligand gated ion channels in male mice, thereby blocking sympathetically mediated sperm transport through the vas deferens during the emission phase of ejaculation. This modification produced 100% infertility without effects on sexual behavior or function. Sperm taken from the cauda epididymides of double knockout mice were microscopically normal and motile. Furthermore, double knockout sperm were capable of producing normal offspring following intracytoplasmic sperm injection into wild-type ova and implantation of the fertilized eggs into foster mothers. Blood pressure and baroreflex function was reduced in double knockout mice, but no more than single knockout of α1A-adrenoceptors alone. These results suggest that this autonomic method of male contraception appears free of major physiological and behavioral side effects. In addition, they provide conclusive proof of concept that pharmacological antagonism of the P2X1-purinoceptor and α1A-adrenoceptor provides a safe and effective therapeutic target for a nonhormonal, readily reversible male contraceptive.
Development of a male contraceptive is a major medical challenge with numerous barriers in its path. Historically, most therapeutic targets have been hormonal and therefore likely to have intolerable sexual, behavioral, physiological, and psychological side effects. More recently, efforts have concentrated on spermatogenic mechanisms that can render sperm dysfunctional and therefore incapable of fertilization (1, 2). These reports identified a number of problems associated with such strategies, including (i) the enormous number of spermatozoa produced by men (∼1,000 per s) (1); (ii) the difficulty in suppressing all of these many sperm compared with only one ovum per female (1); (iii) concern that affecting production of cells in the germ line may alter genetics in offspring (1); (iv) finding a molecule that is able to cross the blood–testis barrier (1); and (v) being able to readily reverse this process (2). These challenges are in addition to the usual issues faced in the development of pharmaceuticals such as large financial expenditure, regulatory hurdles, preference for oral availability, and unexpected adverse events.
An alternate mechanism is to block sperm transport. During ejaculation, sperm is transported from its storage site in the cauda epididymis to the urethra via the vas deferens (3, 4). This process of propulsion of sperm into the ejaculate is essential for males to produce an adequate sperm count for fertilization. Sympathetically innervated smooth muscle cells surround the vas deferens and contract in response to ATP and noradrenaline due to activation of P2X1-purinoceptor ligand-gated ion channels and α1A-adrenergic G protein-coupled receptors (adrenoceptors), respectively (5–7). Consequently, conjoint pharmacological antagonism of these receptors has been shown to inhibit nerve-mediated contraction of the vas deferens in isolated tissue experiments. This suggests a potential mechanism for male contraception, but critical studies in the context of whole-organism physiology are yet to be performed.
Administration of the selective α1A-adrenoceptor antagonist tamsulosin (8) to male rats decreases the number of sperm in the ejaculate as well as reducing the number of embryos per pregnancy; however, full infertility has not been achieved. Furthermore, whereas genetic deletion of α1A-adrenoceptors (9) or P2X1-purinoceptors (10) impairs fertility in mice, the observed percentage of infertile male mice was only 50% for α1A-adrenoceptors (9) and 86% for P2X1-purinoceptors (10). Such impairment of fertility suggests that these receptor targets are indeed important for reproduction. However, male infertility would have to be 100% in preclinical studies for a novel therapeutic target for male contraception to be considered viable in humans.
It has been proposed that simultaneous blockade of both of these receptors may produce adequate inhibition of sperm transport for male contraception (11); however, this strategy is also likely to fail if compensatory mechanisms arise to counteract α-adrenoceptor blockade, as has been observed following genetic deletion of P2X1-purinoceptors in male mice (10). Furthermore, it is unknown whether dual pharmacological blockade of receptors can be exploited as a safe and effective male contraceptive, because the distribution of P2X1-purinoceptors and α1A-adrenoceptors is widespread and they act in concert to control the diameter of resistance arteries and thus blood pressure homeostasis (12, 13). This study demonstrates that dual knockout of α1A-adrenoceptors and P2X1-purinoceptors renders male mice infertile without compromising physiology and behavior.
Results
Generation of Double Knockout α1A (−/−)/P2X1 (−/−) Mice.
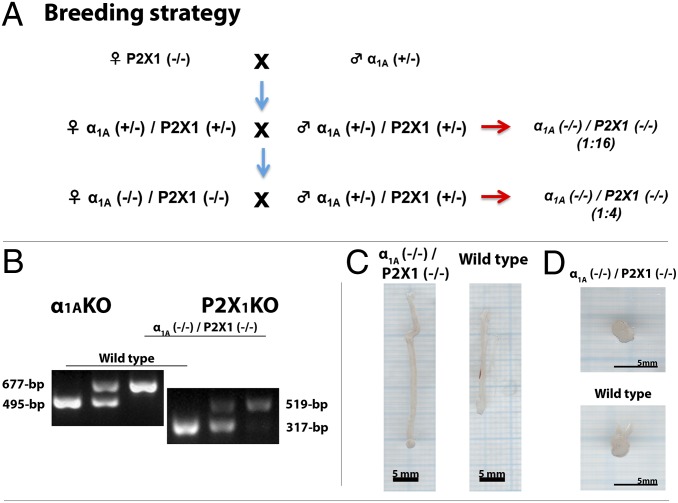
First-generation mice were produced by crossing female α1A-adrenoceptor knockout (−/−) mice with male P2X1-purinoceptor heterozygous (+/−) mice. Double knockout mice were then generated at a Mendelian frequency of 1:16 in the second generation by breeding female α1A (+/−)/P2X1 (+/−) with male α1A (+/−)/P2X1 (+/−) mice (Fig. 1A). Thereafter, because double knockout female α1A (−/−)/P2X1 (−/−) mice were fertile, they were crossed with double heterozygous male α1A (+/−)/P2X1 (+/−) mice (Fig. 1A), producing an average litter size of 6.6. The resulting double knockout α1A (−/−)/P2X1 (−/−) mice occurred at an expected Mendelian genotype frequency of 1:4 (sex ratio 1.11 male to 1 female; n = 240), indicating that there is no selective fertilization or mortality in utero and that female fertility is unaffected by this simultaneous gene deletion.
Fig. 1.
Breeding and gross morphological effects of α1A (−/−)/P2X1 (−/−) double knockout in male mice. (A) Breeding strategy. (B) Genotyping by PCR. (C) Photos of vas deferens from wild-type and double knockout α1A (−/−)/P2X1 (−/−) mice. (D) Photos of whole prostates from wild-type and double knockout α1A (−/−)/P2X1 (−/−) mice.
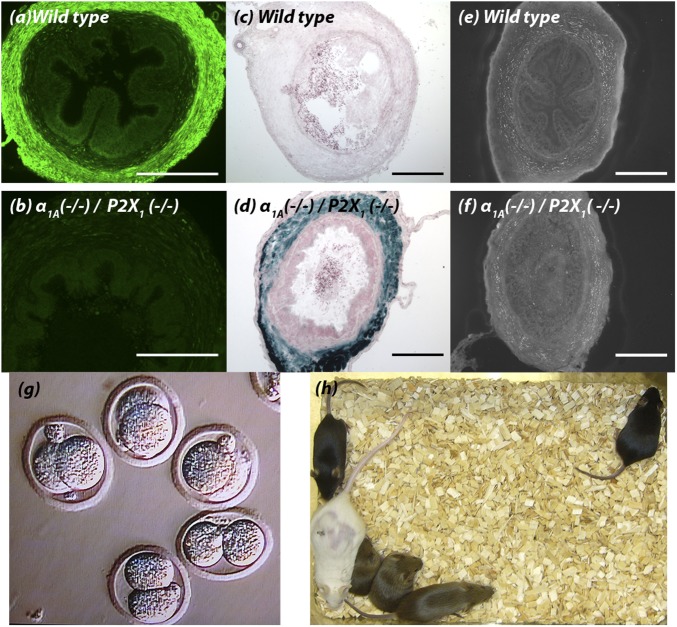
Confirmation of the deficiency of both the α1A-adrenoceptor and P2X1-purinoceptor in double knockout mutant α1A (−/−)/P2X1 (−/−) mice was obtained at the genomic and protein levels. PCR genotyping as previously described for α1A-adrenoceptor knockout α1A (−/−) (13) and P2X1-purinoceptor knockout P2X1 (−/−) (10) mice indicated that mRNA for either gene is not amplified in double knockout α1A (−/−)/P2X1 (−/−) mice (Fig. 1B). A commercially available P2X1-purinoceptor antibody (Alomone Labs) produced high levels of immunoreactivity in the smooth muscle layer of wild-type vas deferens (Fig. 2A), as previously reported (10). However, as observed in P2X1-purinoceptor single knockout P2X1 (−/−) mice, no P2X1-purinoceptor immunoreactivity was seen in vasa deferentia taken from double knockout α1A (−/−)/P2X1 (−/−) mice (Fig. 2B). Similarly, β-galactosidase staining of the Lac Z reporter gene that had been inserted at the α1A/C gene translational start site of the α1A-adrenoceptor gene in these knockout α1A (−/−) mice (13) showed positive staining in vasa deferentia from double knockout α1A (−/−)/P2X1 (−/−) mice (Fig. 2D), which was absent in vasa deferentia taken from wild-type mice (Fig. 2C). This was despite the continued presence of noradrenaline-containing nerve fibers within the smooth muscle of the vas deferens of double knockout α1A (−/−)/P2X1 (−/−) mice (Fig. 2 E and F), as demonstrated by sucrose potassium glyoxylic acid (SPG) histochemistry (14).
Fig. 2.
Vas deferens histochemistry and sperm viability. P2X1-purinoceptor immunohistochemistry of cross-sections of vas deferens from (A) wild-type male mice (n = 3) and (B) double knockout α1A (−/−)/P2X1 (−/−) male mice (n = 3). X-gal staining for β-galactosidase in cross-sections of vas deferens from (C) wild-type male mice (n = 3) and (D) double knockout α1A (−/−)/P2X1 (−/−) male mice (n = 3). SPG histochemical fluorescence of cross-sections of vas deferens from (E) wild-type male mice (n = 3) and (F) double knockout α1A (−/−)/P2X1 (−/−) male mice (n = 3). (Scale bars, 100 μm.) (G) Fertilization of wild-type ova in vitro by intracytoplasmic injection of sperm extracted from cauda epididymides of male double knockout α1A (−/−)/P2X1 (−/−) mice; the photomicrograph was taken at 3 d postinjection. (H) Weanlings (black/agouti) 7 wk after implantation of fertilized ova into a foster mother (white).
Sexual Behavior.
In contrast to the lack of effect of this simultaneous gene deletion on female fertility, when double knockout α1A (−/−)/P2X1 (−/−) males were mated with wild-type α1A (+/+)/P2X1 (+/+) females, no pregnancies resulted [n = 29 matings of 17 double knockout α1A (−/−)/P2X1 (−/−) male mice]. This was despite coitus having taken place to the point of ejaculation during 28/29 matings (Movie S1). In addition, when paired with wild-type α1A (+/+)/P2X1 (+/+) female mice, all double knockout α1A (−/−)/P2X1 (−/−) male mice exhibited normal sexual behavior for this background strain of mouse in terms of precoital chasing, sniffing, mounting of mates, pelvic thrusting with appropriate vigor, and frequency, followed by a period of postejaculation latency and loss of interest in the female mate (Movie S1).
Fertility Evaluation.
Vaginal coagulum plugs that are normally formed in rodents after copulation were not observed in any wild-type females where copulation to the point of ejaculation had been confirmed via video surveillance following matings with male double knockout α1A (−/−)/P2X1 (−/−) mice (n = 29). The male infertility in double knockout α1A (−/−)/P2X1 (−/−) male mice did not result from problems with spermatogenesis or sperm quality, because sperm were recovered from the cauda epididymides of wild-type and double knockout mice in similar numbers and with similar motility (Movies S2 and S3). Furthermore, sperm extracted from the cauda epididymides of double knockout α1A (−/−)/P2X1 (−/−) mice were able to fertilize wild-type ova in vitro following intracytoplasmic sperm injection [Fig. 2G; success rate at 3 d, 78.0 ± 6.5%; n = 140 embryo injections of sperm from three double knockout α1A (−/−)/P2X1 (−/−) mice]. In addition, implantation of fertilized ova into wild-type α1A (+/+)/P2X1 (+/+) foster mothers produced normal litters that survived until weaning (28 d after birth) (Fig. 2H; n = 3).
Vas Deferens Pharmacology.
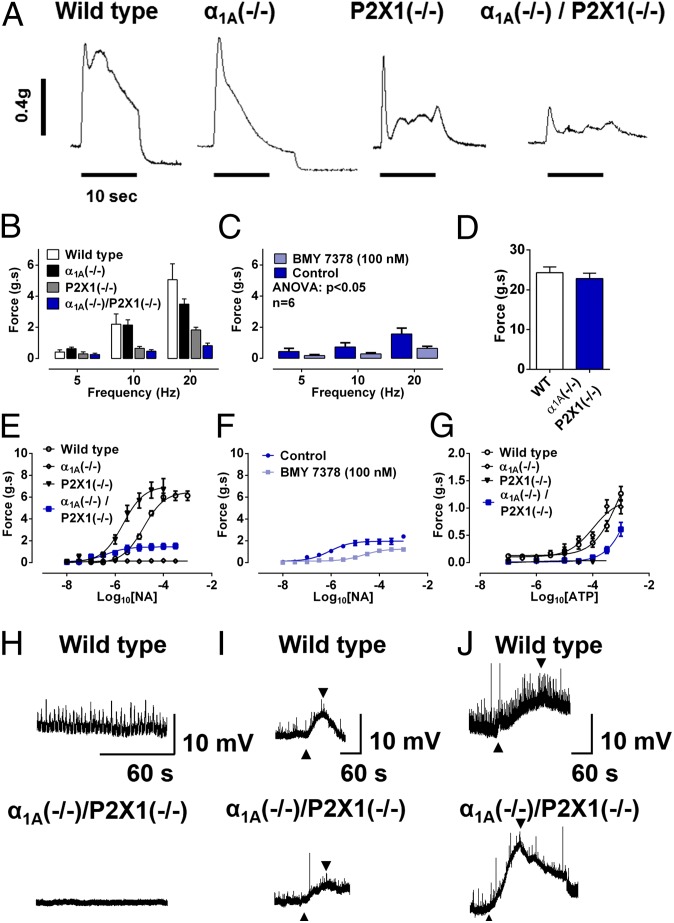
As has been observed previously (9, 10), both α1A (−/−) and P2X1 (−/−) single knockout mice have compromised vas deferens contractility following electrical nerve stimulation (Fig. 3 A and B), and these previously observed effects were additive in double knockout α1A (−/−)/P2X1 (−/−) mice (Fig. 3 A and B). Interestingly, a residual contractile response to electrical field stimulation remained in double knockout α1A (−/−)/P2X1 (−/−) mice that was abolished by the noradrenergic neuron-blocking drug guanethidine (10 μM) and inhibited by the selective α1D-adrenoceptor antagonist BMY 7378 (100 nM) (Fig. 3C).
Fig. 3.
Vas deferens contractility and electrical activity. (A) Typical recording of contractile responses to electrical field stimulation of isolated vasa deferentia taken from wild-type, α1A (−/−) single knockout, P2X1 (−/−) single knockout, and double knockout α1A (−/−)/P2X1 (−/−) mice. (B) Mean frequency–response curve to electrical field stimulation of isolated vasa deferentia taken from wild-type (n = 7), α1A (−/−) single knockout (n = 10), P2X1 (−/−) single knockout (n = 9), and double knockout α1A (−/−)/P2X1 (−/−) (n = 21) mice. (C) Mean frequency–response curve to electrical field stimulation of isolated vasa deferentia taken from double knockout α1A (−/−)/P2X1 (−/−) mice in the absence and presence of BMY 7378 (n = 7). (D) Mean contractile response to 80 mM KCl of isolated vasa deferentia taken from wild-type (n = 10; open columns) and double knockout α1A (−/−)/P2X1 (−/−) (n = 21; filled columns) mice. (E) Mean concentration–response curve to exogenous administration of noradrenaline (NA) in isolated vasa deferentia taken from wild-type (n = 6), α1A (−/−) single knockout (n = 10), P2X1 (−/−) single knockout (n = 10), and double knockout α1A (−/−)/P2X1 (−/−) (n = 5) mice. (F) Mean concentration–response curve to exogenous administration of noradrenaline in isolated vasa deferentia taken from double knockout α1A (−/−)/P2X1 (−/−) mice in the absence and presence of BMY 7378 (n = 6). (G) Mean concentration–response curve to exogenous administration of ATP in isolated vasa deferentia taken from wild-type (n = 10), α1A (−/−) single knockout (n = 7), P2X1 (−/−) single knockout (n = 4), and double knockout α1A (−/−)/P2X1 (−/−) (n = 5) mice. (B–G) Y-axes: Force (g.s) = integral of force (g) × time (s). Error bars represent SEM. (H) Representative intracellular spontaneous electrical activity recordings of vas deferens smooth muscle from wild-type and double knockout α1A (−/−)/P2X1 (−/−) mice. (I) Representative intracellular electrical responses to exogenous administration of ATP (upward arrow, 1 mM) of vas deferens smooth muscle from wild-type and double knockout α1A (−/−)/P2X1 (−/−) mice. (J) Representative intracellular electrical responses to exogenous administration of K+ (upward arrow, 20 mM) of vas deferens smooth muscle from wild-type and double knockout α1A (−/−)/P2X1 (−/−) mice. (I, J) Downward arrows signify washout.
In isolated vasa deferentia taken from wild-type α1A (+/+)/P2X1 (+/+) mice, the purinoceptor agonist ATP and the adrenoceptor agonist noradrenaline evoked concentration-dependent contractions, as previously described (9, 10) (Fig. 3 E and G). Noradrenaline also elicited concentration-dependent contractile responses in vasa deferentia taken from P2X1 (−/−) knockout and to a much lesser extent in double knockout α1A (−/−)/P2X1 (−/−) mice but not α1A (−/−) single knockout mice (Fig. 3E). As was observed previously in single P2X1 (−/−) knockout mice (10), noradrenaline was more potent in vasa deferentia taken from P2X1 (−/−) knockout (−log effective concentration required to produce 50% of maximal response: pEC50, 6.11 ± 0.14) than wild-type mice (pEC50, 4.78 ± 0.06); however, the maximum responses produced were not different (Fig. 3E). In contrast, despite no change in the maximum contractile response mediated by KCl (80 mM; Fig. 3D), the maximum contractile response mediated by noradrenaline in double knockout α1A (−/−)/P2X1 (−/−) mice was only 24% of that observed in wild-type mice (Fig. 3E) and was inhibited by the selective α1D-adrenoceptor antagonist BMY 7378 (100 nM; Fig. 3F; P < 0.001), as was the residual response to electrical field stimulation in double knockout α1A (−/−)/P2X1 (−/−) mice (Fig. 3C). Furthermore, expression of mRNA that encodes for the α1D-adrenoceptor is up-regulated in vasa deferentia from double knockout α1A (−/−)/P2X1 (−/−) mice Fig. S1).
Similarly, ATP elicited concentration-dependent contractile responses in vasa deferentia taken from α1A (−/−) knockout mice (pEC50, 3.90 ± 0.14) more potently than in wild-type mice (pEC50, 2.50 ± 0.48) (Fig. 3G), whereas ATP did not produce contractile responses in vasa deferentia taken from P2X1 (−/−) knockout mice (Fig. 3G). However, a small contractile response was observed to high concentrations of ATP in vasa deferentia taken from double knockout α1A (−/−)/P2X1 (−/−) mice (Fig. 3G).
Vas Deferens Electrophysiology.
Using intracellular electrophysiological techniques (15), vas deferens smooth muscle cells were characterized by resting membrane potential that was −65.2 ± 1.7 mV for tissue taken from wild-type (n = 25 impalements from 10 vasa deferentia) and −64.9 ± 1.7 for tissue taken from double knockout α1A (−/−)/P2X1 (−/−) (n = 19 impalements from 8 vasa deferentia) mice, respectively. Spontaneous excitatory junction potentials (EJPs) (amplitude, 5.3 ± 0.5 mV; frequency, 14.3 ± 4.9 events per min) were recorded from greater than 80% of impalements of the wild-type vas deferens (n = 20 impalements from vasa deferentia from five mice) (Fig. 3H). In addition, exogenously applied ATP (1 mM) evoked electrical depolarizations in vasa deferentia taken from wild-type mice (Fig. 3I) with an amplitude 39 ± 7% (n = 7) of the depolarization evoked by 20 mM K+ (Fig. 3J) and a rise time of 9 mV/s (Fig. 3I). In contrast, vasa deferentia from double knockout α1A (−/−)/P2X1 (−/−) mice evoked smaller electrical depolarizations to ATP (1 mM) (Fig. 3I) that were only 14 ± 3% (P < 0.05 compared with wild type; n = 4) of that evoked by 20 mM K+ (Fig. 3J), with a slower rise time of only 1 mV/s (Fig. 3I). In contrast to what has previously been described for P2X1 (−/−) single knockout mice (10), spontaneous EJPs of similar frequency and amplitude to wild type were still detected in 3 of 19 impalements of vasa deferentia from four double knockout α1A (−/−)/P2X1 (−/−) mice.
Cardiovascular Function.
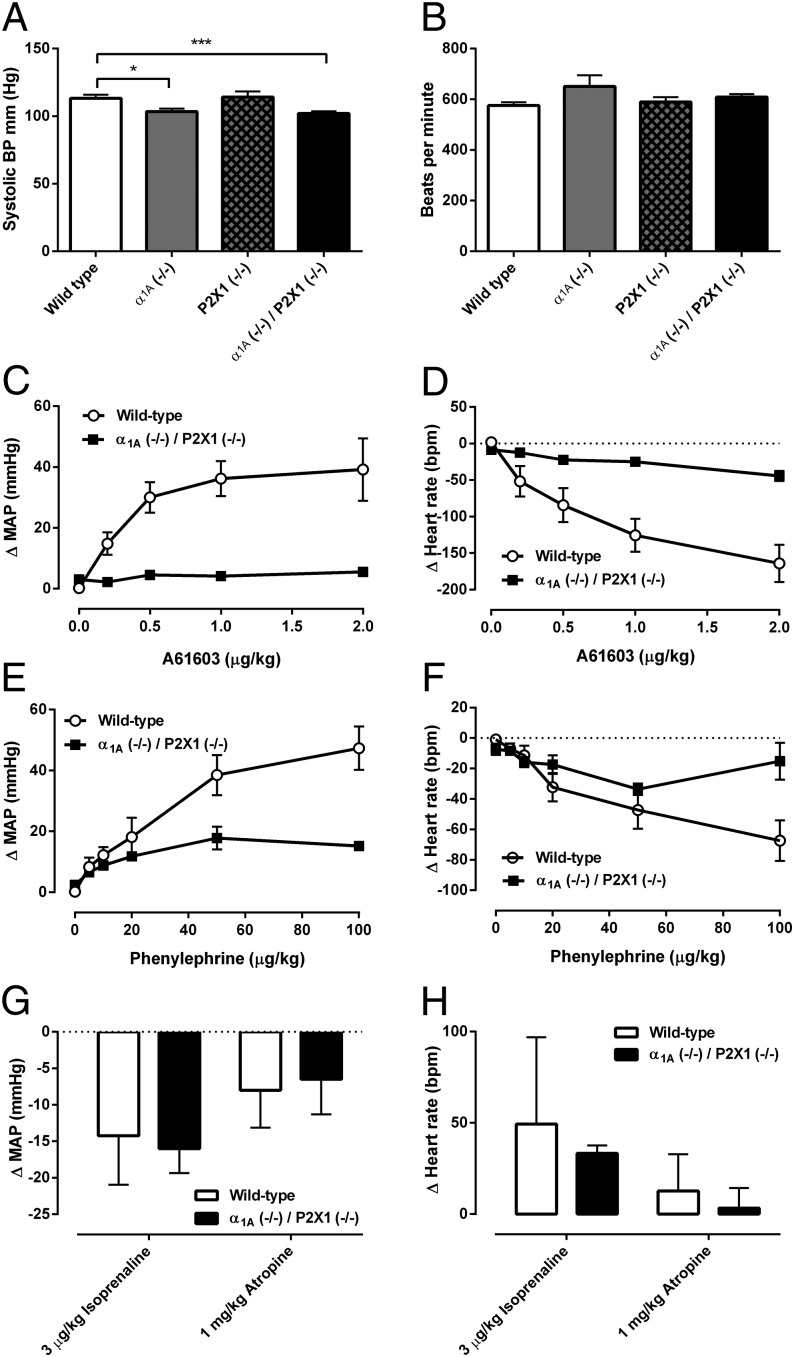
Resting heart rate of double knockout α1A (−/−)/P2X1 (−/−) mice was not different from wild type when measured consciously using a tail cuff method (Fig. 4B). In contrast, there was a small decrease (∼10%) in resting systolic arterial pressure when measured consciously using the tail cuff method (Fig. 4A). However, this decrease in arterial pressure was not different from the decrease in resting arterial pressure seen in single α1A (−/−) knockout mice (13) (Fig. 4A). In double knockout α1A (−/−)/P2X1 (−/−) mice, the pressor response to the selective α1A-adrenoceptor agonist A61603 (0.2–2.0 μg/kg) was absent (Fig. 4C), as had been previously reported in single α1A (−/−) knockout mice (13). Consequently, A61603 (0.2–2.0 μg/kg) had only a minimal effect on heart rate (Fig. 4D). The subtype nonselective α1-adrenoceptor agonist phenylephrine (5–100 μg/kg) produced pressor responses in double knockout α1A (−/−)/P2X1 (−/−) mice that were ∼60% lower than those produced in wild-type mice (Fig. 4E), again similar to what has been reported in single α1A (−/−) knockout mice (14).
Fig. 4.
Cardiovascular responses in double knockout α1A (−/−)/P2X1 (−/−) mice. (A) Resting systolic arterial blood pressure (BP) and (B) resting pulse rate as measured by the tail cuff method in conscious wild-type (n = 8), α1A (−/−) single knockout (n = 11), P2X1 (−/−) single knockout (n = 11), and double knockout α1A (−/−)/P2X1 (−/−) (n = 14) male mice. Asterisks represent a significant difference, *P , 0.05, ***P < 0.001. Mean effect of A61603 (0.2–2.0 μg/kg) in wild-type (n = 6; open circles) and double knockout α1A (−/−)/P2X1 (−/−) (n = 5; filled squares) male mice on (C) carotid artery pressure and (D) heart rate. bpm, beats per min; MAP, mean arterial pressure. Mean effect of phenylephrine (5–100 μg/kg) in wild-type (n = 6; open circles) and double knockout α1A (−/−)/P2X1 (−/−) (n = 5; filled squares) male mice on (E) carotid artery pressure and (F) heart rate. Mean changes to isoprenaline (3 μg/kg) and atropine (1 mg/kg) in wild-type (n = 6) and double knockout α1A (−/−)/P2X1 (−/−) (n = 5) male mice to (G) mean arterial pressure and (H) heart rate. Error bars represent SEM.
The baroreflex heart rate response to phenylephrine (5–100 μg/kg) was also altered in double knockout α1A (−/−)/P2X1 (−/−) mice (Fig. 4F), as seen in single α1A (−/−) knockout mice (13). Cardiovascular responses to the muscarinic receptor antagonist atropine and the β-adrenoceptor agonist isoprenaline were not different in double knockout α1A (−/−)/P2X1 (−/−) mice compared with wild type (Fig. 4 G and H), indicating that the baroreflex resetting that occurs in double knockout α1A (−/−)/P2X1 (−/−) mice does not mask differences in pressor responsiveness or signaling in other systems.
Gross Anatomy.
In nonvascular tissues, there were no observable differences between double knockout α1A (−/−)/P2X1 (−/−) and wild type (Table S1), with the exceptions of the vas deferens and seminal vesicles. The vas deferens was longer and heavier in double knockout α1A (−/−)/P2X1 (−/−) mice (Fig. 1 C and E) and the seminal vesicles were heavier in double knockout α1A (−/−)/P2X1 (−/−) mice (Table S1). The increased weight of seminal vesicles appeared to be due to engorgement with fluid, presumably caused by a loss of contractile function, as in pharmacological electrical field stimulation studies the contractile response of isolated seminal vesicles to 10-Hz stimulation was reduced by 47% [wild-type response, 0.25 ± 0.04 g, compared with double knockout α1A (−/−)/P2X1 (−/−) response, 0.13 ± 0.06 g; P < 0.001; n = 12 and 6, respectively]. A smaller 26% decrease in the contractile response to electrical field stimulation of the coagulating gland was also observed (at 10 Hz: wild-type response, 59.5 ± 7.3 mg, compared with double knockout α1A (−/−)/P2X1 (−/−) response, 44.2 ± 12.5 mg; P < 0.001; n = 9 and 6, respectively). The prostate gland (Fig. 1D and Table S1) and urinary bladder (Table S1) were of similar weight in wild-type and double knockout α1A (−/−)/P2X1 (−/−) mice and appeared to function in a relatively normal fashion, with decreases of only 32% and 31%, respectively, in the contractile response to electrical field stimulation at 10 Hz (prostate responses: wild type, 0.13 ± 0.01 g, compared with double knockout α1A (−/−)/P2X1 (−/−), 0.09 ± 0.01 g; P < 0.001; n = 8 and 14, respectively; bladder responses: wild type, 0.95 ± 0.14 g, compared with double knockout α1A (−/−)/P2X1 (−/−), 0.66 ± 0.13 g; P < 0.001; n = 12 for each).
Discussion
Both α1A (−/−) and P2X1 (−/−) single knockout mice have compromised vas deferens contractility following electrical nerve stimulation (9, 10), and these previously observed effects are additive in double knockout α1A (−/−)/P2X1 (−/−) mice. This explains why in all wild-type females mated with double knockout α1A (−/−)/P2X1 (−/−) male mice no evidence of ejaculated sperm in the form of a vaginal plug was found. Thus, the infertility of double knockout α1A (−/−)/P2X1 (−/−) male mice observed in this study was not due to dysfunctional sperm but rather from a lack of sperm being ejaculated. Coagulum plugs form in rodents following copulation to occlude the vaginal opening. They are formed by the catalytic action of type IV transglutaminase from the coagulating gland (16, 17) cross-linking with semenoclotin (seminal vesicle secretion 2 protein) from the seminal vesicles (18, 19). The lack of a coagulum plug following mating of double knockout α1A (−/−)/P2X1 (−/−) mice with wild-type females indicates a lack of transport of not only sperm but also secretions from the seminal vesicle, coagulating gland, and, to a lesser extent, the prostate gland. This absence of ejaculated sperm circumvents a major problem associated with other strategies for producing male contraception, which is that no sperm at all are likely to find their way to the female reproductive tract. A lack of ejaculate has the potential to be disconcerting. Use of α1A-adrenoceptor antagonists, in particular tamsulosin, have previously been associated with decreased ejaculate volume and occasional dry ejaculation. The dry ejaculation was not due to retrograde ejaculation but rather a lack of seminal emission (20). The vasodilator actions of α1A-adrenoceptors and P2X1-purinoceptors suggest that blockers of these receptors are likely to improve erectile function and premature ejaculation. Indeed, improved sexual performance has been reported in men taking the α1-adrenoceptor antagonist phenoxybenzamine despite dry ejaculation in early trials of this drug for male contraception (21). Notably, all participants in this small trial wished to continue the medication.
The avoidance of tampering with sperm function suggests that this contraceptive process will be readily reversible with no loss of sperm quality, as observed with the high rate of fertilization following intracytoplasmic sperm injection. Subsequent implantation of fertilized embryos into foster mothers to produce normal offspring indicates that this contraceptive method is not associated with detrimental alterations in the genetics of offspring, as may occur with germ-line strategies (1). The challenge of overcoming the often intolerable side effects associated with hormonal treatments has similarly been avoided by this contraceptive method. Importantly, the complete loss of fertility in male double knockout α1A (−/−)/P2X1 (−/−) mice did not affect normal sexual activity.
Another advantage of inhibiting sperm transport over targeting spermatogenesis as a means of male contraception is that it no longer requires the drug to cross the blood–testis barrier. The cauda epididymis and vas deferens lie on the abdominal side of the blood–testis barrier in mice and receive their blood supply predominantly from branches of the deferential artery (22). This is anterior to the blood–testis barrier, and the vas deferens subsequently receives similar blood to other genitourinary and visceral organs. Orally administered selective α1A-adrenoceptor antagonists such as tamsulosin and alfuzosin are already widely used for the treatment of benign prostatic hyperplasia (BPH). This suggests that oral administration of such medicines has no problem reaching genitourinary organs to produce therapeutic effects. Further obstacles associated with the development of pharmaceuticals that are avoided by using medicines already on the market include financial burden, regulatory hurdles, and occurrence of unexpected side effects. Only development of a suitable orally available P2X1-purinoceptor antagonist is required before an appropriate medical preparation can be trialed.
In addition to the vas deferens and other genitourinary organs, α1A-adrenoceptors or P2X1-purinoceptors are present on many other smooth muscle tissues, including blood vessels and parts of the central as well as peripheral nervous systems. The widespread distribution of these receptors may therefore represent a source of possible adverse side effects that requires rigorous safety testing in humans. Of particular concern is the vital role played by both α1A-adrenoceptors and P2X1-purinoceptors in the vascular control of systemic blood pressure via their influence on resistance artery diameter (12, 13). Indeed, before this study it would have been predicted by many physiologists that mice with simultaneous genetic deletion of both of these receptors would most likely be incapable of survival. It had been previously reported that single α1A (−/−) knockout mice have hypotension due to reduced vascular resistance rather than compromised cardiac output (13). A similar reduction in resting blood pressure was seen in double knockout α1A (−/−)/P2X1 (−/−) mice compared with wild type in this study, whereas heart rate was unaffected. In addition, a similar compromise in baroreflex responses was observed in double knockout α1A (−/−)/P2X1 (−/−) and single knockout α1A (−/−) mice (13). Therefore, given the current long-term chronic and widespread use of α1A-adrenoceptor antagonists in the community, it seems that our proposed dual therapeutic target for male contraception is unlikely to produce vascular side effects that are dose-limiting.
The ineffectiveness of single knockout α1A (−/−) (9) or P2X1 (−/−) (10) male mice to be 100% infertile may have been partly associated with the development of compensatory contractile mechanisms in the vas deferens. In single P2X1 (−/−) knockout mice there was an increase in sensitivity to noradrenaline, whereas in single α1A (−/−) knockout mice there was an increase in sensitivity to ATP. The danger exists that even further compensation may have occurred in double knockout α1A (−/−)/P2X1 (−/−) male mice, which has the potential to lead to less than 100% contraception. Indeed, further compensatory changes were indicated by the increased responses to noradrenaline and ATP in double knockout α1A (−/−)/P2X1 (−/−) mice compared with their respective single knockout mice. This compensation was further supported by our PCR data. The contractile response mediated by noradrenaline in double knockout α1A (−/−)/P2X1 (−/−) mice was inhibited by the selective α1D-adrenoceptor antagonist BMY 7378, as was the residual response to electrical field stimulation in double knockout α1A (−/−)/P2X1 (−/−) mice. The residual contractile response to electrical field stimulation that remained in double knockout α1A (−/−)/P2X1 (−/−) mice was also abolished by guanethidine. Together, these observations suggest that this residual nerve-mediated response was sympathetic in nature and at least partially mediated by neuronally released noradrenaline acting at α1D-adrenoceptors.
There also seemed to be a purinergic component to this residual response. ATP did not produce contractile responses in vasa deferentia taken from P2X1 (−/−) knockout mice. However, a small contractile response was observed to high concentrations of ATP in vasa deferentia taken from double knockout α1A (−/−)/P2X1 (−/−) mice. P2X1-purinoceptors mediate contraction of the vas deferens via EJPs that can be recorded from smooth muscle (5, 23). It is likely that the small mechanical and electrical responses seen to exogenously administered ATP in vasa deferentia from double knockout α1A (−/−)/P2X1 (−/−) mice may not be receptor-mediated, because the rise time of the electrical depolarization mediated by ATP was much slower than in vasa deferentia taken from wild-type mice. This slower rise time is not typical of a ligand-gated ion-channel response. Nevertheless, the compensatory contraction that remains following electrical field stimulation of vasa deferentia taken from double knockout α1A (−/−)/P2X1 (−/−) male mice would appear to be insufficient for sperm transport to the urethra, because no ejaculate in the form of a coagulum plug was present following all matings with double knockout α1A (−/−)/P2X1 (−/−) male mice and no pregnancies were observed.
As is observed in all laboratory animals, the human vas deferens has dual purinergic and adrenergic control of smooth muscle contractility (24), with a similar contribution by each mechanism. By genetically deleting these two receptors to produce male mice that are 100% infertile, this study demonstrates that this concept is indeed a feasible mechanism of producing male contraception. In addition, such a pharmacological treatment would be nonhormonal, with mutant male mice exhibiting normal libido and sexual activity. This bypasses perhaps the greatest stumbling block in the quest for a socially acceptable male contraceptive. The proposed mechanism would also appear readily reversible, while the nontargeting of sperm germ lines indicates that genetic abnormalities in future offspring are unlikely. It is still possible that the combined blockade of α1A-adrenoceptor and P2X1-purinoceptors may not be viable due to the widespread distribution and importance of these receptors, particularly in the physiology of vascular control. However, we have shown that double knockout α1A (−/−)/P2X1 (−/−) mice appear normal and their cardiovascular control is no more compromised than in single α1A-adrenoceptor knockout mice (13). The long-standing and widespread safe use of α1-adrenoceptor antagonists as therapeutic treatments for BPH and hypertension also means that development of only a suitable P2X1-purinoceptor antagonist is required before this pharmacological strategy for male contraception can be trialed.
Materials and Methods
Study Approval.
Prior approval for animal breeding and experimentation was granted by the Monash University Standing Committee on Animal Ethics, ethics nos. BCSV 2009.03 for the maintenance and breeding of the knockout mouse colonies and VCPA 2009.14 and VCPA 2009.15 for the use of genetically modified and wild-type mice, respectively. All studies conformed to the National Health and Medical Research Council Australian Code of Practice for the Care and Use of Animals for Scientific Purposes.
Breeding.
Breeding pairs of α1A-adrenoceptor knockout mice purchased from JAX Mice (Jackson Laboratory) and breeding pairs of P2X1-purinoceptor knockout mice generated in-house were used to establish a colony of double α1A-adrenoceptor/P2X1-purinoceptor double knockout mice by selective cross-breeding of mice containing the mutated Adra1a or P2rx1 gene (Fig. 1A). All mice had C57BL/6 genetic backgrounds. Mice for experimentation were produced by crossing heterozygous α1A (+/−)/P2X1 (+/−) males with double knockout α1A (−/−)/P2X1 (−/−) females generated by the initial crossings of mice from the parental colonies maintained on a C57BL/6 background (Fig. 1A). Wild-type controls and, where required, single knockout controls were obtained from the parental single receptor knockout mouse colonies. Offspring were genotyped by PCR (10, 13). Male mice were used when sexually mature (10–12 wk old).
Histochemistry.
Detection of P2X1-purinoceptors by immunohistochemistry used a commercially available antibody (Alomone Labs; APR-001) as previously described (25). X-gal staining for β-galactosidase was as described (13). SPG fluorescence histochemistry was as described (14).
Behavioral Studies.
Double knockout male mice were mated with wild-type female mice in cages fitted with infrared video-recording equipment for 2 h/d for up to 9 consecutive days or until copulation and ejaculation had occurred. Following ejaculation, male mice were given 2 d of rest before being mated with another wild-type female. Female mice were checked for vaginal plugs immediately after mating sessions. Following mating sessions in which copulation and ejaculation had taken place, female mice were kept for 14 d before being killed and their uteri checked for pregnancies.
Sperm Analysis.
Sperm were extracted from cauda epididymides and placed on single concave microscope slides with two drops of physiological saline before coverslipping and viewing under a conventional light microscope (Olympus BX60) fitted with a SPOT RT slider digital camera. Intracytoplasmic sperm injections and implantation of fertilized ova into foster mothers were conducted at the Monash Animal Research Platform, Monash University.
Physiological Studies.
Isolated organ bath experiments were conducted as described (25, 26). Intracellular electrical recordings of vas deferens smooth muscle were as described (15). Resting blood pressure and pulse rate were measured in conscious mice using the tail cuff method with a noninvasive blood pressure analysis system (SC1000; Hatteras Instruments). Analysis was performed over a period of 4–5 consecutive days, with each day comprising 20 repeat blood pressure and heart rate measurements. The first and/or second day was used for acclimatization. Intraarterial pressure under isofluorane anesthesia was measured via a carotid artery catheter with the jugular vein catheterized for i.v. drug infusion, as described (27).
Statistical Methods.
In all cases, means were calculated from data pooled from n experiments, where n is equal to the number of mice used. Results are expressed as mean ± SEM. Mean data were analyzed by ANOVA, followed by Bonferroni posttest for multiple comparisons where required or Student t test. Tests were carried out using GraphPad Prism version 6.00, and P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Dr. Jose Gonzalez of the Monash Animal Research Platform for performing intracytoplasmic sperm injections and implantation of fertilized ova into foster mothers. We thank Ms. Nilushi Karunaratne for technical assistance with behavioral experiments. We thank Drs. Anupa Dey and Dan-Thanh Nguyen as well as Mr. Basu Chakrabarty for technical assistance with electrophysiological recording experiments, and Ms. Brenda Cotter for assistance with mouse colony maintenance. We are grateful to Profs. Patrick Sexton, Nigel Bunnett, Arthur Christopoulos, and Roger Summers for comments and financial support. This study was supported by the National Health and Medical Research Council (Australia). The initial targeted deletion of the P2X1-purinoceptor in mice was funded by Wellcome Trust Grant 080487/Z/06/Z (to R.J.E.). C.W.W. is a recipient of a Monash University Postgraduate Publications Award.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1318624110/-/DCSupplemental.
References
- 1.Matzuk MM, et al. Small-molecule inhibition of BRDT for male contraception. Cell. 2012;150(4):673–684. doi: 10.1016/j.cell.2012.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lo JC, et al. RAB-like 2 has an essential role in male fertility, sperm intra-flagellar transport, and tail assembly. PLoS Genet. 2012;8(10):e1002969. doi: 10.1371/journal.pgen.1002969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruschini H, Schmidt RA, Tanagho EA. Neurologic control of prostatic secretion in the dog. Invest Urol. 1978;15(4):288–290. [PubMed] [Google Scholar]
- 4.Steers WD. Physiology of the vas deferens. World J Urol. 1994;12(5):281–285. doi: 10.1007/BF00191208. [DOI] [PubMed] [Google Scholar]
- 5.Sneddon P, Burnstock G. Inhibition of excitatory junction potentials in guinea-pig vas deferens by alpha, beta-methylene-ATP: Further evidence for ATP and noradrenaline as cotransmitters. Eur J Pharmacol. 1984;100(1):85–90. doi: 10.1016/0014-2999(84)90318-2. [DOI] [PubMed] [Google Scholar]
- 6.Furukawa K, et al. Alpha 1A-adrenoceptor-mediated contractile responses of the human vas deferens. Br J Pharmacol. 1995;116(1):1605–1610. doi: 10.1111/j.1476-5381.1995.tb16380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burt RP, Chapple CR, Marshall I. Alpha1A-adrenoceptor mediated contraction of rat prostatic vas deferens and the involvement of ryanodine stores and Ca2+ influx stimulated by diacylglycerol and PKC. Br J Pharmacol. 1998;123(2):317–325. doi: 10.1038/sj.bjp.0701588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ratnasooriya WD, Wadsworth RM. Tamsulosin, a selective alpha 1-adrenoceptor antagonist, inhibits fertility of male rats. Andrologia. 1994;26(2):107–110. doi: 10.1111/j.1439-0272.1994.tb00766.x. [DOI] [PubMed] [Google Scholar]
- 9.Sanbe A, et al. Alpha1-adrenoceptors are required for normal male sexual function. Br J Pharmacol. 2007;152(3):332–340. doi: 10.1038/sj.bjp.0707366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mulryan K, et al. Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature. 2000;403(6765):86–89. doi: 10.1038/47495. [DOI] [PubMed] [Google Scholar]
- 11.Dunn PM. Fertility: Purinergic receptors and the male contraceptive pill. Curr Biol. 2000;10(8):R305–R307. doi: 10.1016/s0960-9822(00)00436-x. [DOI] [PubMed] [Google Scholar]
- 12.Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- 13.Rokosh DG, Simpson PC. Knockout of the alpha 1A/C-adrenergic receptor subtype: The alpha 1A/C is expressed in resistance arteries and is required to maintain arterial blood pressure. Proc Natl Acad Sci USA. 2002;99(14):9474–9479. doi: 10.1073/pnas.132552699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torre JC, Surgeon JW. A methodological approach to rapid and sensitive monoamine histofluorescence using a modified glyoxylic acid technique: The SPG method. Histochemistry. 1976;49(2):81–93. doi: 10.1007/BF00495672. [DOI] [PubMed] [Google Scholar]
- 15.Exintaris B, Klemm MF, Lang RJ. Spontaneous slow wave and contractile activity of the guinea pig prostate. J Urol. 2002;168(1):315–322. [PubMed] [Google Scholar]
- 16.Williams-Ashman HG. Transglutaminases and the clotting of mammalian seminal fluids. Mol Cell Biochem. 1984;58(1-2):51–61. doi: 10.1007/BF00240604. [DOI] [PubMed] [Google Scholar]
- 17.Tseng HC, Lin HJ, Sudhakar Gandhi PS, Wang CY, Chen YH. Purification and identification of transglutaminase from mouse coagulating gland and its cross-linking activity among seminal vesicle secretion proteins. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;876(2):198–202. doi: 10.1016/j.jchromb.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 18.Lundwall A. The cloning of a rapidly evolving seminal-vesicle-transcribed gene encoding the major clot-forming protein of mouse semen. Eur J Biochem. 1996;235(1-2):424–430. doi: 10.1111/j.1432-1033.1996.00424.x. [DOI] [PubMed] [Google Scholar]
- 19.Kawano N, Yoshida M. Semen-coagulating protein, SVS2, in mouse seminal plasma controls sperm fertility. Biol Reprod. 2007;76(3):353–361. doi: 10.1095/biolreprod.106.056887. [DOI] [PubMed] [Google Scholar]
- 20.Hellstrom WJ, Sikka SC. Effects of acute treatment with tamsulosin versus alfuzosin on ejaculatory function in normal volunteers. J Urol. 2006;176(4 Pt 1):1529–1533. doi: 10.1016/j.juro.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Homonnai ZT, Shilon M, Paz GF. Phenoxybenzamine—An effective male contraceptive pill. Contraception. 1984;29(5):479–491. doi: 10.1016/0010-7824(84)90022-2. [DOI] [PubMed] [Google Scholar]
- 22.Chubb C, Desjardins C. Vasculature of the mouse, rat, and rabbit testis-epididymis. Am J Anat. 1982;165(4):357–372. doi: 10.1002/aja.1001650402. [DOI] [PubMed] [Google Scholar]
- 23.Allcorn RJ, Cunnane TC, Kirkpatrick K. Actions of alpha, beta-methylene ATP and 6-hydroxydopamine on sympathetic neurotransmission in the vas deferens of the guinea-pig, rat and mouse: Support for cotransmission. Br J Pharmacol. 1986;89(4):647–659. doi: 10.1111/j.1476-5381.1986.tb11169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banks FC, et al. The purinergic component of human vas deferens contraction. Fertil Steril. 2006;85(4):932–939. doi: 10.1016/j.fertnstert.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 25.Gray KT, Ventura S. Evaluation of the mouse prostate as a suitable model for the study of human prostate function. J Pharmacol Toxicol Methods. 2005;51(1):41–50. doi: 10.1016/j.vascn.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 26.White CW, Short JL, Haynes JM, Evans RJ, Ventura S. The residual nonadrenergic contractile response to nerve stimulation of the mouse prostate is mediated by acetylcholine but not ATP in a comparison with the mouse vas deferens. J Pharmacol Exp Ther. 2010;335(2):489–496. doi: 10.1124/jpet.110.172130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen D, et al. Changes in angiotensin type 1 receptor binding and angiotensin-induced pressor responses in the rostral ventrolateral medulla of angiotensinogen knockout mice. Am J Physiol Regul Integr Comp Physiol. 2010;298(2):R411–R418. doi: 10.1152/ajpregu.00462.2009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.