Abstract
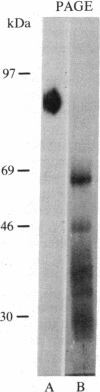
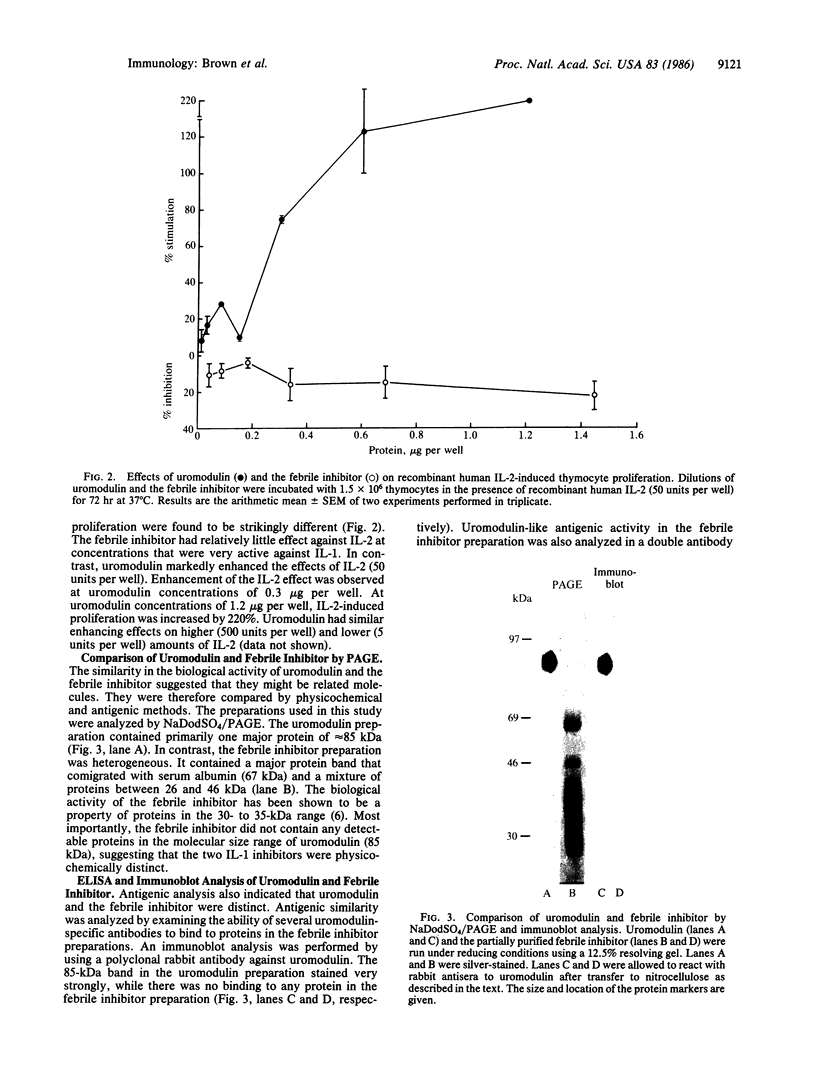
Uromodulin, an 85-kDa glycoprotein isolated from pregnancy urine, has been shown to inhibit antigen-induced proliferation of human lymphocytes in vitro. The present investigation was undertaken to determine its mechanism of action. Uromodulin was found to be a potent inhibitor of interleukin 1 (IL-1)-induced thymocyte proliferation. Uromodulin was compared to a previously described 30- to 35-kDa IL-1 inhibitor isolated from urine of febrile patients (febrile inhibitor). Uromodulin and the febrile inhibitor blocked the effects of both human IL-1 and recombinant murine IL-1, but the activity of uromodulin was greater than that of the only partially purified febrile inhibitor preparation. However, in contrast to the febrile inhibitor, uromodulin markedly enhanced interleukin 2-induced thymocyte proliferation. Antigenic analysis of the two preparations by ELISA and immunoblot analysis demonstrated that the febrile inhibitor did not cross-react with uromodulin using monoclonal or polyclonal antisera. These findings indicate that uromodulin is a potent IL-1 inhibitor that is probably distinct from the IL-1 inhibitor derived from the urine of febrile individuals. Whether this IL-1 inhibitory activity underlies its immunosuppressive activity on human lymphocytes remains to be established.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amento E. P., Kurnick J. T., Epstein A., Krane S. M. Modulation of synovial cell products by a factor from a human cell line: T lymphocyte induction of a mononuclear cell factor. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5307–5311. doi: 10.1073/pnas.79.17.5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amento E. P., Kurnick J. T., Krane S. M. Interleukin 1 production by the human monocyte cell line U937 requires a lymphokine induction signal distinct from interleukin 2 or interferons. J Immunol. 1985 Jan;134(1):350–357. [PubMed] [Google Scholar]
- Arend W. P., Joslin F. G., Massoni R. J. Effects of immune complexes on production by human monocytes of interleukin 1 or an interleukin 1 inhibitor. J Immunol. 1985 Jun;134(6):3868–3875. [PubMed] [Google Scholar]
- Bohn H., Kraus W. Isolierung und Charakterisierung des schwangerschafts-spezifischen beta1-Glykoproteins aus dem Urin schwangerer Frauen. Arch Gynakol. 1977 Aug 31;223(1):33–39. doi: 10.1007/BF00675081. [DOI] [PubMed] [Google Scholar]
- Cerni C., Tatra G., Bohn H. Immunosuppression by human placenta lactogen (HPL) and the pregnancy-specific beta 1-glycoprotein (SP-1). Inhibition of mitogen-induced lymphocyte transformation. Arch Gynakol. 1977 Aug 31;223(1):1–7. doi: 10.1007/BF00675078. [DOI] [PubMed] [Google Scholar]
- Dempsey R. A., Dinarello C. A., Mier J. W., Rosenwasser L. J., Allegretta M., Brown T. E., Parkinson D. R. The differential effects of human leukocyte pyrogen/lymphocyte-activating factor, T cell growth factor, and interferon on human natural killer activity. J Immunol. 1982 Dec;129(6):2504–2510. [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Rosenwasser L. J., Wolff S. M. Demonstration of a circulating suppressor factor of thymocyte proliferation during endotoxin fever in humans. J Immunol. 1981 Dec;127(6):2517–2519. [PubMed] [Google Scholar]
- Dower S. K., Kronheim S. R., March C. J., Conlon P. J., Hopp T. P., Gillis S., Urdal D. L. Detection and characterization of high affinity plasma membrane receptors for human interleukin 1. J Exp Med. 1985 Aug 1;162(2):501–515. doi: 10.1084/jem.162.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkoff R. J., Muraguchi A., Hong J. X., Butler J. L., Dinarello C. A., Fauci A. S. The effects of interleukin 1 on human B cell activation and proliferation. J Immunol. 1983 Aug;131(2):801–805. [PubMed] [Google Scholar]
- Hoffmann M. K., Watson J. Helper T cell-replacing factors secreted by thymus-derived cells and macrophages: cellular requirements for B cell activation and synergistic properties. J Immunol. 1979 Apr;122(4):1371–1375. [PubMed] [Google Scholar]
- Kimball E. S., Pickeral S. F., Oppenheim J. J., Rossio J. L. Interleukin 1 activity in normal human urine. J Immunol. 1984 Jul;133(1):256–260. [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Liao Z., Grimshaw R. S., Rosenstreich D. L. Identification of a specific interleukin 1 inhibitor in the urine of febrile patients. J Exp Med. 1984 Jan 1;159(1):126–136. doi: 10.1084/jem.159.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Z., Haimovitz A., Chen Y., Chan J., Rosenstreich D. L. Characterization of a human interleukin 1 inhibitor. J Immunol. 1985 Jun;134(6):3882–3886. [PubMed] [Google Scholar]
- Lomedico P. T., Gubler U., Hellmann C. P., Dukovich M., Giri J. G., Pan Y. C., Collier K., Semionow R., Chua A. O., Mizel S. B. Cloning and expression of murine interleukin-1 cDNA in Escherichia coli. 1984 Nov 29-Dec 5Nature. 312(5993):458–462. doi: 10.1038/312458a0. [DOI] [PubMed] [Google Scholar]
- Morse J. H., Stearns G., Arden J., Agosto G. M., Canfield R. E. The effects of crude and purified human gonadotropin on in vitro stimulated human lymphocyte cultures. Cell Immunol. 1976 Aug;25(2):178–188. doi: 10.1016/0008-8749(76)90108-8. [DOI] [PubMed] [Google Scholar]
- Muchmore A. V., Decker J. M., Blaese R. M. Evidence that specific oligosaccharides block early events necessary for the expression of antigen-specific proliferation by human lymphocytes. J Immunol. 1980 Sep;125(3):1306–1311. [PubMed] [Google Scholar]
- Muchmore A. V., Decker J. M., Blaese R. M., Nilsson B. Purification and characterization of a mannose-containing disaccharide obtained from human pregnancy urine. A new immunoregulatory saccharide. J Exp Med. 1984 Dec 1;160(6):1672–1685. doi: 10.1084/jem.160.6.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchmore A. V., Decker J. M. Uromodulin: a unique 85-kilodalton immunosuppressive glycoprotein isolated from urine of pregnant women. Science. 1985 Aug 2;229(4712):479–481. doi: 10.1126/science.2409603. [DOI] [PubMed] [Google Scholar]
- Scala G., Kuang Y. D., Hall R. E., Muchmore A. V., Oppenheim J. J. Accessory cell function of human B cells. I. Production of both interleukin 1-like activity and an interleukin 1 inhibitory factor by an EBV-transformed human B cell line. J Exp Med. 1984 Jun 1;159(6):1637–1652. doi: 10.1084/jem.159.6.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimson W. H. Are pregnancy-associated serum proteins responsible for the inhibition of lymphocyte transformation by pregnancy serum? Clin Exp Immunol. 1980 Apr;40(1):157–160. [PMC free article] [PubMed] [Google Scholar]
- Wilkins J. A., Sigurdson L., Jordon Y., Rutherford W. J., Warrington R. J. Immunoregulatory factors from a human macrophage-like cell line. II. A human T-cell lymphokine-induced suppressor factor for lymphocyte proliferation. Cell Immunol. 1983 Apr 15;77(2):329–337. doi: 10.1016/0008-8749(83)90033-3. [DOI] [PubMed] [Google Scholar]
- Wilkins J. A., Sigurdson S. L., Rutherford W. J., Jordan Y., Warrington R. J. The production of immunoregulatory factors by a human macrophage-like cell line. I. Characterization of an inhibitor of lymphocyte DNA synthesis. Cell Immunol. 1983 Feb 1;75(2):328–336. doi: 10.1016/0008-8749(83)90330-1. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]