Abstract
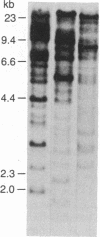
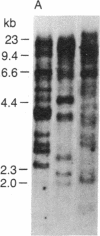
The diversity of the genes encoding mammalian proteoglycan peptide cores was explored using a cDNA clone that encodes the partial sequence of a cell surface/pericellular matrix-localized chondroitin sulfate proteoglycan. Thus we were able to detect the expression of the gene(s) encoding the intracellular chondroitin sulfate proteoglycan produced by a variety of rat and mouse mucosal-like mast cells and the intracellular heparin proteoglycan synthesized by rat serosal mast cells. The cDNA from the proteoglycan cDNA clone pPG-1 was fractionated into two discrete fragments, one of which contained the nucleotides encoding the serine-glycine repeat sequence (pPG-B) and the other of which contained sequences on the 3' side of the repeat (pPG-M). As assessed by Southern blot analysis, pPG-B identified a large gene family, whereas pPG-M identified a single DNA fragment in the rat genome. When the pPG-1 insert and the two subcloned probes pPG-B and pPG-M were used to analyze RNA extracted from the rat and mouse mucosal-like mast cells and the rat serosal mast cells, the same major RNA species was detected at 1.3 kilobases with both probes. These data suggest that the gene responsible for the peptide core of the extracellular chondroitin sulfate proteoglycan synthesized by the rat yolk sac cell line is also the gene that encodes the core peptides of the secretory granule-localized chondroitin sulfate and heparin proteoglycans.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourdon M. A., Oldberg A., Pierschbacher M., Ruoslahti E. Molecular cloning and sequence analysis of a chondroitin sulfate proteoglycan cDNA. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1321–1325. doi: 10.1073/pnas.82.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Eccleston E., Leonard B. J., Lowe J. S., Welford H. J. Basophilic leukaemia in the albino rat and a demonstration of the basopoietin. Nat New Biol. 1973 Jul 18;244(133):73–76. doi: 10.1038/newbio244073b0. [DOI] [PubMed] [Google Scholar]
- Holgate S. T., Lewis R. A., Austen K. F. 3',5'-Cyclic adenosine monophosphate-dependent protein kinase of the rat serosal mast cell and its immunologic activation. J Immunol. 1980 May;124(5):2093–2099. [PubMed] [Google Scholar]
- Ihle J. N., Pepersack L., Rebar L. Regulation of T cell differentiation: in vitro induction of 20 alpha-hydroxysteroid dehydrogenase in splenic lymphocytes from athymic mice by a unique lymphokine. J Immunol. 1981 Jun;126(6):2184–2189. [PubMed] [Google Scholar]
- Jackson F. R., Bargiello T. A., Yun S. H., Young M. W. Product of per locus of Drosophila shares homology with proteoglycans. Nature. 1986 Mar 13;320(6058):185–188. doi: 10.1038/320185a0. [DOI] [PubMed] [Google Scholar]
- Mayrhofer G., Bazin H. Nature of the thymus dependency of mucosal mast cells. III. Mucosal mast cells in nude mice and nude rats, in B rats and in a child with the Di George syndrome. Int Arch Allergy Appl Immunol. 1981;64(3):320–331. doi: 10.1159/000232710. [DOI] [PubMed] [Google Scholar]
- Metcalfe D. D., Smith J. A., Austen K. F., Silbert J. E. Polydispersity of rat mast cell heparin. Implications for proteoglycan assembly. J Biol Chem. 1980 Dec 25;255(24):11753–11758. [PubMed] [Google Scholar]
- Oldberg A., Hayman E. G., Ruoslahti E. Isolation of a chondroitin sulfate proteoglycan from a rat yolk sac tumor and immunochemical demonstration of its cell surface localization. J Biol Chem. 1981 Nov 10;256(21):10847–10852. [PubMed] [Google Scholar]
- Pierce J. H., Di Fiore P. P., Aaronson S. A., Potter M., Pumphrey J., Scott A., Ihle J. N. Neoplastic transformation of mast cells by Abelson-MuLV: abrogation of IL-3 dependence by a nonautocrine mechanism. Cell. 1985 Jul;41(3):685–693. doi: 10.1016/s0092-8674(85)80049-0. [DOI] [PubMed] [Google Scholar]
- Razin E., Cordon-Cardo C., Good R. A. Growth of a pure population of mouse mast cells in vitro with conditioned medium derived from concanavalin A-stimulated splenocytes. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2559–2561. doi: 10.1073/pnas.78.4.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin E., Ihle J. N., Seldin D., Mencia-Huerta J. M., Katz H. R., LeBlanc P. A., Hein A., Caulfield J. P., Austen K. F., Stevens R. L. Interleukin 3: A differentiation and growth factor for the mouse mast cell that contains chondroitin sulfate E proteoglycan. J Immunol. 1984 Mar;132(3):1479–1486. [PubMed] [Google Scholar]
- Razin E., Stevens R. L., Akiyama F., Schmid K., Austen K. F. Culture from mouse bone marrow of a subclass of mast cells possessing a distinct chondroitin sulfate proteoglycan with glycosaminoglycans rich in N-acetylgalactosamine-4,6-disulfate. J Biol Chem. 1982 Jun 25;257(12):7229–7236. [PubMed] [Google Scholar]
- Robinson H. C., Horner A. A., Hök M., Ogren S., Lindahl U. A proteoglycan form of heparin and its degradation to single-chain molecules. J Biol Chem. 1978 Oct 10;253(19):6687–6693. [PubMed] [Google Scholar]
- Schwartz L. B., Bradford T. R. Regulation of tryptase from human lung mast cells by heparin. Stabilization of the active tetramer. J Biol Chem. 1986 Jun 5;261(16):7372–7379. [PubMed] [Google Scholar]
- Seldin D. C., Adelman S., Austen K. F., Stevens R. L., Hein A., Caulfield J. P., Woodbury R. G. Homology of the rat basophilic leukemia cell and the rat mucosal mast cell. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3871–3875. doi: 10.1073/pnas.82.11.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seldin D. C., Austen K. F., Stevens R. L. Purification and characterization of protease-resistant secretory granule proteoglycans containing chondroitin sulfate di-B and heparin-like glycosaminoglycans from rat basophilic leukemia cells. J Biol Chem. 1985 Sep 15;260(20):11131–11139. [PubMed] [Google Scholar]
- Shin H. S., Bargiello T. A., Clark B. T., Jackson F. R., Young M. W. An unusual coding sequence from a Drosophila clock gene is conserved in vertebrates. Nature. 1985 Oct 3;317(6036):445–448. doi: 10.1038/317445a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stevens R. L., Lee T. D., Seldin D. C., Austen K. F., Befus A. D., Bienenstock J. Intestinal mucosal mast cells from rats infected with Nippostrongylus brasiliensis contain protease-resistant chondroitin sulfate di-B proteoglycans. J Immunol. 1986 Jul 1;137(1):291–295. [PubMed] [Google Scholar]
- Stevens R. L., Otsu K., Austen K. F. Purification and analysis of the core protein of the protease-resistant intracellular chondroitin sulfate E proteoglycan from the interleukin 3-dependent mouse mast cell. J Biol Chem. 1985 Nov 15;260(26):14194–14200. [PubMed] [Google Scholar]
- Stevens R. L., Razin E., Austen K. F., Hein A., Caulfield J. P., Seno N., Schmid K., Akiyama F. Synthesis of chondroitin sulfate E glycosaminoglycan onto p-nitrophenyl-beta-D-xyloside and its localization to the secretory granules of rat serosal mast cells and mouse bone marrow-derived mast cells. J Biol Chem. 1983 May 10;258(9):5977–5984. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Yurt R. W., Leid R. W., Jr, Austen K. F. Native heparin from rat peritoneal mast cells. J Biol Chem. 1977 Jan 25;252(2):518–521. [PubMed] [Google Scholar]
- Yurt R., Austen K. F. Preparative purification of the rat mast cell chymase: characterization and interaction with granule components. J Exp Med. 1977 Nov 1;146(5):1405–1419. doi: 10.1084/jem.146.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]