Abstract
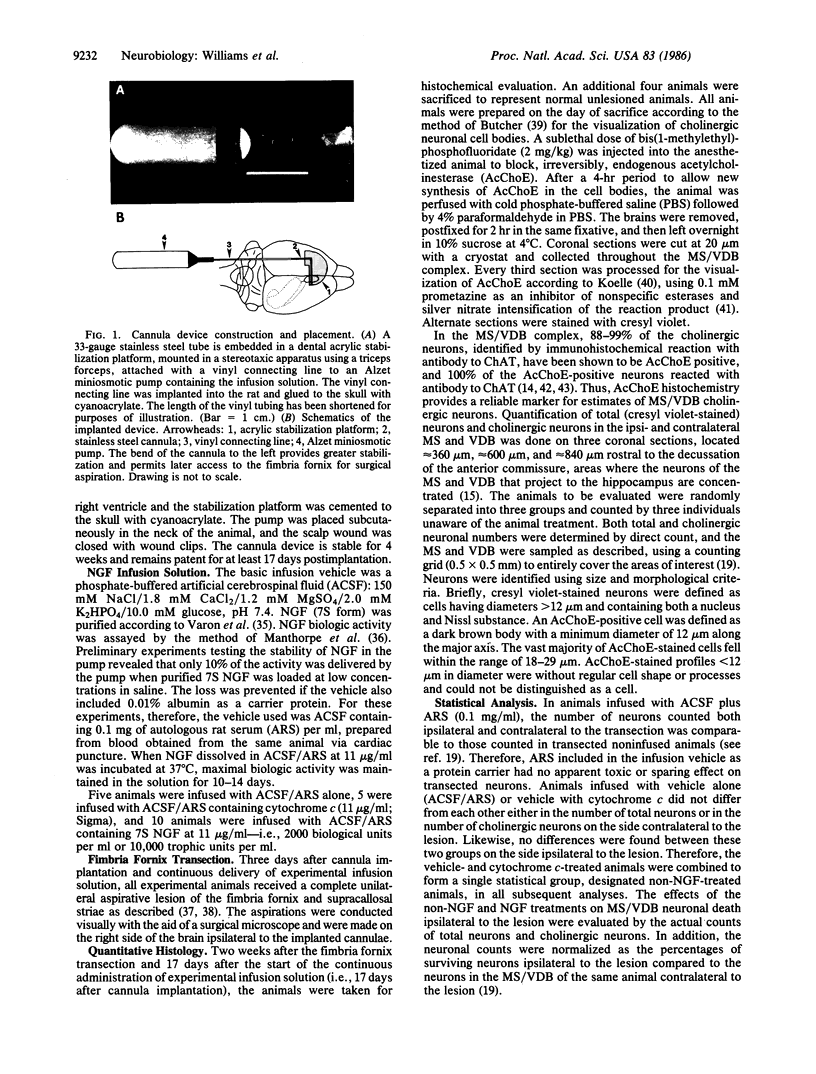
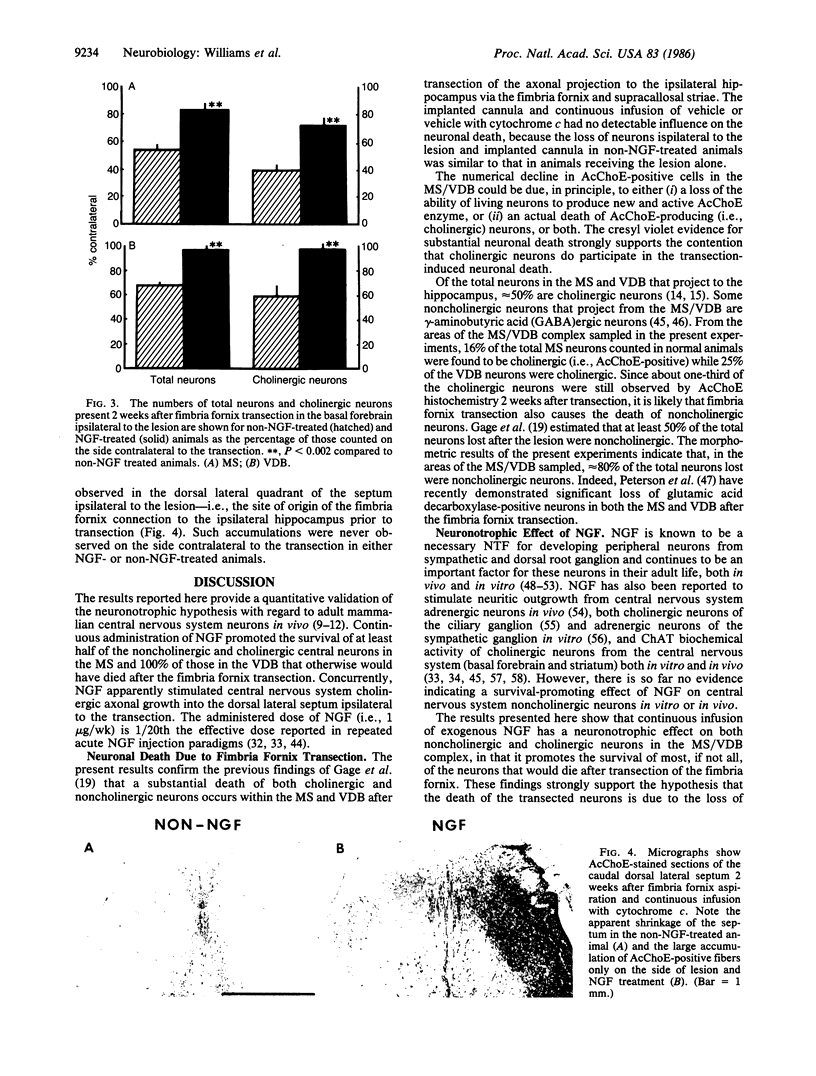
Neurons in the rat medial septum (MS) and vertical limb of the diagonal band of Broca (VDB) undergo a rapid and severe cell death after transection of their dorsal projection to the hippocampus by aspiration of the ipsilateral fimbria fornix and supracallosal striae. By 2 weeks posttransection, the extent of neuronal loss was 50% of the total neurons and 70% of the cholinergic neurons in the MS and 30% of the total neurons and 40% of the cholinergic neurons in the VDB. We hypothesized that (i) the death was due to the loss of a hippocampus-derived neuronotrophic factor, and (ii) exogenous nerve growth factor (NGF) might provide trophic support to the MS/VDB cholinergic neurons, in light of recent reports that the septal diagonal band cholinergic neurons are responsive to NGF and that NGF is present and produced in the hippocampus. In the present study, we attempted to prevent the transection-induced neuronal death by continuous infusion of exogenous 7S NGF (1 microgram/wk) through an intraventricular cannula device. We report here that NGF treatment significantly reduces both the total neuronal and cholinergic neuronal death found 2 weeks after fimbria fornix transection; there was a sparing of 50% of the neurons in the MS and essentially 100% of those in the VDB that otherwise would have died. We conclude that NGF also has a protective effect on noncholinergic neurons since calculations indicate that 80% of the NGF-affected neurons are noncholinergic.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amaral D. G., Kurz J. An analysis of the origins of the cholinergic and noncholinergic septal projections to the hippocampal formation of the rat. J Comp Neurol. 1985 Oct 1;240(1):37–59. doi: 10.1002/cne.902400104. [DOI] [PubMed] [Google Scholar]
- Appel S. H. A unifying hypothesis for the cause of amyotrophic lateral sclerosis, parkinsonism, and Alzheimer disease. Ann Neurol. 1981 Dec;10(6):499–505. doi: 10.1002/ana.410100602. [DOI] [PubMed] [Google Scholar]
- Bartus R. T., Dean R. L., 3rd, Beer B., Lippa A. S. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982 Jul 30;217(4558):408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- Björklund A., Stenevi U. In vivo evidence for a hippocampal adrenergic neuronotrophic factor specifically released on septal deafferentation. Brain Res. 1981 Dec 21;229(2):403–428. doi: 10.1016/0006-8993(81)91004-0. [DOI] [PubMed] [Google Scholar]
- Björklund A., Stenevi U. Regeneration of monoaminergic and cholinergic neurons in the mammalian central nervous system. Physiol Rev. 1979 Jan;59(1):62–100. doi: 10.1152/physrev.1979.59.1.62. [DOI] [PubMed] [Google Scholar]
- Campenot R. B. Local control of neurite development by nerve growth factor. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4516–4519. doi: 10.1073/pnas.74.10.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. An effect of nerve growth factor on the parasympathetic ciliary ganglion. J Neurosci. 1984 May;4(5):1281–1288. doi: 10.1523/JNEUROSCI.04-05-01281.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crutcher K. A., Collins F. In vitro Evidence for two distinct hippocampal growth factors: basis of neuronal plasticity? Science. 1982 Jul 2;217(4554):67–68. doi: 10.1126/science.7089542. [DOI] [PubMed] [Google Scholar]
- DAITZ H. M., POWELL T. P. Studies of the connexions of the fornix system. J Neurol Neurosurg Psychiatry. 1954 Feb;17(1):75–82. doi: 10.1136/jnnp.17.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P., Maloney A. J. Selective loss of central cholinergic neurons in Alzheimer's disease. Lancet. 1976 Dec 25;2(8000):1403–1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- Deutsch J. A. The cholinergic synapse and the site of memory. Science. 1971 Nov 19;174(4011):788–794. doi: 10.1126/science.174.4011.788. [DOI] [PubMed] [Google Scholar]
- Eckenstein F., Sofroniew M. V. Identification of central cholinergic neurons containing both choline acetyltransferase and acetylcholinesterase and of central neurons containing only acetylcholinesterase. J Neurosci. 1983 Nov;3(11):2286–2291. doi: 10.1523/JNEUROSCI.03-11-02286.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage F. H., Björklund A. Enhanced graft survival in the hippocampus following selective denervation. Neuroscience. 1986;17(1):89–98. doi: 10.1016/0306-4522(86)90227-7. [DOI] [PubMed] [Google Scholar]
- Gage F. H., Björklund A., Stenevi U. Cells of origin of the ventral cholinergic septohippocampal pathway undergoing compensatory collateral sprouting following fimbria-fornix transection. Neurosci Lett. 1984 Feb 10;44(2):211–216. doi: 10.1016/0304-3940(84)90083-1. [DOI] [PubMed] [Google Scholar]
- Gage F. H., Björklund A., Stenevi U. Denervation releases a neuronal survival factor in adult rat hippocampus. Nature. 1984 Apr 12;308(5960):637–639. doi: 10.1038/308637a0. [DOI] [PubMed] [Google Scholar]
- Gage F. H., Björklund A., Stenevi U. Reinnervation of the partially deafferented hippocampus by compensatory collateral sprouting from spared cholinergic and noradrenergic afferents. Brain Res. 1983 May 23;268(1):27–37. doi: 10.1016/0006-8993(83)90387-6. [DOI] [PubMed] [Google Scholar]
- Gage F. H., Wictorin K., Fischer W., Williams L. R., Varon S., Bjorklund A. Retrograde cell changes in medial septum and diagonal band following fimbria-fornix transection: quantitative temporal analysis. Neuroscience. 1986 Sep;19(1):241–255. doi: 10.1016/0306-4522(86)90018-7. [DOI] [PubMed] [Google Scholar]
- Gage N. H., Dunnett S. B., Stenevi U., Björklund A. Aged rats: recovery of motor impairments by intrastriatal nigral grafts. Science. 1983 Sep 2;221(4614):966–969. doi: 10.1126/science.6879196. [DOI] [PubMed] [Google Scholar]
- Geneser-Jensen F. A., Blackstad T. W. Distribution of acetyl cholinesterase in the hippocampal region of the guinea pig. I. Entorhinal area, parasubiculum, and presubiculum. Z Zellforsch Mikrosk Anat. 1971;114(4):460–481. doi: 10.1007/BF00325634. [DOI] [PubMed] [Google Scholar]
- Gnahn H., Hefti F., Heumann R., Schwab M. E., Thoenen H. NGF-mediated increase of choline acetyltransferase (ChAT) in the neonatal rat forebrain: evidence for a physiological role of NGF in the brain? Brain Res. 1983 Jul;285(1):45–52. doi: 10.1016/0165-3806(83)90107-4. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Shooter E. M. The nerve growth factor: biochemistry, synthesis, and mechanism of action. Annu Rev Neurosci. 1980;3:353–402. doi: 10.1146/annurev.ne.03.030180.002033. [DOI] [PubMed] [Google Scholar]
- Gundersen R. W. Sensory neurite growth cone guidance by substrate adsorbed nerve growth factor. J Neurosci Res. 1985;13(1-2):199–212. doi: 10.1002/jnr.490130114. [DOI] [PubMed] [Google Scholar]
- Hefti F., Dravid A., Hartikka J. Chronic intraventricular injections of nerve growth factor elevate hippocampal choline acetyltransferase activity in adult rats with partial septo-hippocampal lesions. Brain Res. 1984 Feb 20;293(2):305–311. doi: 10.1016/0006-8993(84)91237-x. [DOI] [PubMed] [Google Scholar]
- Hefti F. Is Alzheimer disease caused by lack of nerve growth factor? Ann Neurol. 1983 Jan;13(1):109–110. doi: 10.1002/ana.410130127. [DOI] [PubMed] [Google Scholar]
- Hefti F. Nerve growth factor promotes survival of septal cholinergic neurons after fimbrial transections. J Neurosci. 1986 Aug;6(8):2155–2162. doi: 10.1523/JNEUROSCI.06-08-02155.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honegger P., Lenoir D. Nerve growth factor (NGF) stimulation of cholinergic telencephalic neurons in aggregating cell cultures. Brain Res. 1982 Feb;255(2):229–238. doi: 10.1016/0165-3806(82)90023-2. [DOI] [PubMed] [Google Scholar]
- KOELLE G. B. The histochemical localization of cholinesterases in the central nervous system of the rat. J Comp Neurol. 1954 Feb;100(1):211–235. doi: 10.1002/cne.901000108. [DOI] [PubMed] [Google Scholar]
- Korsching S., Auburger G., Heumann R., Scott J., Thoenen H. Levels of nerve growth factor and its mRNA in the central nervous system of the rat correlate with cholinergic innervation. EMBO J. 1985 Jun;4(6):1389–1393. doi: 10.1002/j.1460-2075.1985.tb03791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler C., Chan-Palay V., Wu J. Y. Septal neurons containing glutamic acid decarboxylase immunoreactivity project to the hippocampal region in the rat brain. Anat Embryol (Berl) 1984;169(1):41–44. doi: 10.1007/BF00300585. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. Developmental neurobiology and the natural history of nerve growth factor. Annu Rev Neurosci. 1982;5:341–362. doi: 10.1146/annurev.ne.05.030182.002013. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor: its mode of action on sensory and sympathetic nerve cells. Harvey Lect. 1966;60:217–259. [PubMed] [Google Scholar]
- Manthorpe M., Fagnani R., Skaper S. D., Varon S. An automated colorimetric microassay for neuronotrophic factors. Brain Res. 1986 Mar;390(2):191–198. doi: 10.1016/s0006-8993(86)80227-x. [DOI] [PubMed] [Google Scholar]
- Manthorpe M., Nieto-Sampedro M., Skaper S. D., Lewis E. R., Barbin G., Longo F. M., Cotman C. W., Varon S. Neuronotrophic activity in brain wounds of the developing rat. Correlation with implant survival in the wound cavity. Brain Res. 1983 May 9;267(1):47–56. doi: 10.1016/0006-8993(83)91038-7. [DOI] [PubMed] [Google Scholar]
- Mesulam M. M., Mufson E. J., Wainer B. H., Levey A. I. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6). Neuroscience. 1983 Dec;10(4):1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Mobley W. C., Rutkowski J. L., Tennekoon G. I., Buchanan K., Johnston M. V. Choline acetyltransferase activity in striatum of neonatal rats increased by nerve growth factor. Science. 1985 Jul 19;229(4710):284–287. doi: 10.1126/science.2861660. [DOI] [PubMed] [Google Scholar]
- Nieto-Sampedro M., Lewis E. R., Cotman C. W., Manthorpe M., Skaper S. D., Barbin G., Longo F. M., Varon S. Brain injury causes a time-dependent increase in neuronotrophic activity at the lesion site. Science. 1982 Aug 27;217(4562):860–861. doi: 10.1126/science.7100931. [DOI] [PubMed] [Google Scholar]
- Nieto-Sampedro M., Manthrope M., Barbin G., Varon S., Cotman C. W. Injury-induced neuronotrophic activity in adult rat brain: correlation with survival of delayed implants in the wound cavity. J Neurosci. 1983 Nov;3(11):2219–2229. doi: 10.1523/JNEUROSCI.03-11-02219.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojika K., Appel S. H. Neurotrophic effects of hippocampal extracts on medial septal nucleus in vitro. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2567–2571. doi: 10.1073/pnas.81.8.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panula P., Revuelta A. V., Cheney D. L., Wu J. Y., Costa E. An immunohistochemical study on the location of GABAergic neurons in rat septum. J Comp Neurol. 1984 Jan 1;222(1):69–80. doi: 10.1002/cne.902220107. [DOI] [PubMed] [Google Scholar]
- Pearson R. C., Sofroniew M. V., Cuello A. C., Powell T. P., Eckenstein F., Esiri M. M., Wilcock G. K. Persistence of cholinergic neurons in the basal nucleus in a brain with senile dementia of the Alzheimer's type demonstrated by immunohistochemical staining for choline acetyltransferase. Brain Res. 1983 Dec 19;289(1-2):375–379. doi: 10.1016/0006-8993(83)90046-x. [DOI] [PubMed] [Google Scholar]
- Satoh K., Armstrong D. M., Fibiger H. C. A comparison of the distribution of central cholinergic neurons as demonstrated by acetylcholinesterase pharmacohistochemistry and choline acetyltransferase immunohistochemistry. Brain Res Bull. 1983 Dec;11(6):693–720. doi: 10.1016/0361-9230(83)90013-8. [DOI] [PubMed] [Google Scholar]
- Schonfeld A. R., Heacock A. M., Katzman R. Enhancement of central cholinergic sprouting by prior injury: correlation with endogenous trophic content of hippocampus. Brain Res. 1984 Nov 12;321(2):377–380. doi: 10.1016/0006-8993(84)90197-5. [DOI] [PubMed] [Google Scholar]
- Schwab M. E., Otten U., Agid Y., Thoenen H. Nerve growth factor (NGF) in the rat CNS: absence of specific retrograde axonal transport and tyrosine hydroxylase induction in locus coeruleus and substantia nigra. Brain Res. 1979 Jun 8;168(3):473–483. doi: 10.1016/0006-8993(79)90303-2. [DOI] [PubMed] [Google Scholar]
- Seiler M., Schwab M. E. Specific retrograde transport of nerve growth factor (NGF) from neocortex to nucleus basalis in the rat. Brain Res. 1984 May 21;300(1):33–39. doi: 10.1016/0006-8993(84)91338-6. [DOI] [PubMed] [Google Scholar]
- Storm-Mathisen J. Choline acetyltransferase and acetylcholinesterase in fascia dentata following lesion of the entorhinal afferents. Brain Res. 1974 Nov 15;80(2):181–197. doi: 10.1016/0006-8993(74)90683-0. [DOI] [PubMed] [Google Scholar]
- Terry R. D., Davies P. Dementia of the Alzheimer type. Annu Rev Neurosci. 1980;3:77–95. doi: 10.1146/annurev.ne.03.030180.000453. [DOI] [PubMed] [Google Scholar]
- Thoenen H., Barde Y. A. Physiology of nerve growth factor. Physiol Rev. 1980 Oct;60(4):1284–1335. doi: 10.1152/physrev.1980.60.4.1284. [DOI] [PubMed] [Google Scholar]
- Varon S., Manthorpe M., Williams L. R. Neuronotrophic and neurite-promoting factors and their clinical potentials. Dev Neurosci. 1983;6(2):73–100. doi: 10.1159/000112334. [DOI] [PubMed] [Google Scholar]
- Varon S. Nerve growth factor and its mode of action. Exp Neurol. 1975 Sep;48(3 Pt 2):75–92. doi: 10.1016/0014-4886(75)90172-7. [DOI] [PubMed] [Google Scholar]
- Wainer B. H., Levey A. I., Rye D. B., Mesulam M. M., Mufson E. J. Cholinergic and non-cholinergic septohippocampal pathways. Neurosci Lett. 1985 Feb 28;54(1):45–52. doi: 10.1016/s0304-3940(85)80116-6. [DOI] [PubMed] [Google Scholar]
- Whitehouse P. J., Price D. L., Struble R. G., Clark A. W., Coyle J. T., Delon M. R. Alzheimer's disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982 Mar 5;215(4537):1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]