Abstract
The protease fibroblast activation protein (FAP) is a specific marker of activated mesenchymal cells in tumour stroma and fibrotic liver. A specific, reliable FAP enzyme assay has been lacking. FAP's unique and restricted cleavage of the post proline bond was exploited to generate a new specific substrate to quantify FAP enzyme activity. This sensitive assay detected no FAP activity in any tissue or fluid of FAP gene knockout mice, thus confirming assay specificity. Circulating FAP activity was ∼20- and 1.3-fold less in baboon than in mouse and human plasma, respectively. Serum and plasma contained comparable FAP activity. In mice, the highest levels of FAP activity were in uterus, pancreas, submaxillary gland and skin, whereas the lowest levels were in brain, prostate, leukocytes and testis. Baboon organs high in FAP activity included skin, epididymis, bladder, colon, adipose tissue, nerve and tongue. FAP activity was greatly elevated in tumours and associated lymph nodes and in fungal-infected skin of unhealthy baboons. FAP activity was 14- to 18-fold greater in cirrhotic than in non-diseased human liver, and circulating FAP activity was almost doubled in alcoholic cirrhosis. Parallel DPP4 measurements concorded with the literature, except for the novel finding of high DPP4 activity in bile. The new FAP enzyme assay is the first to be thoroughly characterised and shows that FAP activity is measurable in most organs and at high levels in some. This new assay is a robust tool for specific quantitation of FAP enzyme activity in both preclinical and clinical samples, particularly liver fibrosis.
Keywords: Fibroblast, Dipeptidyl peptidase, Protease substrates, Protease activity, Liver disease, Fibrosis, Biomarker
Abbreviations: ALD, alcoholic liver disease; AMC, amino-4-methylcoumarin; DPP4, dipeptidyl peptidase 4; DMSO, dimethyl sulfoxide; EDTA, ethylene diamine tetra acetic acid; FAP, fibroblast activation protein-α; gko, gene knock out; HCV, hepatitis C virus; het, heterozygous; LDS, lithium dodecyl sulphate; LN, lymph node; mAb, monoclonal antibody; ND, non-diseased; PBC, primary biliary cirrhosis; PBMC, peripheral blood mononuclear cells; PBS, phosphate-buffered saline; PEP, prolyl endopeptidase; PVDF, polyvinylidene fluoride; STLV, simian T-cell lymphotrophic virus; wt, wild type; yrs, years
Graphical abstract
Highlights
-
•
A novel synthetic fluorogenic substrate is proven to be FAP-specific.
-
•
Mice have higher levels of circulating FAP activity compared to baboons or humans.
-
•
No FAP activity was detected in urine or bile but bile contained high DPP4 activity.
-
•
FAP activity is greatest in pancreas, uterus, salivary gland, skin and lymph node.
-
•
FAP activity and protein is elevated in both serum and liver in human liver disease.
1. Introduction
Proteases are increasingly recognised as important regulatory molecules [1]. Fibroblast activation protein (FAP) belongs to the S9 family of proteases, which also contains the similar enzymes dipeptidyl peptidase 4 (DPP4), DPP8, DPP9 and prolyl endopeptidase (PEP) [2]. All of these enzymes share the unique ability to cleave the post proline bond, which is usually resistant to degradation. Recently, this enzyme family has stimulated great pharmaceutical interest, as DPP4 inhibitors are a successful therapy for type 2 diabetes [3] and have the potential to treat other conditions [4,5].
FAP is a constitutively active serine protease that exists as a dimer both on the cell surface and in a soluble, circulating form in the blood. FAP can hydrolyse both dipeptidase and endopeptidase substrates, which include natural X-Pro-containing bioactive peptides [6] and denatured collagen [7,8] and α2-antiplasmin [9,10]. It is thought that FAP is generally absent from normal adult tissue but has increased expression during embryogenesis [11], tumourigenesis [12–14], tissue damage and wound healing, fibrosis [7,15] and inflammation [16,17]. As FAP is up-regulated in stromal fibroblasts of over 90% of malignant epithelial tumours but not in benign tumours [18], it has become a potential biomarker and therapeutic target for tumour stroma [19–21].
DPP4 is related transmembrane dimeric glycoprotein that cleaves the post proline bond but acts only as a dipeptidyl peptidase. It is a ubiquitous enzyme, found on most epithelial cells, especially in liver, kidney and gut, on capillary endothelial cells in most organs and on most lymphocytes in immune organs such as thymus, spleen and lymph node [22–25]. In contrast to FAP, many natural DPP4 substrates are known, including gastric hormones [26], neuropeptides [27,28], and chemokines [29,30]. Soluble DPP4 is present in plasma, serum, seminal and synovial fluids, as has been reviewed [22,31] and soluble DPP4 levels are associated with a variety of human conditions such as psoriasis [32], chronic fatigue [33], tuberculosis [34] and hepatitis C virus (HCV) [35,36]. Circulating DPP4 activity is elevated in some tumours [37,38] but reduced in others [39–41], so the level may be tumour-type dependent. It has also been reported to be both increased [42,43] and decreased [44,45] in type 2 diabetes patients. As with FAP, the regulatory process or sheddase activity by which the soluble form of the enzyme is released from the cell is unknown.
In contrast to DPP4, little is known about the normal physiological function of either cellular or circulating FAP. Specifically identifying both the source and activity of soluble FAP has been difficult due to its dual enzyme activity, and to date all FAP synthetic substrates are also hydrolysed by DPP4 and/or PEP [46]. Up to now, analysis of FAP content in tissue samples has relied on mRNA measurements [47,48] or the use of antibodies, where few are reliable for mouse FAP [49]. FAP has been targeted in cancer models using chemical inhibitors [50], antibodies [51], toxins [52], pro-drugs [19], T-cells [53] and RNA interference [54], but basal, endogenous FAP expression has only recently been examined in normal tissue [55,56] and this has challenged the dogma that FAP is only expressed in diseased tissue. Thus, FAP's basal expression pattern and normal physiological activity profile require close examination to better understand the role of this protease.
Our hypothesis is that FAP is present and active in measureable amounts in some normal tissues and the aim of the present study was to utilise a new FAP-specific substrate, 3144-AMC, to quantify FAP expression in mouse, baboon and human fluids and tissues. We sought to show that this assay is specific, sensitive and efficient at quantifying both the soluble and cell-bound forms of FAP from a range of tissues in three different mammalian species. The poor availability of fresh human tissue was overcome by obtaining an extensive range of fresh tissues from a closely-related primate, the baboon. We also quantified FAP activity in diseased baboon and human tissue and plasma as a preliminary exploration of its use as a biomarker, while DPP4 activity was measured as a comparator. FAP is an intriguing protein and the heavy focus on targeting it in cancer therapy needs to be reconciled with its normal physiological function, which is not fully understood. Here we delineate locations and relative quantities of FAP enzyme activity in a wide range of settings to develop an understanding of the roles of this unique enzyme.
2. Results
FAP is present in a soluble form in human plasma [10]. A novel FAP substrate, 3144-AMC [57], was used in this study to quantify FAP activity in non-diseased plasma from three species. During optimisation of this soluble assay we found that the greatest FAP activity came from volumes of mouse, baboon or human plasma of 0.5–1 μl (Fig. 1A–C), so 1 μl was used for all subsequent assays. Clear inverse dose responses were produced by plasma volumes that were greater than optimal. All samples tested for enzyme activity in mouse, baboon and humans are listed in Supplemental Tables 1–3, respectively.
Fig. 1.
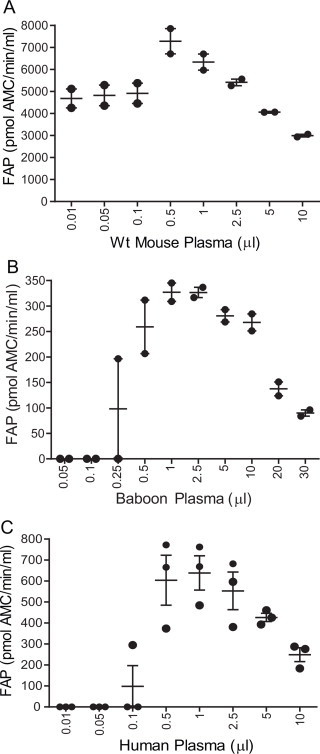
Volume optimization for circulating FAP activity assay. Determining an optimal volume of mouse (A), baboon (B) and human plasma (C) in FAP enzyme assays. The minimum volume was 5 μl of diluted or undiluted plasma. FAP activity was expressed as pmol AMC released from substrate 3144-AMC/min/ml. 1 μl was chosen as an optimal volume for fluids of all species. Horizontal bars are means and error bars depict SEM. n = 2 (A and B) or 3 (C).
The second aim was to verify the specificity of substrate 3144-AMC for FAP, using FAP-deficient samples including plasma from FAP gene knockout (gko) mice [58] and plasma from humans carrying a FAP gene variant [59]. Similar quantities of FAP activity were detected in wt and DPP4 gko mouse plasma (Fig. 2A), which is consistent with our previous data showing no compensatory change in FAP expression in liver when DPP4 is absent [60]. In contrast, FAP gko mouse plasma had no detectable activity while heterozygous (het) mice had reduced FAP enzymatic levels (Fig. 2A). We separately identified FAP-deficient individuals expressing a Ser363Leu variant FAP protein that lacks functional FAP activity [59]. Plasma FAP activity levels from the heterozygous person (FAP+/−) were less than half that of healthy volunteers, whereas neither of the two FAP Ser363Leu homozygous individuals (FAP−/−) had detectable FAP activity (Fig. 2B). The four points on the graph for the mutation-affected individuals are from four separate blood draws over a 3-h period post glucose bolus ingestion. All FAP activity data for mouse, baboon and human fluids is given in Supplemental Table 4.
Fig. 2.
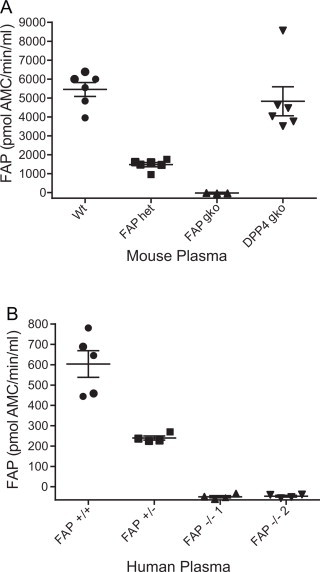
Specificity of substrate 3144-AMC for circulating FAP. FAP activity was assayed in 1 μl of plasma from (A) mice, (n = 3–6) from four genetic backgrounds and (B) healthy adult humans (FAP+/+, n = 5), a heterozygous FAP activity-deficient individual (FAP+/−) and two separate homozygous individuals (FAP−/− 1, FAP−/− 2). FAP activity was expressed as pmol AMC released from substrate 3144-AMC/min/ml. Horizontal bars are means and error bars depict SEM.
3144-AMC was then used to assess FAP activity in other primate fluids. Enzymatic levels in baboon plasma and serum were approximately 300 pmol AMC/min/ml but no detectable FAP activity was present in baboon urine or bile from three animals (Fig. 3A), despite various volumes of both fluids (0.1–50 μl) being tested (data not shown). Although there was ∼2-fold variation between individuals, humans had greater average FAP activity than baboons. Interestingly, it was found that FAP activity levels were equal between plasma and serum in both baboons and humans (Fig. 3A), indicating that the presence of platelets, fibrinogen and other clotting factors do not affect this FAP assay. Subsequent FAP graphs contain average data for plasma and serum if both were analysed from one individual. The activity levels of DPP4 in these samples were then measured using the fluorogenic substrate H-Gly-Pro-AMC. Baboon plasma and serum had DPP4 activity of ∼2785 pmol AMC/min/ml and human plasma and serum had similar levels to baboon (Fig. 3B). Baboon urine had barely detectable DPP4 activity, whereas bile had high levels. Further examination of bile from three species showed that mice had higher levels of bile DPP4 activity than a mixed cohort of baboons, with human bile from liver transplant recipients having the least DPP4 activity (one-way ANOVA p-value = 0.0024; Fig. 3C). Both soluble FAP and DPP4 quantitation was unaffected by 12 freeze/thaw cycles of plasma (Supplemental Fig. 1). All DPP4 activity data for mouse, baboon and human fluids is given in Supplemental Table 5.
Fig. 3.
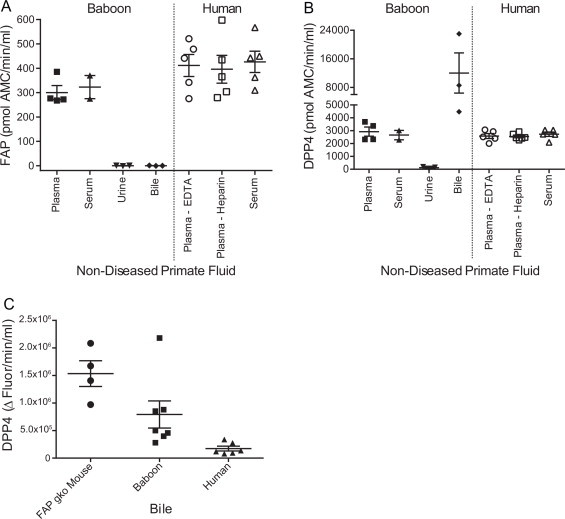
Quantitation of FAP and DPP4 activity in fluids. An optimal volume of 1 μl of each fluid was used to examine FAP (A) and DPP4 (B, C) levels in plasma, serum, urine and bile. (C) DPP4 activity in bile from non-diseased FAP gko mice (n = 4), a mixed cohort of baboons (n = 7) and human cirrhotic liver transplant recipients (n = 6). Horizontal bars are means and error bars depict SEM.
A direct comparison shows that circulating FAP activity levels differ between species, with wt mice having approximately 19- and 15-fold greater FAP activity than baboons and humans, respectively (Fig. 4A). Baboon and human plasma, with average FAP activity levels of 315 and 404 pmol AMC/min/ml, respectively, are comparable. Total plasma protein quantitation in all three species was in line with published values, with protein concentration for primate and mouse plasma at ∼70 and ∼42 mg/ml, respectively (data not shown). DPP4 enzymatic levels were then measured in these fluids and wt and FAP gko mice showed equal levels of plasma DPP4 activity while DPP4 gko mice had little activity (Fig. 4B). The average DPP4 activities in baboon and human plasma were similar, at ∼3100 and ∼2590 pmol AMC/min/ml, respectively (Fig. 4B). In contrast to FAP, where mice exhibited the highest circulating activity levels, serum DPP4 activity was greater in primates than in mice (p = 0.0012 for human DPP4 compared to wt mouse).
Fig. 4.
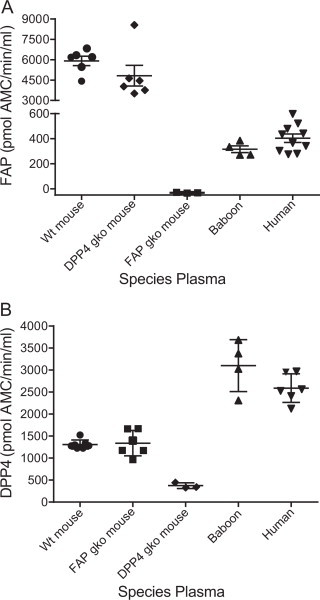
Species differences in circulating FAP and DPP4 activity. (A) FAP enzyme activity in plasma from mouse, baboon and human. (B) DPP4 enzyme levels in the same set of samples. Horizontal bars are means and error bars depict SEM.
The differing activity levels of soluble FAP across species prompted an investigation into protein sequence similarities. The predicted baboon FAP protein sequence was aligned with that of human, mouse, chimpanzee and rat (Supplemental Fig. 2). Human FAP was calculated to be 99.61% identical to baboon FAP and 89.61% identical to mouse FAP by ClustalW analysis. Considering the enzymatic hydrolase domain alone, the identity between human FAP and that of baboon and mouse rose to 100% and 92.61%, respectively.
Having shown that 3144-AMC can specifically detect circulating FAP, the aim was then to examine tissue-derived FAP activity in organ homogenates. FAP activity was measured in previously homogenised [25] organs from wt and FAP gko mice (Fig. 5). Initial examination showed greatest FAP activity in mouse uterus, pancreas, submaxillary gland and skin, followed by lymph node, ovary, skeletal muscle, adrenal and bone marrow. Greater numbers of these high-FAP organs were then analysed and uterus, pancreas and submaxillary gland remained the three organs in which FAP activity was most abundant (Fig. 5). Notably, these high-FAP organs showed no enzyme activity in the FAP gko mouse (Fig. 5). Indeed, no FAP enzyme activity was detected in any organ from the FAP gko mice (data not shown), which provided additional surety of its specificity. Organs with the least FAP activity included prostate, peripheral blood mononuclear cells (PBMCs) and red blood cells as well as the large vital organs such as brain, heart, liver and lung. All enzyme activity data for mouse, baboon and human organ homogenates are in Supplemental Table 6. DPP4 levels in these mouse organs have been published [25].
Fig. 5.
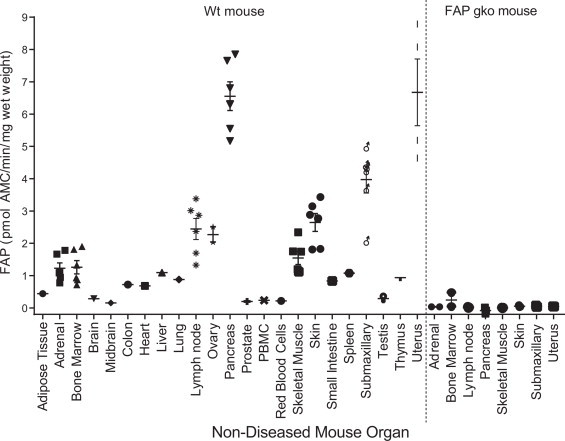
Specificity of substrate 3144-AMC for tissue-derived mouse FAP. FAP activity was measured in organ homogenates from wt (n = 1–6) and FAP gko (n = 2–3) mice. Horizontal bars are means and error bars depict SEM. PBMC = peripheral blood mononuclear cells.
To investigate other mammals, non-diseased baboon and human organs were assayed for FAP activity. Variability among baboons was evident; however FAP activity was high in skin, similar to mouse and also high in baboon epididymis, bladder, adipose tissue and nerve (Fig. 6A). The large vital organs such as heart, kidney, liver and lung were shown to be lacking in FAP activity, as was observed in mice. Human adipose (subcutaneous and omental) and human liver tissue were analysed alongside baboon organs for direct comparison (Fig. 6A). Human adipose contained less FAP activity than baboon adipose, but the variability amongst baboon adipose was great. Human liver had low FAP activity (Fig. 6A), similar to baboon and mouse liver.
Fig. 6.
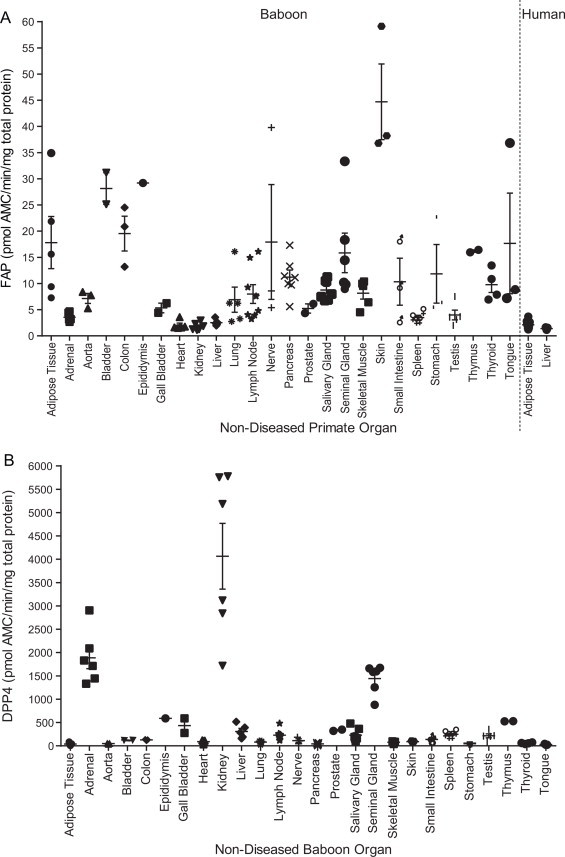
Quantitation of tissue-derived baboon and human FAP and DPP4 activity. (A) FAP enzyme activity was quantified in organ homogenates derived from non-diseased baboons and humans, showing highest FAP levels in baboon skin, bladder, epididymis, tongue and nerve. (B) DPP4 activity levels in the same baboon organs, showing highest DPP4 levels in baboon kidney, adrenal, seminal gland, epididymis and thymus. Horizontal bars are means and error bars depict SEM.
All of these baboon organs were then tested for DPP4 activity (Fig. 6B). As expected, [22], DPP4 activity was most abundant in kidney, followed by adrenal, seminal gland, thymus and epididymis, while tongue, adipose, pancreas and aorta contained the least DPP4 activity. Some baboon organs were from different regions: (a) adipose came from epididymal, pericardial, subcutaneous, urogenital and visceral regions, (b) lymph nodes were from the neck and mesentery, (c) parotid and submaxillary salivary glands, (d) skeletal muscle included bicep, gastrocnemius and gluteus muscles and (e) small intestine included duodenum and jejunum. In each of these five organs, FAP and DPP4 enzyme activity levels were not significantly different between regions and thus activity data from each are presented together as a single organ type.
Assured of the specificity of 3144-AMC, we then sought to use this substrate in a FAP biomarker assay in altered or diseased states. Examining mice, no significant FAP activity differences were detected in blood drawn in the morning or the evening or during pregnancy (Fig. 7A). Circulating FAP activity levels in the plasma/serum of all available baboons showed that the diseased and non-diseased baboons were not significantly different (Fig. 7B), however one diseased male animal, with bronchitis and enlarged lymph nodes, had significantly more circulating FAP activity than all the others. The diseased cohort were simian T-cell lymphotropic virus (STLV)-positive and were all euthanased when very ill, often following prolonged weight loss, along with other chronic or complicating factors. There was significantly less circulating FAP activity in the diabetic baboons than in the non-diseased animals (p-value = 0.0317) (Fig. 7B).
Fig. 7.
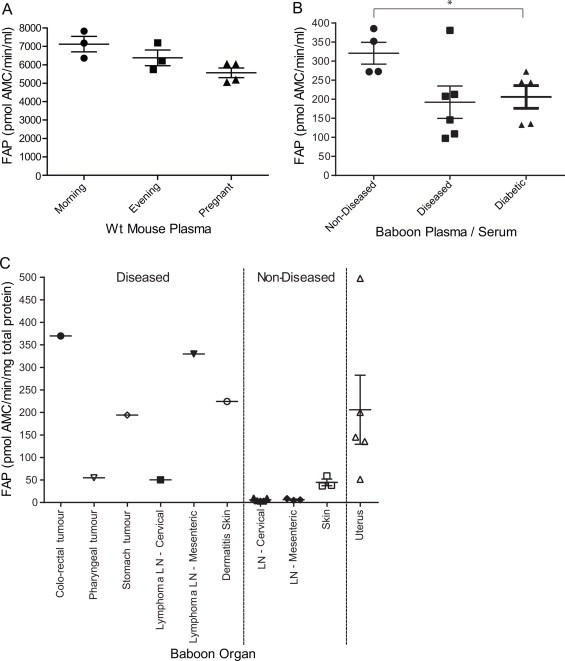
FAP enzyme activity in pregnancy, time of day and disease. (A) Circulating mouse FAP activity. (B) Circulating baboon FAP activity. (C) Tissue-derived FAP activity from baboon tumour mass and related lymph nodes (LN), skin and uterus from diseased baboons compared with non-diseased. FAP activity is expressed as pmol AMC released from substrate 3144-AMC/min/ml (fluids) or per mg of total protein (organs). Horizontal bars are means and error bars depict SEM. *Denotes p-value < 0.05.
As FAP is known to be elevated in solid tumours and other diseases involving tissue damage, specific enzyme activity was then examined in selected diseased baboon organs (Supplemental Table 2). A 19-year old female baboon was euthanased due to a large colorectal tumour, which proved to be high in FAP activity (Fig. 7C). Similarly, a diseased 13-year old male baboon was euthanased due to chronic weight loss from a T-cell lymphoma. Two tumour masses on his pharynx and stomach contained high FAP activity (Fig. 7C). In addition, cervical lymph nodes near the pharyngeal tumour mass had approximately 8-fold more FAP activity than non-diseased cervical lymph nodes and mesenteric lymph nodes near the stomach tumour had 52-fold greater FAP activity than non-diseased mesenteric lymph nodes. The affected skin of an 18-year old female baboon with a chronic dermatophyte infection showed 5-fold more FAP activity compared to non-diseased skin (Fig. 7C). The uterus samples were all from diseased females of ages 10, 12, 18, 19 and 21 and all showed variable but high FAP activity levels (Fig. 7C), as was observed in mice (Fig. 5).
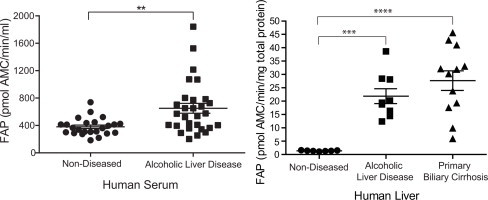
Finally, human liver disease was examined (Fig. 8). FAP activity in serum from patients with alcoholic liver disease (ALD) (Supplemental Table 7) was significantly increased compared to non-diseased (ND) sera (Fig. 8A; p-value = 0.0024). This cirrhotic cohort showed no significant difference in DPP4 serum activity (data not shown), highlighting the important role of FAP in liver disease. FAP enzyme activity was also significantly increased in the liver homogenates of patients with two aetiologies of chronic liver disease with the p-value for ALD and primary biliary cirrhosis (PBC) of 0.0002 and <0.0001, respectively, compared to non-diseased (ND) livers (Fig. 8B).
Fig. 8.
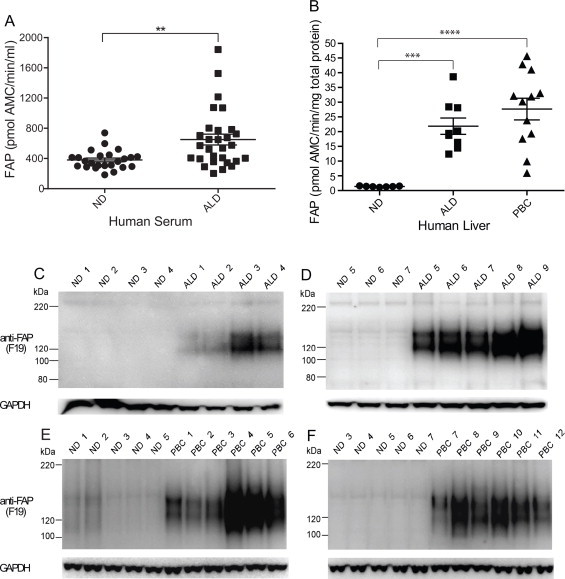
Elevated FAP activity and protein in human liver disease. (A) Circulating FAP activity in patients with alcoholic liver disease (ALD) compared to age-, gender- and alcohol intake-matched non-diseased (ND) patients. **Denotes p-value < 0.01. (B) FAP activity in non-diseased (ND) and cirrhotic liver explants from patients ALD or PBC. ***,****Denotes p-value < 0.001 and <0.0001, respectively. (C–F) FAP protein in ALD and PBC human livers. Unreduced/unboiled 3–8% Tris-Acetate SDS–PAGE with Western blot analysis using anti-FAP monoclonal antibody, F19 showed FAP bands at 120 and 150 kDa. GAPDH served as a loading control.
The increased FAP activity detected in human liver homogenates by enzyme assays with 3144-AMC concorded with Western blot data using F19 antibody (Fig. 8C–F). On unreduced and unboiled SDS–PAGE Western blot, F19 detected FAP bands at 150 and 120 kDa in ALD and PBC livers which were very faint or absent from non-diseased (ND) livers. Densitometry analysis showed that FAP protein levels were more than 30-fold greater in ALD livers and approximately 15-fold greater in PBC livers compared to non-diseased livers.
3. Discussion
FAP has been extensively studied in the cancer field but has been difficult to reliably quantify in normal tissues. In this study, we showed that the novel FAP substrate, 3144-AMC, can form the basis of a specific and sensitive assay to quantify endogenous FAP enzyme activity in diverse mammalian fluids and organs. We proved assay specificity by showing no activity in any tissue or fluid from FAP-deficient mice and humans. Organs rich and poor in FAP activity were identified and inter-species differences observed. The diagnostic potential of this FAP assay was also explored.
Most previously published data has indicated that FAP is not detectable in normal adult tissue, but readily detectable only in embryos, tumours, fibrosis and sites of chronic injury and wound healing [61]. Here, we have shown that FAP is indeed present and active in normal adult mammalian organs. As previously published, FAP is active in mouse pancreas [62], but is also active in uterus, submaxillary gland and skin. The high FAP activity in skin is likely due to expression by skin fibroblasts and, while the high FAP activity in baboon lymph node and ovary is novel, it is poorly understood in terms of activated mesenchymal cell expression. Some organs showed differential activity between the species. Adipose tissue had more FAP activity in baboon than human, whereas pancreas had the most FAP activity in mouse. The high FAP protein sequence identity across the three species suggests that measurements of FAP activity likely reflect the concentration of FAP protein molecules present. However, supporting studies using purified recombinant protein from all three species are required to confirm this.
Skin, uterus and diseased liver are FAP-active organs of particular interest. While non-diseased skin was itself high in FAP activity, the chronic inflammation and skin regeneration caused by a dermatophyte infection may have caused the increased FAP activity in the skin of baboon Gi. The high uterine FAP activity may result from the tissue remodelling and angiogenesis [63] of oestrus cycle-driven regeneration. The increased FAP expression in diseased liver, which was shown here to be enzymatically active, may be pro-fibrotic [15,61] and our assays also showed elevated circulating FAP activity in ALD patients, suggesting that FAP may become a serum biomarker for liver cirrhosis.
The cellular origin of soluble FAP is unknown. Some cells may translate it specifically for secretion or it could be shed from the surface of certain cells depending upon the local concentration of an appropriate sheddase. In contrast to DPP4, circulating FAP activity was 15- to 20-fold greater in mice than in primates, suggesting that mice may have greater expression of such a sheddase. The origin of soluble DPP4 is also unknown, but is thought to be shed from many cells including lymphocytes [22], hepatocytes [64] and adipocytes [65]. The important species differences between soluble FAP and DPP4, as well as their potential sheddases, should be further investigated and may be exploited in the selective targeting of each enzyme in murine or human studies.
The DPP4 activity assay data aligns with the literature. However, the high level of DPP4 activity observed in bile is novel and consistent with the potential of DPP4 as a liver injury biomarker [36,66,67], suggesting that circulating DPP4 may be most elevated in diseases involving impaired bile excretion, such as cholestasis. In a non-diseased setting, hepatocytes express DPP4 on their biliary surface [24] and thus can shed the enzyme into the bile but the loss of cell polarity by damaged hepatocytes in cirrhotic liver [68] may cause some hepatocyte-derived DPP4 to enter the blood rather than the bile.
The inverse FAP activity dose response that occurred with greater than optimal plasma volumes may indicate that there is a fluorophore quencher or a natural FAP inhibitor that is more active at higher plasma concentrations, or perhaps that the substrate is sequestered in plasma/serum. Equal levels of FAP and DPP4 in plasma compared to serum suggest that both the proteases and any potential inhibitors are not depleted by the blood clotting process. This is an important observation as FAP-mediated cleavage of α2-antiplasmin increases its activity, which inhibits fibrinolysis [10,69].
Whether elevated FAP in tumour tissue is reflected in matched serum samples is of diagnostic interest. We observed high cellular FAP activity in baboon tumour masses as well as in nearby lymph nodes but the animal with stomach and pharyngeal tumours had one of the lowest circulating FAP measurements. This concords with prior reports that circulating FAP levels are lower in cancer patients [40,70]. In contrast, FAP activity was increased in both the serum and the liver of patients with chronic liver disease, which aligns with previous studies showing up-regulation of FAP in cirrhotic human liver [7,71]. Such data underscores the need to measure both tissue and circulating forms of FAP in disease studies and, given that soluble FAP is in a functional and active form, its role outside the cell needs to be considered as this information may influence outcomes of FAP-targeted interventions.
Finally, we explored the diagnostic potential of this assay and showed that it is a sensitive, simple, robust and specific system to measure elevated soluble or cellular FAP activity in disease states. Only a very small volume of fluid is required, making it useful in clinical or preclinical settings with limited sample resources and the enzyme activity was preserved over numerous freeze–thaw events, suggesting that both fresh and archived samples can provide accurate data.
The importance of FAP in epithelial cancers and wound healing is established and now these new data indicate that measurable FAP activity is in resting states also. The most useful measure of a protease is by its enzyme activity and we are now equipped to detect FAP in a sensitive, accurate and specific manner. In doing so, we can gain a greater understanding of the role and diagnostic and therapeutic potential of FAP.
4. Materials and methods
4.1. Reagents
Substrate 3144-aminomethylcoumarin (AMC) is a low molecular weight fluorogenic substrate [57]. The lyophilised powder was reconstituted in dimethyl sulfoxide (DMSO) to a stock concentration of 10 mM and aliquoted for storage at −20 °C. 30 μl used per well of a working solution of 0.5 mM in PBS gave a final substrate concentration of 150 μM per assay. Substrate H-Gly-Pro-AMC (Mimotopes, VIC, Australia and Bachem, Switzerland) detects DPP4 preferentially in non-reducing conditions at pH 7.8 and room temperature. This substrate was prepared to a stock concentration of 2 mM in 10% methanol in Tris-EDTA buffer [25]. 50 μl of substrate was added per well to give a final assay substrate concentration of 1 mM. A stock concentration of 5 mM AMC standards (Mimotopes, VIC, Australia) in DMSO was prepared and stored at 4 °C. From a 20 μM working solution in PBS, 30 μl was added to 70 μl of PBS to create the first AMC standard of 600 pmol AMC. Doubling dilutions were made in PBS in 96-well plates to prepare a linear standard curve of AMC fluorescence, from which all samples were interpolated.
4.2. Sample collection and preparation
All fluids and organs tested for mouse, baboon and human are summarised in Supplemental Tables 1–3, respectively. Mouse: C57Bl/6 mouse blood was drawn into EDTA- or heparin-coated collection tubes and cells were removed by centrifugation to collect clear yellow plasma. For serum, blood was collected in clotting tubes before centrifugation. Bile was collected from gall bladders using a 25 gauge needle and brief centrifugation. C57Bl/6 mouse organ collection and homogenisation have been described previously [25] and all mouse studies conformed to Australian National Health and Medical Research Council (NHMRC) regulations and were approved by the University of Sydney Animal Ethics Committee. Baboon: Papio hamadryas baboon tissue and fluid samples from the Australian National Baboon Colony were collected at autopsy after euthanasia in accordance with the Australian NHMRC guidelines under Sydney Local Health District Animal Welfare Committee approvals. Fluids were prepared as described for mice above. The veterinary post-mortem and histopathology reports are summarised in Supplemental Table 2. Type 1 diabetes was induced in these animals as described previously [72]. Human: Non-diseased human serum/plasma derived from male and female local volunteers aged between 21 and 55. The plasma from FAP Ser363Leu variant individuals was obtained with written informed consent and was in accordance with the Declaration of Helsinki protocols and approved by the local institution [59]. Human bile (n = 6) was collected from explant gall bladders of liver transplant recipients. Sera from 29 alcoholic liver cirrhotic patients (age range 33–71 and three quarters male) were compared with control samples from heavy drinkers without liver disease and matched for gender, age and alcohol consumption (Supplemental Table 4). Human cirrhotic liver tissues were explant biopsies obtained from liver transplant recipients, all at end-stage Child-Pugh class C cirrhosis with published pathology parameters [73]. ALD patients had an average age 49.3 ± 8 (range 34–60; 9 males) and PBC patients had an average age 51.7 ± 13.3 (range 27–67; 10 females and 2 males). Non-diseased liver donors had an age range of 6–58 and mixed genders, as described previously [7,73]. All samples from humans were obtained after written informed consent and in accordance with Australian NHMRC guidelines under Sydney Local Health District Human Research Ethics Committee (HREC) approvals and were in accordance with the Helsinki Declaration of 1975.
Tissue Lysis: Fresh frozen tissue was homogenized in ice-cold lysis buffer: 50 mM Tris-HCl pH 7.6, 1 mM EDTA, 10% glycerol, 1% Triton-X114, with complete protease inhibitors (Roche, Basel, Switzerland). Protease inhibitors are required to preserve FAP enzyme activity by preventing proteolytic breakdown during cell lysis. Lysis buffer was added at a ratio of 1:10 wet weight:total volume and homogenised using a PT2100 Polytron homogeniser (Kinematica, Switzerland). Samples were then centrifuged at 14,000 rpm at 4 °C for 20 min and the soluble supernatant was transferred into a fresh tube, taking care to avoid the lipid layer. Total protein in baboon and human tissue lysates was quantified using the BCA assay (Thermo Scientific, Waltham, MA, USA). FAP activity was calculated as pmol AMC released from the 3144-AMC substrate per min per mg of total protein (baboon, human). Mouse organ homogenates were not quantified for total protein concentration [25], so FAP activity was calculated as pmol AMC/min/mg wet weight of tissue.
4.3. Enzyme assays
Black 96-well plates (Greiner Bio-one, Germany) contained duplicate AMC standards ranging from 600 to 0 pmol with fluid and organs assayed in triplicate. All fluid samples were added at a minimum volume of 5 μl. For volumes below 5 μl, pre-dilutions were made. Specifically, 5 μl of a 1/100, 1/50, 1/10, 1/5 and 1/2 dilution gave a total plasma volume of 0.05, 0.1, 0.5, 1.0, 2.5 μl, respectively. After optimisation, 5 μl of a 1/5 dilution of plasma/serum was routinely assayed. Negative control/blank wells contained lysis buffer or PBS for organ homogenates or plasma, respectively, along with substrate. The plate was read in a Polarstar plate reader (BMG Labtech, Germany) at excitation of 355 nm and emission of 450 nm every 5 min for 1 h at 37 °C (FAP assay) or every 2 min for 30 min at 24 °C (DPP4 assay).
For FAP Enzyme Activity Assay of baboon and human tissue samples, the volume required to give 100 μg of total protein was added per well. For mouse tissue samples, 10 μl of tissue lysate was added per well. In all cases, the volume was made to a total of 70 μl with PBS before adding 30 μl of 500 μM substrate 3144-AMC to detect soluble, intracellular and membrane bound forms of active FAP from tissue lysates. The limit of detection of this substrate on recombinant human FAP (R&D Systems, Minneapolis, USA) was 15 pg, which is equivalent to 0.3 pmol AMC/min/ml. Replicate analysis showed inter- and intra-assay coefficient of variation of 19.7 ± 8.35% and 6.2 ± 3.5%, respectively for the soluble human FAP activity assay.
For DPP4 Enzyme Activity Assay of baboon tissue samples, the volume required to give measureable DPP4 activity was optimized as follows: total protein amounts of 10 μg (adrenal and kidney), 20 μg (spleen), 30 μg (seminal gland), 40 μg (gall bladder, small intestine, skeletal muscle and pancreas), 50 μg (bladder, colon, liver, lung, lymph node, ovary, prostate, salivary gland, stomach, testis, thymus and thyroid), 60 μg (epididymis and heart) and 80 μg (aorta, adipose, nerve, skin, tongue) were added per well. In all cases, the volume was made to a total of 50 μl with PBS before adding 50 μl of 2 mM H-Gly-Pro-AMC (Mimotopes, Australia and Bachem, Switzerland).
4.4. Bile assay
5 μl of a 1/5 dilution of bile was added to 45 μl of TE buffer and 50 μl of H-Ala-Pro-AFC (Bachem, Switzerland). Fluorescence generated was measured at excitation 400 nm and emission 510 nm and DPP4 levels were expressed as the change in fluorescence over time (Δ Fluor/min/ml).
4.5. Data analysis
Using the BMG plate reader software (Omega Mars, BMG Labtech, Germany), the values for each well were blank-corrected and triplicates were averaged. The standard curve for fluorescence of AMC standards was linear for the concentration range of 0–600 pmol AMC and there was no change in fluorescence over the time-course of the assay. All samples were then interpolated from within this range and enzyme activity was expressed as pmol AMC released per min. This value was then corrected for volume of plasma/serum, per mg of total protein (baboon and human) or per mg of wet weight of tissue (mouse). Statistical analysis and graphing including standard curves used GraphPad Prism 6. Data were analysed by unpaired t-test (Mann–Whitney) and significance was assigned to p-values less than 0.05.
4.6. Multiple sequence alignment
The FAP protein sequences for human (Q12884), chimpanzee (H2QIW2), mouse (P97321) and rat (Q8R492) were obtained from www.uniprot.org. The baboon genome is undergoing sequencing (https://www.hgsc.bcm.edu/non-human-primates/baboon-genome-project), so the FAP protein sequence predicted from the gene sequence from the olive baboon (Papio anubis) was obtained from NCBI (Gene ID:101023556). All five sequences were aligned by ClustalW (http://www.ebi.ac.uk/Tools/msa/clustalw2/) and pair wise identity scores for both the full length protein and the enzymatic hydrolase domain alone were calculated.
4.7. Human liver Western blotting
Frozen human liver tissues were weighed and homogenized on ice as above. Protein was quantified and incubated on ice for 10 min in the presence of 1× lithium dodecyl sulphate (LDS) sample buffer (Invitrogen, Carlsbad, CA, USA) prior to gel loading. Protein samples were subjected to unreduced and unboiled 3–8% Tris-Acetate SDS–PAGE at 150 V for 60 min in 1× Tris-Acetate SDS–PAGE running buffer (NuPAGE, Invitrogen), then transferred to a polyvinylidene fluoride (PVDF) membrane (Schleicher & Schull, Dassel, Germany) and immunoblotted with mouse anti-FAP monoclonal antibody (mAb), F19. After detection of FAP protein, membranes were blocked overnight and re-probed with anti-GAPDH mAb. Densitometry analysis used the program ImageJ.
Declaration
Professor William W. Bachovchin is a director of Arisaph Pharmaceuticals Inc., which provided substrate 3144-AMC.
Acknowledgements
This work was supported by grants from the Australian National Health and Medical Research Council (NHMRC) (GWM and MDG), the Rebecca L. Cooper Medical Research Foundation (MDG), Perpetual Trustees (MDG), the Australian Centre for HIV and Hepatitis Virology Research (MDG), University of Sydney (MDG) and National Institutes of Health (WWB). We thank Arisaph Pharmaceuticals Inc for substrate 3144-AMC, Louise Cooney for human adipose tissue collection and Kaitlyn Preece, Suzanne Pears and Scott Heffernan for technical assistance. We also acknowledge the Juvenile Diabetes Research Foundation, and NHMRC for financial support of the National Baboon Colony.
Grant Numbers and Sources of Support: MDG - NHMRC 512282 (Sydney); GWM – NHMRC 571408 (Sydney) and WWB – NIH R01 CA163930–01A1 (Boston).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplementary material
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.fob.2013.12.001.
Appendix. Supplementary Materials
Supplementary materials for Quantitation of fibroblast activation protein (FAP)-specific protease activity in mouse, baboon and human fluids and organs.
References
- 1.Turk B., Turk D., Turk V. Protease signalling: the cutting edge. EMBO J. 2012;31:1630–1643. doi: 10.1038/emboj.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorrell MD., Park J.E. Fibroblast activation protein alpha. In: Rawlings N.L., Salvesen G., editors. third ed. Elsevier; San Diego: 2013. pp. 3395–3401. (Handbook of Proteolytic Enzymes). [Google Scholar]
- 3.Keane F.M., Chowdhury S., Yao T.-W., Nadvi N.A., Gall M.G., Chen Y., Osborne B., Vieira de Ribeiro A.J., Church W.B., McCaughan G.W. Targeting dipeptidyl peptidase-4 (DPP-4) and fibroblast activation protein (FAP) for diabetes and cancer therapy. In: Dunn B., editor. Proteinases as Drug Targets. Royal Society of Chemistry; Cambridge, UK: 2012. pp. 119–145. [Google Scholar]
- 4.Gorrell M.D. Dipeptidyl peptidase IV and related enzymes in cell biology and liver disorders. Clin. Sci. (Lond) 2005;108:277–292. doi: 10.1042/CS20040302. [DOI] [PubMed] [Google Scholar]
- 5.Kim Y.O., Schuppan D. When GLP-1 hits the liver: a novel approach for insulin resistance and NASH. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;302:G759–G761. doi: 10.1152/ajpgi.00078.2012. [DOI] [PubMed] [Google Scholar]
- 6.Keane F.M., Nadvi N.A., Yao T.-W., Gorrell M.D. Neuropeptide Y, B-type natriuretic peptide, substance P and peptide YY are novel substrates of fibroblast activation protein-α. FEBS. J. 2011;278:1316–1332. doi: 10.1111/j.1742-4658.2011.08051.x. [DOI] [PubMed] [Google Scholar]
- 7.Levy M.T., McCaughan G.W., Abbott C.A., Park J.E., Cunningham A.M., Muller E., Rettig W.J., Gorrell M.D. Fibroblast activation protein: a cell surface dipeptidyl peptidase and gelatinase expressed by stellate cells at the tissue remodelling interface in human cirrhosis. Hepatology. 1999;29:1768–1778. doi: 10.1002/hep.510290631. [DOI] [PubMed] [Google Scholar]
- 8.Park J.E., Lenter M.C., Zimmermann R.N., Garin-Chesa P., Old L.J., Rettig W.J. Fibroblast activation protein, a dual specificity serine protease expressed in reactive human tumor stromal fibroblasts. J. Biol. Chem. 1999;274:36505–36512. doi: 10.1074/jbc.274.51.36505. [DOI] [PubMed] [Google Scholar]
- 9.Lee K.N., Jackson K.W., Christiansen V.J., Chung K.H., McKee P.A. A novel plasma proteinase potentiates alpha2-antiplasmin inhibition of fibrin digestion. Bloodd. 2004;103:3783–3788. doi: 10.1182/blood-2003-12-4240. [DOI] [PubMed] [Google Scholar]
- 10.Lee K.N., Jackson K.W., Christiansen V.J., Lee C.S., Chun J.G., McKee P.A. Antiplasmin-cleaving enzyme is a soluble form of fibroblast activation protein. Blood. 2006;107:1397–1404. doi: 10.1182/blood-2005-08-3452. [DOI] [PubMed] [Google Scholar]
- 11.Niedermeyer J., Garin-Chesa P., Kriz M., Hilberg F., Mueller E., Bamberger U., Rettig W.J., Schnapp A. Expression of the fibroblast activation protein during mouse embryo development. Int. J. Dev. Biol. 2001;45:445–447. [PubMed] [Google Scholar]
- 12.Hua X., Yu L., Huang X., Liao Z., Xian Q. Expression and role of fibroblast activation protein-alpha in microinvasive breast carcinoma. Diagn. Pathol. 2011;6:111. doi: 10.1186/1746-1596-6-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mentlein R., Hattermann K., Hemion C., Jungbluth A.A., Held-Feindt J. Expression and role of the cell surface protease seprase/fibroblast activation protein-alpha (FAP-alpha) in astroglial tumors. Biol. Chem. 2011;392:199–207. doi: 10.1515/BC.2010.119. [DOI] [PubMed] [Google Scholar]
- 14.Shi M., Yu D.H., Chen Y., Zhao C.Y., Zhang J., Liu Q.H., Ni C.R., Zhu M.H. Expression of fibroblast activation protein in human pancreatic adenocarcinoma and its clinicopathological significance. World J. Gastroenterol. 2012;18:840–846. doi: 10.3748/wjg.v18.i8.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X.M., Yu D.M., McCaughan G.W., Gorrell M.D. Fibroblast activation protein increases apoptosis, cell adhesion, and migration by the LX-2 human stellate cell line. Hepatology. 2005;42:935–945. doi: 10.1002/hep.20853. [DOI] [PubMed] [Google Scholar]
- 16.Bauer S., Jendro M.C., Wadle A., Kleber S., Stenner F., Dinser R., Reich A., Faccin E., Godde S., Dinges H. Fibroblast activation protein is expressed by rheumatoid myofibroblast-like synoviocytes. Arthritis Res. Ther. 2006;8:R171. doi: 10.1186/ar2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brokopp C.E., Schoenauer R., Richards P., Bauer S., Lohmann C., Emmert M.Y., Weber B., Winnik S., Aikawa E., Graves K. Fibroblast activation protein is induced by inflammation and degrades type I collagen in thin-cap fibroatheromata. Eur. Heart J. 2011;32:2713–2722. doi: 10.1093/eurheartj/ehq519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garin-Chesa P., Old L.J., Rettig W.J. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc. Natl. Acad. Sci. U.S.A. 1990;87:7235–7239. doi: 10.1073/pnas.87.18.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brennen W.N., Isaacs J.T., Denmeade S.R. Rationale behind targeting fibroblast activation protein–expressing carcinoma-associated fibroblasts as a novel chemotherapeutic strategy. Mol. Cancer Ther. 2012;11:257–266. doi: 10.1158/1535-7163.MCT-11-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu R., Li H., Liu L., Yu J., Ren X. Fibroblast activation protein: a potential therapeutic target in cancer. Cancer Biol. Ther. 2012;13:123–129. doi: 10.4161/cbt.13.3.18696. [DOI] [PubMed] [Google Scholar]
- 21.Wen Y., Wang C., Ma T., Li Z., Zhou L., Mu B., Leng F., Shi H., Li Y., Wei Y. Immunotherapy targeting fibroblast activation protein inhibits tumor growth and increases survival in a murine colon cancer model. Cancer Sci. 2010;101:2325–2332. doi: 10.1111/j.1349-7006.2010.01695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorrell M.D., Gysbers V., McCaughan G.W. CD26: a multifunctional integral membrane and secreted protein of activated lymphocytes. Scand. J. Immunol. 2001;54:249–264. doi: 10.1046/j.1365-3083.2001.00984.x. [DOI] [PubMed] [Google Scholar]
- 23.Harstad E.B., Rosenblum J.S., Gorrell M.D., Achanzar W.E., Minimo L., Wu J., Rosini-Marthaler L., Gullo R., Ordway N.D., Kirby M.S. DPP8 and DPP9 expression in cynomolgus monkey and Sprague Dawley rat tissues. Regul. Pept. 2013;186:26–35. doi: 10.1016/j.regpep.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 24.McCaughan G.W., Wickson J.E., Creswick P.F., Gorrell M.D. Identification of the bile canalicular cell surface molecule GP110 as the ectopeptidase dipeptidyl peptidase IV: an analysis by tissue distribution, purification and N-terminal amino acid sequence. Hepatology. 1990;11:534–544. doi: 10.1002/hep.1840110403. [DOI] [PubMed] [Google Scholar]
- 25.Yu D.M., Ajami K., Gall M.G., Park J., Lee C.S., Evans K.A., McLaughlin E.A., Pitman M.R., Abbott C.A., McCaughan G.W. The in vivo expression of dipeptidyl peptidases 8 and 9. J. Histochem. Cytochem. 2009;57:1025–1040. doi: 10.1369/jhc.2009.953760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mentlein R., Gallwitz B., Schmidt W.E. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7–36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur. J. Biochem. 1993;214:829–835. doi: 10.1111/j.1432-1033.1993.tb17986.x. [DOI] [PubMed] [Google Scholar]
- 27.Heymann E., Mentlein R. Liver dipeptidyl aminopeptidase IV hydrolyzes substance P. FEBS Lett. 1978;91:360–364. doi: 10.1016/0014-5793(78)81210-1. [DOI] [PubMed] [Google Scholar]
- 28.Mentlein R., Dahms P., Grandt D., Kruger R. Proteolytic processing of neuropeptide Y and peptide YY by dipeptidyl peptidase IV. Regul. Pept. 1993;49:133–144. doi: 10.1016/0167-0115(93)90435-b. [DOI] [PubMed] [Google Scholar]
- 29.Oravecz T., Pall M., Roderiquez G., Gorrell M.D., Ditto M., Nguyen N.Y., Boykins R., Unsworth E., Norcross M.A. Regulation of the receptor specificity and function of the chemokine RANTES (regulated on activation, normal T cell expressed and secreted) by dipeptidyl peptidase IV (CD26)-mediated cleavage. J. Exp. Med. 1997;186:1865–1872. doi: 10.1084/jem.186.11.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Proost P., Menten P., Struyf S., Schutyser E., De Meester I., Van Damme J. Cleavage by CD26/dipeptidyl peptidase IV converts the chemokine LD78beta into a most efficient monocyte attractant and CCR1 agonist. Blood. 2000;96:1674–1680. [PubMed] [Google Scholar]
- 31.Cordero O.J., Salgado F.J., Nogueira M. On the origin of serum CD26 and its altered concentration in cancer patients. Cancer Immunol. Immunother. 2009;58:1723–1747. doi: 10.1007/s00262-009-0728-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yildirim F.E., Karaduman A., Pinar A., Aksoy Y. CD26/dipeptidyl-peptidase IV and adenosine deaminase serum levels in psoriatic patients treated with cyclosporine, etanercept, and psoralen plus ultraviolet A phototherapy. Int. J. Dermatol. 2011;50:948–955. doi: 10.1111/j.1365-4632.2010.04799.x. [DOI] [PubMed] [Google Scholar]
- 33.Fletcher M.A., Zeng X.R., Maher K., Levis S., Hurwitz B., Antoni M., Broderick G., Klimas N.G. Biomarkers in chronic fatigue syndrome: evaluation of natural killer cell function and dipeptidyl peptidase IV/CD26. PLoS One. 2010;5:e10817. doi: 10.1371/journal.pone.0010817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kupeli E., Karnak D., Elgun S., Arguder E., Kayacan O. Concurrent measurement of adenosine deaminase and dipeptidyl peptidase IV activity in the diagnosis of tuberculous pleural effusion. Diagn. Microbiol. Infect. Dis. 2009;65:365–371. doi: 10.1016/j.diagmicrobio.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Casrouge A., Decalf J., Ahloulay M., Lababidi C., Mansour H., Vallet-Pichard A., Mallet V., Mottez E., Mapes J., Fontanet A. Evidence for an antagonist form of the chemokine CXCL10 in patients chronically infected with HCV (hepatitis C virus) J. Clin. Invest. 2011;121:308–318. doi: 10.1172/JCI40594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Itou M., Kawaguchi T., Taniguchi E., Hirano E., Sumie S., Oriishi T., Mitsuyama K., Tsuruta O., Ueno T., Sata M. Altered expression of glucagon-like peptide and dipeptidyl peptidase IV in patients with HCV-related glucose intolerance. J. Gastroenterol. Hepatol. 2008;23:244–251. doi: 10.1111/j.1440-1746.2007.05183.x. [DOI] [PubMed] [Google Scholar]
- 37.Amatya V.J., Takeshima Y., Kushitani K., Yamada T., Morimoto C., Inai K. Overexpression of CD26/DPPIV in mesothelioma tissue and mesothelioma cell lines. Oncol. Rep. 2011;26:1369–1375. doi: 10.3892/or.2011.1449. [DOI] [PubMed] [Google Scholar]
- 38.Arwert E.N., Mentink R.A., Driskell R.R., Hoste E., Goldie S.J., Quist S., Watt F.M. Upregulation of CD26 expression in epithelial cells and stromal cells during wound-induced skin tumour formation. Oncogene. 2012;31:992–1000. doi: 10.1038/onc.2011.298. [DOI] [PubMed] [Google Scholar]
- 39.Ayude D., Paez de la Cadena M., Cordero O.J., Nogueira M., Ayude J., Fernandez-Briera A., Rodriguez-Berrocal F.J. Clinical interest of the combined use of serum CD26 and alpha-L-fucosidase in the early diagnosis of colorectal cancer. Dis. Markers. 2003;19:267–272. doi: 10.1155/2004/834309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Javidroozi M., Zucker S., Chen W.T. Plasma seprase and DPP4 levels as markers of disease and prognosis in cancer. Dis. Markers. 2012;32:309–320. doi: 10.3233/DMA-2011-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khin E.E., Kikkawa F., Ino K., Kajiyama H., Suzuki T., Shibata K., Tamakoshi K., Nagasaka T., Mizutani S. Dipeptidyl peptidase IV expression in endometrial endometrioid adenocarcinoma and its inverse correlation with tumor grade. Am. J. Obstet. Gynecol. 2003;188:670–676. doi: 10.1067/mob.2003.169. [DOI] [PubMed] [Google Scholar]
- 42.Mannucci E., Pala L., Ciani S., Bardini G., Pezzatini A., Sposato I., Cremasco F., Ognibene A., Rotella C.M. Hyperglycaemia increases dipeptidyl peptidase IV activity in diabetes mellitus. Diabetologia. 2005;48:1168–1172. doi: 10.1007/s00125-005-1749-8. [DOI] [PubMed] [Google Scholar]
- 43.Ryskjaer J., Deacon C.F., Carr R.D., Krarup T., Madsbad S., Holst J., Vilsboll T. Plasma dipeptidyl peptidase-IV activity in patients with type-2 diabetes mellitus correlates positively with HbAlc levels, but is not acutely affected by food intake. Eur. J. Endocrinol. 2006;155:485–493. doi: 10.1530/eje.1.02221. [DOI] [PubMed] [Google Scholar]
- 44.McKillop A.M., Duffy N.A., Lindsay J.R., O’Harte F.P., Bell P.M., Flatt P.R. Decreased dipeptidyl peptidase-IV activity and glucagon-like peptide-1(7–36)amide degradation in type 2 diabetic subjects. Diabetes Res. Clin. Pract. 2008;79:79–85. doi: 10.1016/j.diabres.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Meneilly G.S., Demuth H.U., McIntosh C.H., Pederson R.A. Effect of ageing and diabetes on glucose-dependent insulinotropic polypeptide and dipeptidyl peptidase IV responses to oral glucose. Diabet. Med. 2000;17:346–350. doi: 10.1046/j.1464-5491.2000.00236.x. [DOI] [PubMed] [Google Scholar]
- 46.Jambunathan K., Watson D.S., Endsley A.N., Kodukula K., Galande A.K. Comparative analysis of the substrate preferences of two post-proline cleaving endopeptidases, prolyl oligopeptidase and fibroblast activation protein alpha. FEBS Lett. 2012;586:2507–2512. doi: 10.1016/j.febslet.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niedermeyer J., Scanlan M.J., Garin-Chesa P., Daiber C., Fiebig H.H., Old L.J., Rettig W.J., Schnapp A. Mouse fibroblast activation protein: molecular cloning, alternative splicing and expression in the reactive stroma of epithelial cancers. Int. J. Cancer. 1997;71:383–389. doi: 10.1002/(sici)1097-0215(19970502)71:3<383::aid-ijc14>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 48.Yuan D., Liu B., Liu K., Zhu G., Dai Z., Xie Y. Overexpression of fibroblast activation protein and its clinical implications in patients with osteosarcoma. J. Surg. Oncol. 2013;108:157–162. doi: 10.1002/jso.23368. [DOI] [PubMed] [Google Scholar]
- 49.Jacob M., Chang L., Pure E. Fibroblast activation protein in remodeling tissues. Curr. Mol. Med. 2012;12:1220–1243. doi: 10.2174/156652412803833607. [DOI] [PubMed] [Google Scholar]
- 50.Christiansen V.J., Jackson K.W., Lee K.N., Downs T.D., McKee P.A. Targeting inhibition of fibroblast activation protein-α and prolyl oligopeptidase activities on cells common to metastatic tumor microenvironments. Neoplasia. 2013;15:348–358. doi: 10.1593/neo.121850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fischer E., Chaitanya K., Wuest T., Wadle A., Scott A.M., van den Broek M., Schibli R., Bauer S., Renner C. Radioimmunotherapy of fibroblast activation protein positive tumors by rapidly internalizing antibodies. Clin. Cancer Res. 2012;18:6208–6218. doi: 10.1158/1078-0432.CCR-12-0644. [DOI] [PubMed] [Google Scholar]
- 52.LeBeau A.M., Brennen W.N., Aggarwal S., Denmeade S.R. Targeting cancer stroma with a fibroblast activation protein-activated promelittin protoxin. Mol. Cancer Ther. 2009;8:1378–1386. doi: 10.1158/1535-7163.MCT-08-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kakarla S., Chow K.K., Mata M., Shaffer D.R., Song X.T., Wu M.F., Liu H., Wang L.L., Rowley D.R., Pfizenmaier K. Antitumor effects of chimeric receptor engineered human T cells directed to tumor stroma. Mol. Ther. 2013;21:1611–1620. doi: 10.1038/mt.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cai F., Li Z., Wang C., Xian S., Xu G., Peng F., Wei Y., Lu Y. Short hairpin RNA targeting of fibroblast activation protein inhibits tumor growth and improves the tumor microenvironment in a mouse model. BMB Rep. 2013;46:252–257. doi: 10.5483/BMBRep.2013.46.5.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roberts E.W., Deonarine A., Jones J.O., Denton A.E., Feig C., Lyons S.K., Espeli M., Kraman M., McKenna B., Wells R.J. Depletion of stromal cells expressing fibroblast activation protein-alpha from skeletal muscle and bone marrow results in cachexia and anemia. J. Exp. Med. 2013;210:1137–1151. doi: 10.1084/jem.20122344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tran E., Chinnasamy D., Yu Z., Morgan R.A., Lee C.C., Restifo N.P., Rosenberg S.A. Immune targeting of fibroblast activation protein triggers recognition of multipotent bone marrow stromal cells and cachexia. J. Exp. Med. 2013;210:1125–1135. doi: 10.1084/jem.20130110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Poplawski S.E., Li Y., Shu Y., Zhang M., DiMare M.T., Sanford D.G., Lai J.H., Cheng J.D., Alpaugh K.R., Gorrell M.D. Cancerous tissues from human patients contain far higher levels of fibroblast activation protein enzyme activity than corresponding mouse xenografts as revealed using ARI-3144, a new highly specific substrate assay. Cancer Cell. 2013 Submitted. [Google Scholar]
- 58.Niedermeyer J., Kriz M., Hilberg F., Garin-Chesa P., Bamberger U., Lenter M.C., Park J., Viertel B., Puschner H., Mauz M. Targeted disruption of mouse fibroblast activation protein. Mol. Cell Biol. 2000;20:1089–1094. doi: 10.1128/mcb.20.3.1089-1094.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Osborne B., Yao T.-W., Wang X.M., Kotan L.D., Nadvi N.A., Herdem M., Yu D.M.T., Allen J.D., Topaloglu A.K., Gorrell M.D. A rare variant in human fibroblast activation protein associated with ER stress, loss of function and loss of cell surface localisation. Biochim. Biophys. Acta Proteins Proteomics. 2013 doi: 10.1016/j.bbapap.2014.03.015. Submitted. [DOI] [PubMed] [Google Scholar]
- 60.Chowdhury S., Chen Y., Yao T.-W., Ajami K., Wang X.M., Popov Y., Schuppan D., Bertolino P., McCaughan G.W., Yu D.M. Regulation of dipeptidyl peptidase 8 and 9 expression in activated lymphocytes and injured liver. World J. Gastroenterol. 2013;19:2883–2893. doi: 10.3748/wjg.v19.i19.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang X.M., Yao T.W., Nadvi N.A., Osborne B., McCaughan G.W., Gorrell M.D. Fibroblast activation protein and chronic liver disease. Front. Biosci. 2008;13:3168–3180. doi: 10.2741/2918. [DOI] [PubMed] [Google Scholar]
- 62.Li J., Chen K., Liu H., Cheng K., Yang M., Zhang J., Cheng JD., Zhang Y., Cheng Z. Activatable near-infrared fluorescent probe for in vivo imaging of fibroblast activation protein-alpha. Bioconjug. Chem. 2012;23:1704–1711. doi: 10.1021/bc300278r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Santos A.M., Jung J., Aziz N., Kissil J.L., Pure E. Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J. Clin. Invest. 2010;119:3613–3626. doi: 10.1172/JCI38988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abbott C.A., Gorrell M.D., Kobayashi Y., Kawasaki T., Liddle C., Bishop G.A., McCaughan G.W. Increased serum levels of dipeptidyl peptidase IV (CD26) in rats undergoing liver regeneration. Int. Hepatol. Commun. 1995;4:165–174. [Google Scholar]
- 65.Lamers D., Famulla S., Wronkowitz N., Hartwig S., Lehr S., Ouwens D.M., Eckardt K., Kaufman J.M., Ryden M., Müller S. Dipeptidyl peptidase 4 is a novel adipokine potentially linking obesity to the metabolic syndrome. Diabetes. 2011;60:1917–1925. doi: 10.2337/db10-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gorrell M.D. Editorial: impaired glucose tolerance and incretins in chronic liver disease. J. Gastroenterol. Hepatol. 2008;23:166–167. doi: 10.1111/j.1440-1746.2007.05268.x. [DOI] [PubMed] [Google Scholar]
- 67.Lee S., Macquillan G.C., Keane N.M., Flexman J., Jeffrey G.P., French M.A., Brochier J., Price P. Immunological markers predicting outcome in patients with hepatitis C treated with interferon-alpha and ribavirin. Immunol. Cell. Biol. 2002;80:391–397. doi: 10.1046/j.1440-1711.2002.01102.x. [DOI] [PubMed] [Google Scholar]
- 68.Matsumoto Y., Bishop G.A., McCaughan G.W. Altered zonal expression of the CD26 antigen (dipeptidyl peptidase IV) in human cirrhotic liver. Hepatology. 1992;15:1048–1053. doi: 10.1002/hep.1840150613. [DOI] [PubMed] [Google Scholar]
- 69.Lee K.N., Jackson K.W., Christiansen V.J, Dolence E.K., McKee P.A. Enhancement of fibrinolysis by inhibiting enzymatic cleavage of precursor alpha2-antiplasmin. J. Thromb. Haemost. 2011;9:987–996. doi: 10.1111/j.1538-7836.2011.04195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wild N., Andres H., Rollinger W., Krause F., Dilba P., Tacke M., Karl J. A combination of serum markers for the early detection of colorectal cancer. Clin. Cancer Res. 2010;16:6111–6121. doi: 10.1158/1078-0432.CCR-10-0119. [DOI] [PubMed] [Google Scholar]
- 71.Levy M.T., McCaughan G.W., Marinos G., Gorrell M.D. Intrahepatic expression of the hepatic stellate cell marker fibroblast activation protein correlates with the degree of fibrosis in hepatitis C virus infection. Liver. 2002;22:93–101. doi: 10.1034/j.1600-0676.2002.01503.x. [DOI] [PubMed] [Google Scholar]
- 72.Thomson S.E., McLennan S.V., Hennessy A., Boughton P., Bonner J., Zoellner H., Yue D.K., Twigg S.M. A novel primate model of delayed wound healing in diabetes: dysregulation of connective tissue growth factor. Diabetologia. 2010;53:572–583. doi: 10.1007/s00125-009-1610-6. [DOI] [PubMed] [Google Scholar]
- 73.Song S., Shackel N.A., Wang X.M., Ajami K., McCaughan G.W., Gorrell M.D. Discoidin domain receptor 1: isoform expression and potential functions in cirrhotic human liver. Am. J. Pathol. 2011;178:1134–1144. doi: 10.1016/j.ajpath.2010.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials for Quantitation of fibroblast activation protein (FAP)-specific protease activity in mouse, baboon and human fluids and organs.


