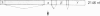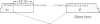Abstract
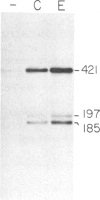
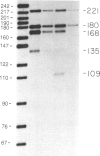
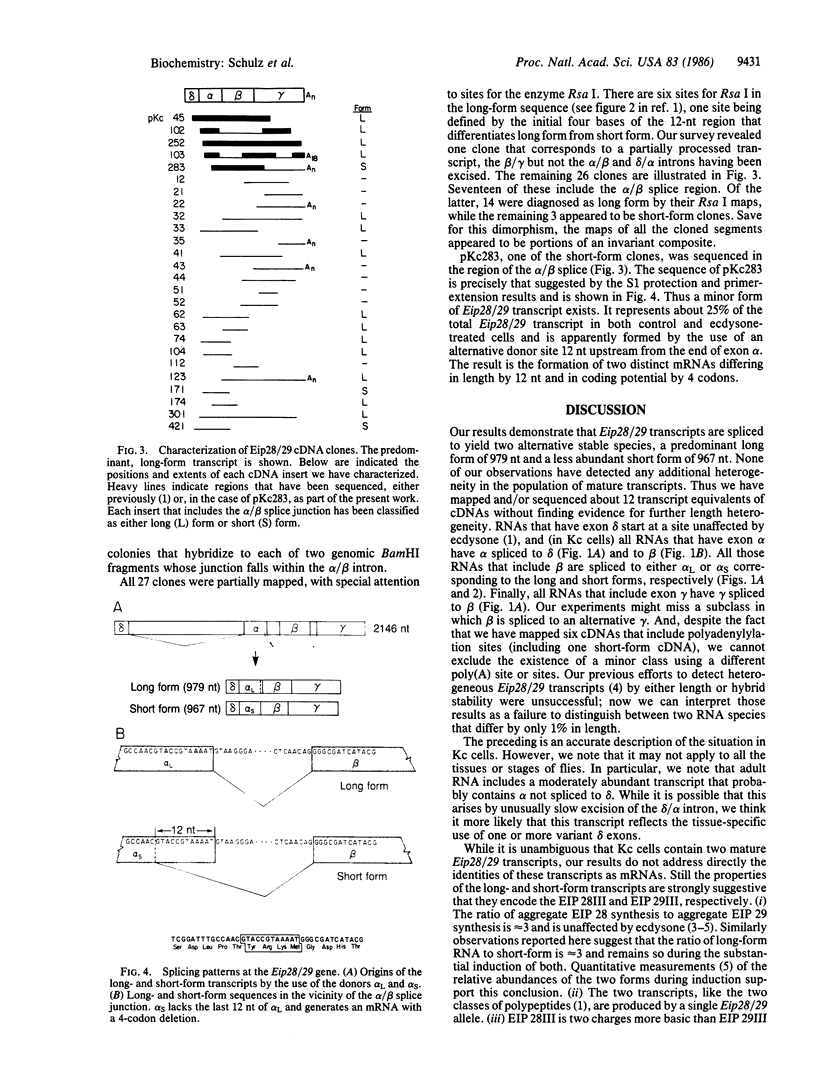
The Drosophila Eip28/29 gene encodes two primary translation products, ecdysone-inducible polypeptide (EIP) 28III and EIP 29III. When cells of the Kc cell line are treated with the steroid hormone ecdysone, the number of Eip28/29 transcripts and the synthesis of the various forms of EIP 28 and 29 increase rapidly. We have reported the sequence of the Eip28/29 gene and of its major transcript. Here we describe a minor or short-form transcript that is about 25% of the total Eip28/29 gene transcripts in both untreated and hormone-treated cells. This transcript is formed by the use of an alternative splice donor sequence 12 nucleotides upstream from the major donor site at the end of the second exon. Evidently the relative abundance of the two products is not hormonally regulated. The short form translation product should lack only an internal dibasic tetrapeptide. The long and short forms probably represent distinct mRNAs for EIP 28III and EIP 29III, respectively.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amara S. G., Evans R. M., Rosenfeld M. G. Calcitonin/calcitonin gene-related peptide transcription unit: tissue-specific expression involves selective use of alternative polyadenylation sites. Mol Cell Biol. 1984 Oct;4(10):2151–2160. doi: 10.1128/mcb.4.10.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherbas L., Schulz R. A., Koehler M. M., Savakis C., Cherbas P. Structure of the Eip28/29 gene, an ecdysone-inducible gene from Drosophila. J Mol Biol. 1986 Jun 20;189(4):617–631. doi: 10.1016/0022-2836(86)90492-4. [DOI] [PubMed] [Google Scholar]
- Douglass J., Civelli O., Herbert E. Polyprotein gene expression: generation of diversity of neuroendocrine peptides. Annu Rev Biochem. 1984;53:665–715. doi: 10.1146/annurev.bi.53.070184.003313. [DOI] [PubMed] [Google Scholar]
- Maurer R. A., Erwin C. R., Donelson J. E. Analysis of 5' flanking sequences and intron-exon boundaries of the rat prolactin gene. J Biol Chem. 1981 Oct 25;256(20):10524–10528. [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J. H. The origin and evolution of retroposons. Int Rev Cytol. 1985;93:187–279. doi: 10.1016/s0074-7696(08)61375-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Savakis C., Demetri G., Cherbas P. Ecdysteroid-inducible polypeptides in a Drosophila cell line. Cell. 1980 Dec;22(3):665–674. doi: 10.1016/0092-8674(80)90542-5. [DOI] [PubMed] [Google Scholar]
- Savakis C., Koehler M. M., Cherbas P. cDNA clones for the ecdysone-inducible polypeptide (EIP) mRNAs of Drosophila Kc cells. EMBO J. 1984 Jan;3(1):235–243. doi: 10.1002/j.1460-2075.1984.tb01790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solnick D. Alternative splicing caused by RNA secondary structure. Cell. 1985 Dec;43(3 Pt 2):667–676. doi: 10.1016/0092-8674(85)90239-9. [DOI] [PubMed] [Google Scholar]
- Stein J. P., Catterall J. F., Kristo P., Means A. R., O'Malley B. W. Ovomucoid intervening sequences specify functional domains and generate protein polymorphism. Cell. 1980 Oct;21(3):681–687. doi: 10.1016/0092-8674(80)90431-6. [DOI] [PubMed] [Google Scholar]
- Treisman R., Orkin S. H., Maniatis T. Specific transcription and RNA splicing defects in five cloned beta-thalassaemia genes. Nature. 1983 Apr 14;302(5909):591–596. doi: 10.1038/302591a0. [DOI] [PubMed] [Google Scholar]
- Wallis M. Growth hormone: deletions in the protein and introns in the gene. Nature. 1980 Apr 10;284(5756):512–512. doi: 10.1038/284512a0. [DOI] [PubMed] [Google Scholar]
- Wieringa B., Hofer E., Weissmann C. A minimal intron length but no specific internal sequence is required for splicing the large rabbit beta-globin intron. Cell. 1984 Jul;37(3):915–925. doi: 10.1016/0092-8674(84)90426-4. [DOI] [PubMed] [Google Scholar]
- Ziff E. B. Splicing in adenovirus and other animal viruses. Int Rev Cytol. 1985;93:327–358. doi: 10.1016/s0074-7696(08)61377-7. [DOI] [PubMed] [Google Scholar]