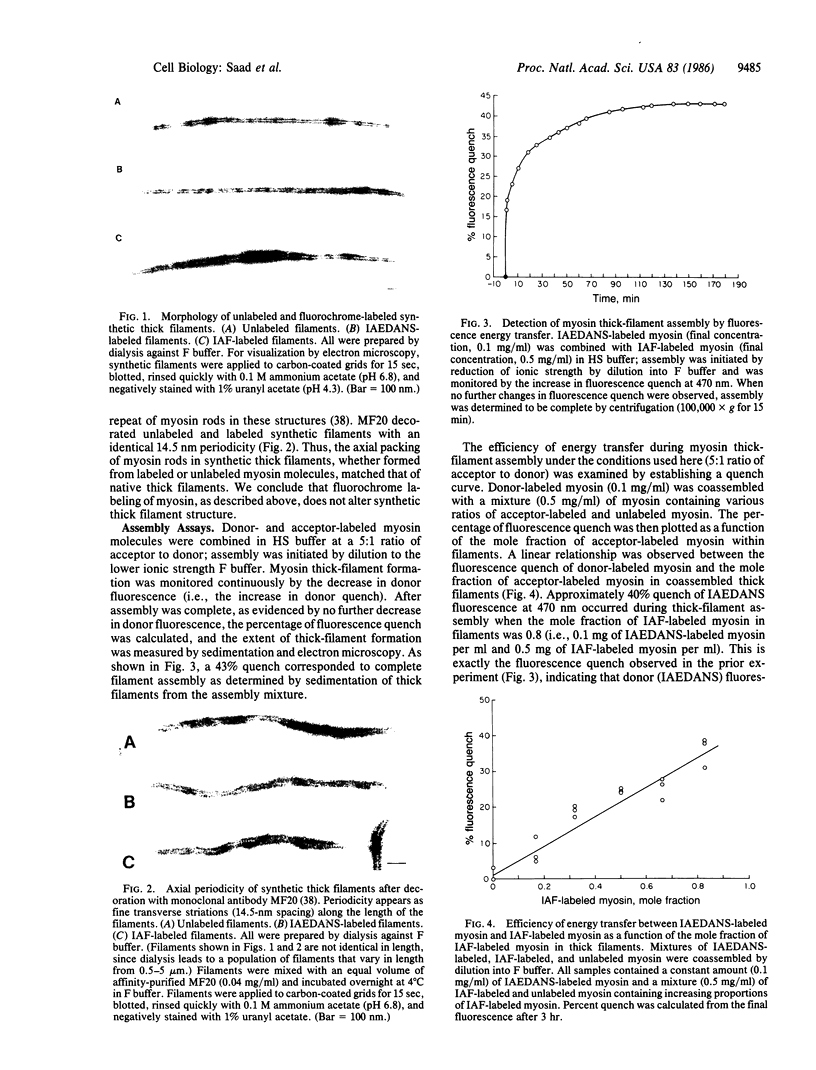
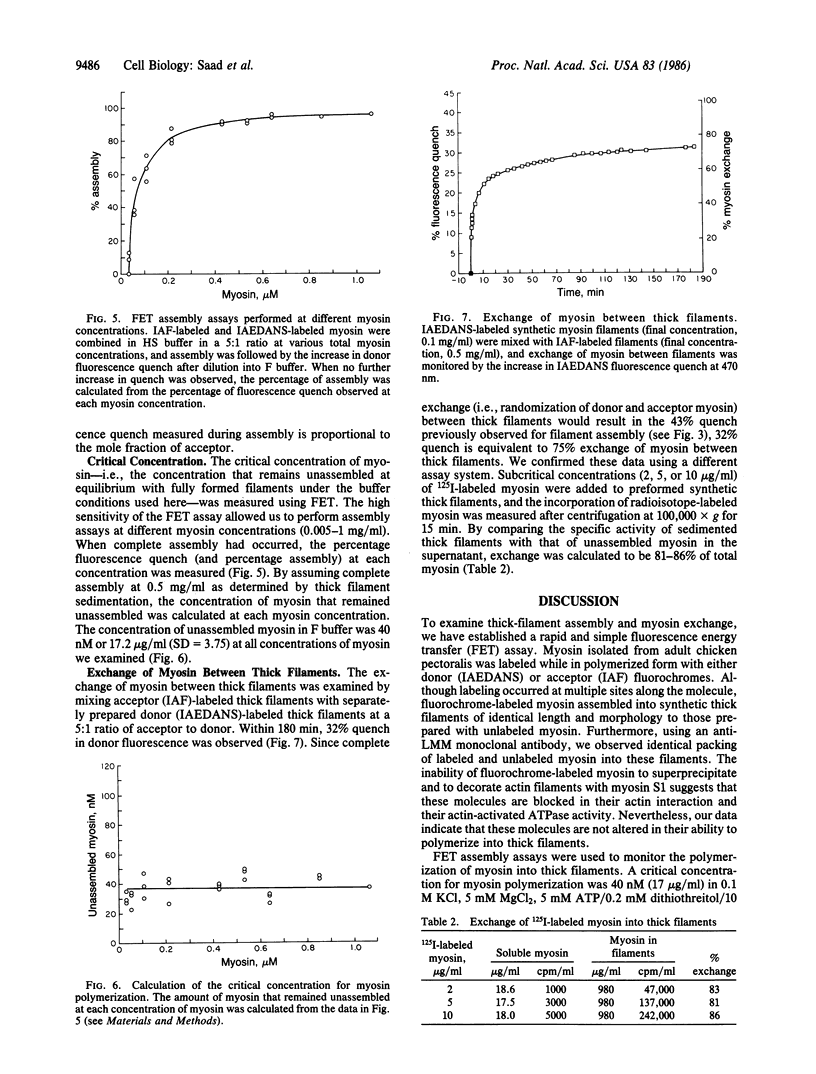
Abstract
To examine thick filament assembly and myosin exchange, a fluorescence energy transfer assay has been established. Assembly-competent myosin molecules labeled with the sulfhydryl-specific fluorochromes 5-(2-[(iodoacetyl)-amino]ethyl)aminonaphthalene-1-sulfonic acids (IAEDANS) or 5-iodoacetamidofluorescein (IAF) were prepared. Using IAEDANS-labeled myosin as fluorescence donor and IAF-labeled myosin as acceptor, thick filament formation was followed by the decrease in donor fluorescence at 0.1 M KCl/10 mM potassium phosphate, pH 6.9. The critical concentration of myosin--i.e., that concentration that remained unassembled at equilibrium with fully formed filaments--was 40 nM. In FET and 125I-labeled myosin incorporation assays, extensive exchange of myosin between thick filaments was observed. The presence of a critical concentration and the measurements of extensive exchange suggest a dynamic equilibrium between fully polymerized myosin and a small pool of soluble myosin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader D., Masaki T., Fischman D. A. Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J Cell Biol. 1982 Dec;95(3):763–770. doi: 10.1083/jcb.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernengo J. C., Cardinaud R. State of myosin in solution. Electric birefringence and dynamic light-scattering studies. J Mol Biol. 1982 Aug 15;159(3):501–517. doi: 10.1016/0022-2836(82)90298-4. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burke M., Harrington W. F. Geometry of the myosin dimer in high-salt media. II. Hydrodynamic studies on macromodels of myosin and its rod segments. Biochemistry. 1972 Apr 11;11(8):1456–1462. doi: 10.1021/bi00758a020. [DOI] [PubMed] [Google Scholar]
- Clark W. A., Jr, Zak R. Assessment of fractional rates of protein synthesis in cardiac muscle cultures after equilibrium labeling. J Biol Chem. 1981 May 25;256(10):4863–4870. [PubMed] [Google Scholar]
- Davis J. S., Buck J., Greene E. P. The myosin dimer: an intermediate in the self-assembly of the thick filament of vertebrate skeletal muscle. FEBS Lett. 1982 Apr 19;140(2):293–297. doi: 10.1016/0014-5793(82)80917-4. [DOI] [PubMed] [Google Scholar]
- Davis J. S. Kinetics and thermodynamics of the assembly of the parallel- and antiparallel-packed sections of synthetic thick filaments of skeletal myosin: a pressure-jump study. Biochemistry. 1985 Sep 10;24(19):5263–5269. doi: 10.1021/bi00340a046. [DOI] [PubMed] [Google Scholar]
- Davis J. S. Pressure-jump studies on the length-regulation kinetics of the self-assembly of myosin from vertebrate skeletal muscle into thick filament. Biochem J. 1981 Aug 1;197(2):309–314. doi: 10.1042/bj1970309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etlinger J. D., Zak R., Fischman D. A. Compositional studies of myofibrils from rabbit striated muscle. J Cell Biol. 1976 Jan;68(1):123–141. doi: 10.1083/jcb.68.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glacy S. D. Pattern and time course of rhodamine-actin incorporation in cardiac myocytes. J Cell Biol. 1983 Apr;96(4):1164–1167. doi: 10.1083/jcb.96.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey J. E., Harrington W. F. Self-association in the myosin system at high ionic strength. II. Evidence for the presence of a monomer--dimer equilibrium. Biochemistry. 1970 Feb 17;9(4):894–908. doi: 10.1021/bi00806a026. [DOI] [PubMed] [Google Scholar]
- Grove B. K., Cerny L., Perriard J. C., Eppenberger H. M. Myomesin and M-protein: expression of two M-band proteins in pectoral muscle and heart during development. J Cell Biol. 1985 Oct;101(4):1413–1421. doi: 10.1083/jcb.101.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington W. F., Burke M. Geometry of the myosin dimer in high-salt media. I. Association behavior of rod segments from myosin. Biochemistry. 1972 Apr 11;11(8):1448–1455. doi: 10.1021/bi00758a019. [DOI] [PubMed] [Google Scholar]
- Harrington W. F., Rodgers M. E. Myosin. Annu Rev Biochem. 1984;53:35–73. doi: 10.1146/annurev.bi.53.070184.000343. [DOI] [PubMed] [Google Scholar]
- Herbert T. J., Carlson F. D. Spectroscopic study of the self-association of myosin. Biopolymers. 1971 Nov;10(11):2231–2252. doi: 10.1002/bip.360101116. [DOI] [PubMed] [Google Scholar]
- Josephs R., Harrington W. F. An unusual pressure dependence for a reversibly associating protein system; sedimentation studies on myosin. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1587–1594. doi: 10.1073/pnas.58.4.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs R., Harrington W. F. On the stability of myosin filaments. Biochemistry. 1968 Aug;7(8):2834–2847. doi: 10.1021/bi00848a020. [DOI] [PubMed] [Google Scholar]
- Josephs R., Harrington W. F. Studies on the formation and physical chemical properties of synthetic myosin filaments. Biochemistry. 1966 Nov;5(11):3474–3487. doi: 10.1021/bi00875a013. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marsh D. J., Lowey S. Fluorescence energey transfer in myosin subfragment-1. Biochemistry. 1980 Feb 19;19(4):774–784. doi: 10.1021/bi00545a025. [DOI] [PubMed] [Google Scholar]
- Masaki T., Takaiti O. M-protein. J Biochem. 1974 Feb;75(2):367–380. doi: 10.1093/oxfordjournals.jbchem.a130403. [DOI] [PubMed] [Google Scholar]
- McKenna N., Meigs J. B., Wang Y. L. Identical distribution of fluorescently labeled brain and muscle actins in living cardiac fibroblasts and myocytes. J Cell Biol. 1985 Jan;100(1):292–296. doi: 10.1083/jcb.100.1.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megerman J., Lowey S. Polymerization of myosin from smooth muscle of the calf aorta. Biochemistry. 1981 Apr 14;20(8):2099–2110. doi: 10.1021/bi00511a006. [DOI] [PubMed] [Google Scholar]
- Morimoto K., Harrington W. F. Isolation and composition of thick filaments from rabbit skeletal muscle. J Mol Biol. 1973 Jun 15;77(1):165–175. doi: 10.1016/0022-2836(73)90370-7. [DOI] [PubMed] [Google Scholar]
- Offer G., Moos C., Starr R. A new protein of the thick filaments of vertebrate skeletal myofibrils. Extractions, purification and characterization. J Mol Biol. 1973 Mar 15;74(4):653–676. doi: 10.1016/0022-2836(73)90055-7. [DOI] [PubMed] [Google Scholar]
- Pardee J. D., Simpson P. A., Stryer L., Spudich J. A. Actin filaments undergo limited subunit exchange in physiological salt conditions. J Cell Biol. 1982 Aug;94(2):316–324. doi: 10.1083/jcb.94.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee J. D., Spudich J. A. Mechanism of K+-induced actin assembly. J Cell Biol. 1982 Jun;93(3):648–654. doi: 10.1083/jcb.93.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinset-Härström I. MgATP specifically controls in vitro self-assembly of vertebrate skeletal myosin in the physiological pH range. J Mol Biol. 1985 Mar 5;182(1):159–172. doi: 10.1016/0022-2836(85)90034-8. [DOI] [PubMed] [Google Scholar]
- Pollard T. D. Structure and polymerization of Acanthamoeba myosin-II filaments. J Cell Biol. 1982 Dec;95(3):816–825. doi: 10.1083/jcb.95.3.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisler E., Cheung P., Oriol-Audit C., Lake J. A. Growth of synthetic myosin filaments from myosin minifilaments. Biochemistry. 1982 Feb 16;21(4):701–707. doi: 10.1021/bi00533a018. [DOI] [PubMed] [Google Scholar]
- Saad A. D., Fischman D. A., Pardee J. D. Fluorescence energy transfer studies of Myosin thick filament assembly. Biophys J. 1986 Jan;49(1):140–142. doi: 10.1016/S0006-3495(86)83626-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger J. W., Mittal B., Sanger J. M. Analysis of myofibrillar structure and assembly using fluorescently labeled contractile proteins. J Cell Biol. 1984 Mar;98(3):825–833. doi: 10.1083/jcb.98.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger J. W., Mittal B., Sanger J. M. Interaction of fluorescently-labeled contractile proteins with the cytoskeleton in cell models. J Cell Biol. 1984 Sep;99(3):918–928. doi: 10.1083/jcb.99.3.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T., Dennis J. E., Masaki T., Fischman D. A. Axial arrangement of the myosin rod in vertebrate thick filaments: immunoelectron microscopy with a monoclonal antibody to light meromyosin. J Cell Biol. 1985 Sep;101(3):1115–1123. doi: 10.1083/jcb.101.3.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. R., Kunes S. M., Schultz D. W., Taylor A., Triman K. L. Structure of chi hotspots of generalized recombination. Cell. 1981 May;24(2):429–436. doi: 10.1016/0092-8674(81)90333-0. [DOI] [PubMed] [Google Scholar]
- Stryer L. Fluorescence energy transfer as a spectroscopic ruler. Annu Rev Biochem. 1978;47:819–846. doi: 10.1146/annurev.bi.47.070178.004131. [DOI] [PubMed] [Google Scholar]
- Taylor D. L., Reidler J., Spudich J. A., Stryer L. Detection of actin assembly by fluorescence energy transfer. J Cell Biol. 1981 May;89(2):362–367. doi: 10.1083/jcb.89.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Cooke R. Orientation of spin-labeled myosin heads in glycerinated muscle fibers. Biophys J. 1980 Dec;32(3):891–906. doi: 10.1016/S0006-3495(80)85024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinick J. A. End-filaments: a new structural element of vertebrate skeletal muscle thick filaments. J Mol Biol. 1981 Sep 15;151(2):309–314. doi: 10.1016/0022-2836(81)90517-9. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Pope B. Studies on the chymotryptic digestion of myosin. Effects of divalent cations on proteolytic susceptibility. J Mol Biol. 1977 Apr;111(2):129–157. doi: 10.1016/s0022-2836(77)80119-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Pardee J. D., Reidler J., Stryer L., Spudich J. A. Mechanism of interaction of Dictyostelium severin with actin filaments. J Cell Biol. 1982 Dec;95(3):711–719. doi: 10.1083/jcb.95.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]