Abstract
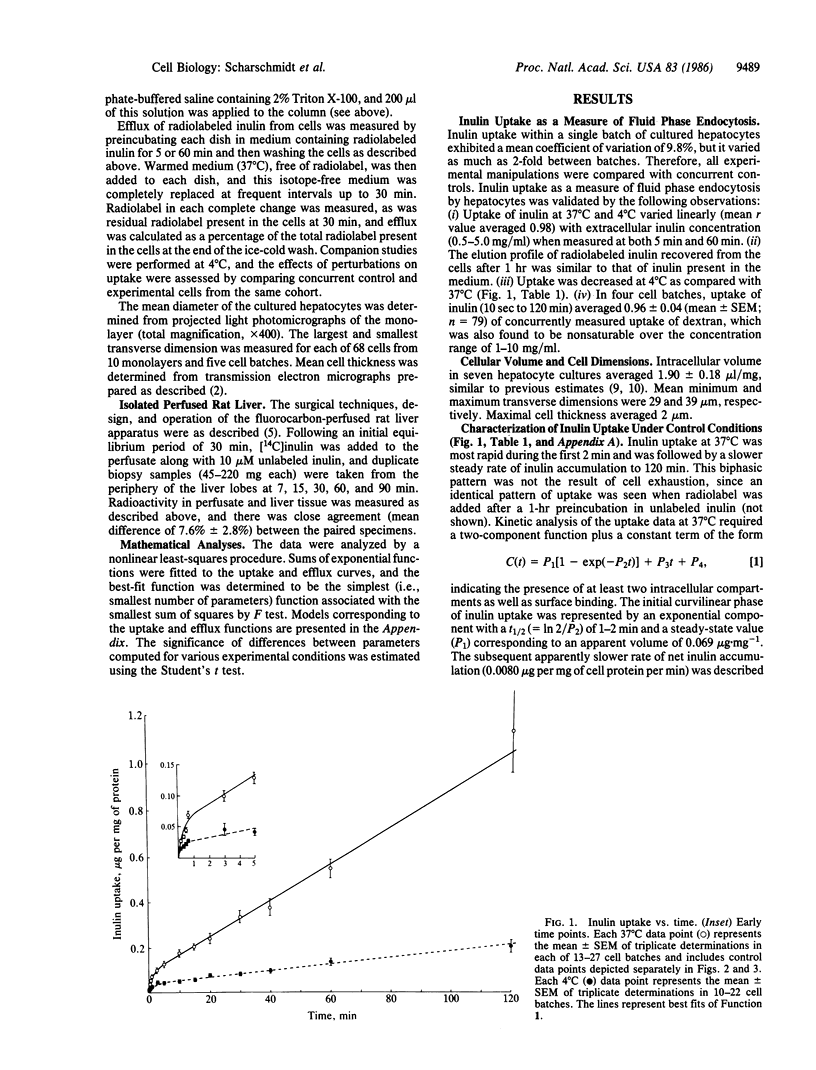
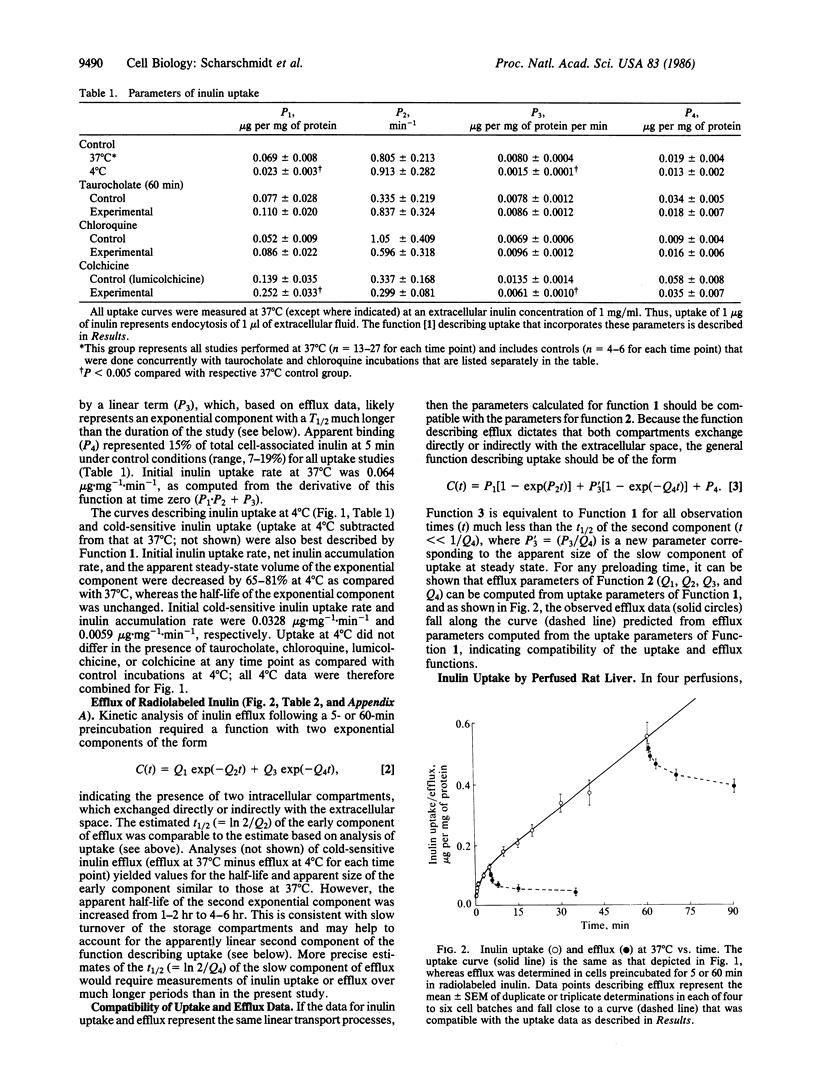
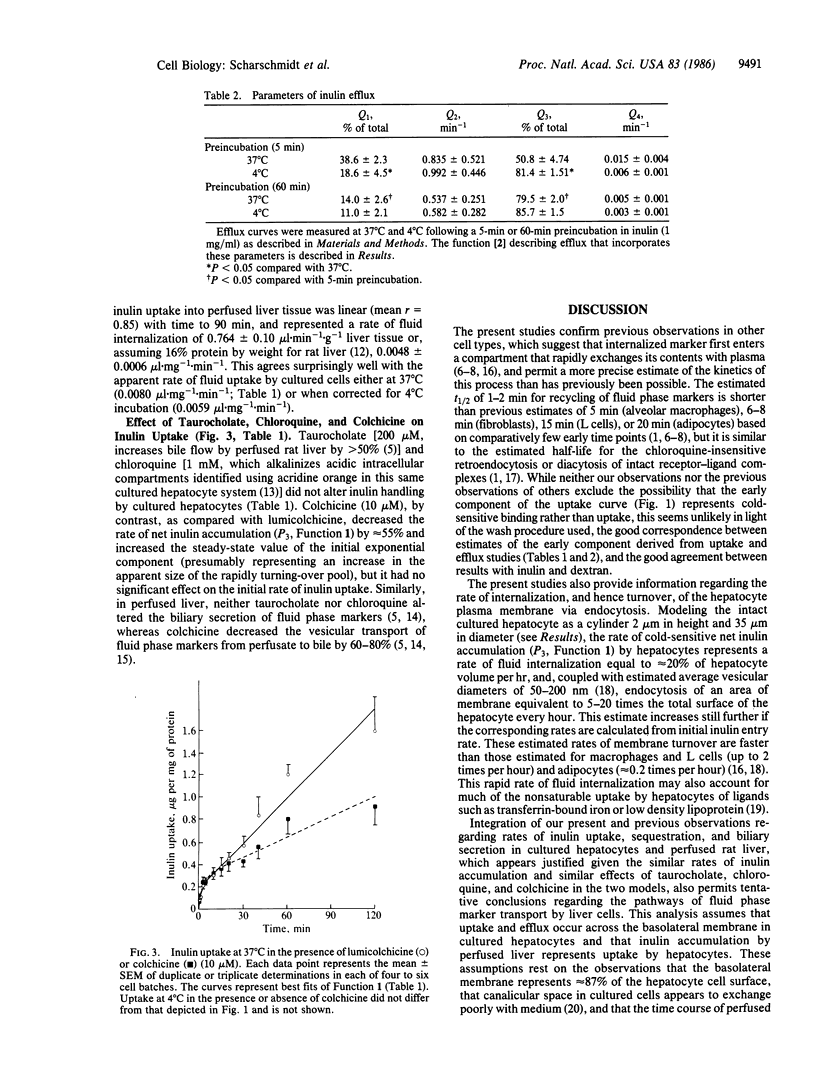
Hepatocytes take up a variety of ligands via receptor-mediated endocytosis, yet little is known regarding either the volume of fluid or the amount of membrane internalized via endocytosis in liver cells. In these studies, we have utilized radiolabeled inulin to characterize fluid phase endocytosis by rat hepatocytes in primary culture and perfused rat liver. Uptake of inulin by cultured hepatocytes was nonlinear with time, occurring most rapidly during the first 2 min. Inulin uptake and efflux in cultured hepatocytes and inulin uptake by perfused rat liver were kinetically compatible with the entry of inulin into a rapidly (t1/2, 1-2 min) turning-over (presumably endosomal) compartment that exchanged contents with the extracellular space and comprised approximately 3% of hepatocyte volume, as well as entry into and concentration of inulin within slowly (t1/2, greater than 1 hr) turning-over storage compartments. Based on inulin uptake, it is estimated that cultured hepatocytes endocytosed the equivalent of 20% or more of their volume and 5 or more times their plasma membrane surface area each hour. Neither chloroquine (1 mM) nor taurocholate (200 microM) affected inulin handling by cultured cells, whereas colchicine (10 microM) inhibited transfer to storage compartments by greater than 50%. In conjunction with our previous observations, the present findings suggest that inulin endocytosed across the basolateral membrane is largely (congruent to 80%) regurgitated back into plasma, with smaller amounts transported to intracellular storage compartments (congruent to 18%) or to bile (congruent to 2%). Transport of inulin via these pathways is unaffected by taurocholate and does not require vesicle acidification, whereas intact microtubular function is required for transfer to storage compartments or biliary secretion.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Besterman J. M., Airhart J. A., Woodworth R. C., Low R. B. Exocytosis of pinocytosed fluid in cultured cells: kinetic evidence for rapid turnover and compartmentation. J Cell Biol. 1981 Dec;91(3 Pt 1):716–727. doi: 10.1083/jcb.91.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell D. M., Guzelian P. S. Phenotypic stability of adult rat hepatocytes in primary monolayer culture. Ann N Y Acad Sci. 1980;349:85–98. doi: 10.1111/j.1749-6632.1980.tb29518.x. [DOI] [PubMed] [Google Scholar]
- Goldman I. S., Jones A. L., Hradek G. T., Huling S. Hepatocyte handling of immunoglobulin A in the rat: the role of microtubules. Gastroenterology. 1983 Jul;85(1):130–140. [PubMed] [Google Scholar]
- Graf J., Gautam A., Boyer J. L. Isolated rat hepatocyte couplets: a primary secretory unit for electrophysiologic studies of bile secretory function. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6516–6520. doi: 10.1073/pnas.81.20.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake J. R., Licko V., Van Dyke R. W., Scharschmidt B. F. Biliary secretion of fluid-phase markers by the isolated perfused rat liver. Role of transcellular vesicular transport. J Clin Invest. 1985 Aug;76(2):676–684. doi: 10.1172/JCI112021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe P. J., Kan K. S., Barnwell S. G., Sharma R. K., Coleman R. Transcytosis and paracellular movements of horseradish peroxidase across liver parenchymal tissue from blood to bile. Effects of alpha-naphthylisothiocyanate and colchicine. Biochem J. 1985 Jul 15;229(2):529–537. doi: 10.1042/bj2290529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall S. Dual pathways for the intracellular processing of insulin. Relationship between retroendocytosis of intact hormone and the recycling of insulin receptors. J Biol Chem. 1985 Nov 5;260(25):13524–13531. [PubMed] [Google Scholar]
- Meier P. J., Sztul E. S., Reuben A., Boyer J. L. Structural and functional polarity of canalicular and basolateral plasma membrane vesicles isolated in high yield from rat liver. J Cell Biol. 1984 Mar;98(3):991–1000. doi: 10.1083/jcb.98.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrill G. A., Kostellow A. B., Weinstein S. P. Endocytosis in the amphibian oocyte. Effect of insulin and progesterone on membrane and fluid internalization during the meiotic divisions. Biochim Biophys Acta. 1984 Feb 17;803(1-2):71–77. doi: 10.1016/0167-4889(84)90056-9. [DOI] [PubMed] [Google Scholar]
- Munniksma J., Noteborn M., Kooistra T., Stienstra S., Bouma J. M., Gruber M., Brouwer A., Praaning-van Dalen Dalen D., Knook D. L. Fluid endocytosis by rat liver and spleen. Experiments with 125I-labelled poly(vinylpyrrolidone) in vivo. Biochem J. 1980 Nov 15;192(2):613–621. doi: 10.1042/bj1920613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ose L., Ose T., Reinertsen R., Berg T. Fluid endocytosis in isolated rat parenchymal and non-parenchymal liver cells. Exp Cell Res. 1980 Mar;126(1):109–119. doi: 10.1016/0014-4827(80)90475-9. [DOI] [PubMed] [Google Scholar]
- Schiff J. M., Fisher M. M., Underdown B. J. Receptor-mediated biliary transport of immunoglobulin A and asialoglycoprotein: sorting and missorting of ligands revealed by two radiolabeling methods. J Cell Biol. 1984 Jan;98(1):79–89. doi: 10.1083/jcb.98.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spady D. K., Turley S. D., Dietschy J. M. Receptor-independent low density lipoprotein transport in the rat in vivo. Quantitation, characterization, and metabolic consequences. J Clin Invest. 1985 Sep;76(3):1113–1122. doi: 10.1172/JCI112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Brodie S. E., Cohn Z. A. Membrane flow during pinocytosis. A stereologic analysis. J Cell Biol. 1976 Mar;68(3):665–687. doi: 10.1083/jcb.68.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Mellman I. S., Muller W. A., Cohn Z. A. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983 Jan;96(1):1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke R. W., Scharschmidt B. F. (Na,K)-ATPase-mediated cation pumping in cultured rat hepatocytes. Rapid modulation by alanine and taurocholate transport and characterization of its relationship to intracellular sodium concentration. J Biol Chem. 1983 Nov 10;258(21):12912–12919. [PubMed] [Google Scholar]
- Van Dyke R. W., Steer C. J., Scharschmidt B. F. Clathrin-coated vesicles from rat liver: enzymatic profile and characterization of ATP-dependent proton transport. Proc Natl Acad Sci U S A. 1984 May;81(10):3108–3112. doi: 10.1073/pnas.81.10.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wileman T., Harding C., Stahl P. Receptor-mediated endocytosis. Biochem J. 1985 Nov 15;232(1):1–14. doi: 10.1042/bj2320001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deurs B., Röpke C., Thorball N. Kinetics of pinocytosis studied by flow cytometry. Eur J Cell Biol. 1984 May;34(1):96–102. [PubMed] [Google Scholar]
- van Tamelen E. E., Webber B. D. Hydrogenative deoxygenation of organic compounds: Direct conversion of amides to alkanes. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1321–1322. doi: 10.1073/pnas.78.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]


