Summary
Our study, conducted within a New York City-based cohort, identified novel interactions between maternal PAH exposure and maternal and newborn genetic haplotypes in key B[a]P metabolism genes on B[a]P-DNA adducts in paired cord blood samples.
Abstract
Polycyclic aromatic hydrocarbons (PAH) are a class of chemicals common in the environment. Certain PAH are carcinogenic, although the degree to which genetic variation influences susceptibility to carcinogenic PAH remains unclear. Also unknown is the influence of genetic variation on the procarcinogenic effect of in utero exposures to PAH. Benzo[a]pyrene (B[a]P) is a well-studied PAH that is classified as a probable human carcinogen. Within our New York City-based cohort, we explored interactions between maternal exposure to airborne PAH during pregnancy and maternal and newborn haplotypes (and in one case, a single-nucleotide polymorphism) in key B[a]P metabolism genes on B[a]P-DNA adducts in paired cord blood samples. The study subjects included non-smoking African-American (n = 132) and Dominican (n = 235) women with available data on maternal PAH exposure, paired cord adducts and genetic data who resided in the Washington Heights, Central Harlem and South Bronx neighborhoods of New York City. We selected seven maternal and newborn genes related to B[a]P metabolism, detoxification and repair for our analyses: CYP1A1, CYP1A2, CYP1B1, GSTM3, GSTT2, NQO1 and XRCC1. We found significant interactions between maternal PAH exposure and haplotype on cord B[a]P-DNA adducts in the following genes: maternal CYP1B1, XRCC1 and GSTM3, and newborn CYP1A2 and XRCC1 in African-Americans; and maternal XRCC1 and newborn NQO1 in Dominicans. These novel findings highlight differences in maternal and newborn genetic contributions to B[a]P-DNA adduct formation, as well as ethnic differences in gene–environment interactions, and have the potential to identify at-risk subpopulations who are susceptible to the carcinogenic potential of B[a]P.
Introduction
Polycyclic aromatic hydrocarbons (PAH) are a class of chemicals, which are ubiquitous in the environment as a result of incomplete combustion reactions. Major sources of these compounds include fossil fuel combustion, cigarette smoking and grilling of meats (1). The common routes of exposure to PAH are inhalation and oral.
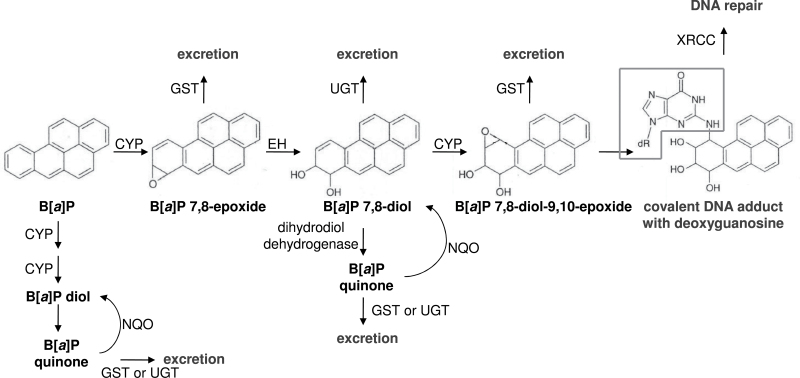
Certain PAH are carcinogenic and of these, the best-studied is benzo[a]pyrene (B[a]P). The metabolic activation of B[a]P to form a compound, which is highly reactive with DNA, has been well elucidated and is diagrammed in Figure 1. The reactive B[a]P 7,8-diol-9,10-epoxide (BPDE) metabolite preferentially forms a covalent adduct at the N2 position of the deoxyguanosine base, thus forming a DNA adduct, which can be considered an early risk biomarker of cancer, as well as a biomarker which integrates multiple B[a]P exposure routes and reflects a biologically effective dose (2–4). The International Agency for Research on Cancer originally designated B[a]P as a Group 2A carcinogen, or a probable human carcinogen (1). However, based on accumulating evidence, the agency more recently promoted the classification of B[a]P to that of a Group 1 carcinogen, or a known human carcinogen (5,6).
Fig. 1.
Metabolic scheme showing activation and detoxification pathways of B[a]P (adapted from Boelsterli, 2003).
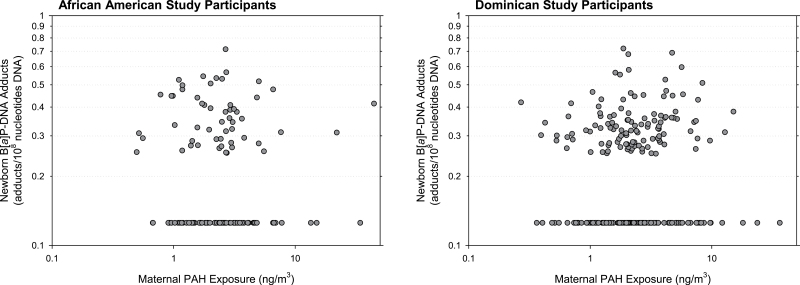
In this study, we have evaluated the interaction between maternal PAH exposure measured by personal air monitoring during pregnancy and both maternal and newborn genetic haplotypes on B[a]P-DNA adducts measured in umbilical cord blood. We have described previously the susceptibility of the human fetus to B[a]P-DNA adduction in our epidemiologic cohorts (7,8). Additionally, the formation and persistence of B[a]P-DNA adducts and their association with cancer have been described in other experimental and epidemiologic studies (9,10). In our New York City-based cohort of mothers and newborns, we observed a lack of correlation between maternal PAH exposure and paired cord blood B[a]P-DNA adducts. These data are shown in Figure 2. There was a weak correlation between maternal B[a]P-DNA adducts and paired cord blood B[a]P-DNA adducts (for African-Americans, the correlation coefficient between the two variables was 0.25, and for Dominicans, the correlation coefficient was also 0.25; data not shown). The variation in percentage of B[a]P across the total maternal PAH exposure measurements is one reason for our observed lack of correlation between maternal PAH exposure and paired cord blood B[a]P-DNA adducts. However, we do not think that this fully explains the lack of correlation and thus we hypothesized that interindividual genetic variability can account for some of the lack of concordance we observed between maternal PAH exposure and paired cord blood adducts. We previously reported on a preliminary gene–environment interaction analysis conducted in our cohort in which we found significant interactions between maternal PAH exposure and several single-nucleotide polymorphisms (SNPs) and haplotypes in relevant metabolism genes on B[a]P-DNA adducts in cord blood (11). This study was limited by small sample size (n = 155 African-American and n = 250 Dominican mothers for whom we had PAH exposure data, and n = 76 African-American and n = 104 Dominican newborns for whom we had B[a]P-DNA adduct data in our New York City cohort). Our current study involved a larger number of subjects (n = 247 African-American and n = 433 Dominican mothers for whom we had PAH exposure data, and n = 133 African-American and n = 241 Dominican newborns for whom we had B[a]P-DNA adduct data) and a greater number of genes for haplotype and also a SNP analysis. We focused on genetic haplotypes in this study because the combination of multiple genetic markers can sometimes provide greater power for detection of an effect than the analysis of a single SNP (12).
Fig. 2.
There is a lack of correlation between maternal PAH exposure and cord blood levels of B[a]P-DNA adducts. Maternal PAH exposure levels were measured and plotted against the amount of B[a]P-DNA adducts measured in the paired umbilical cord blood samples. No correlation was observed between maternal PAH exposure and cord blood measures of B[a]P-DNA adducts. The correlation coefficients between maternal PAH exposure in African-Americans and paired cord blood DNA adducts were <0.01 for both the full dataset and for the dataset excluding values at the limit of detection. The correlation coefficient between maternal PAH exposure in Dominicans and paired cord blood DNA adducts was <0.01 for the full dataset and was 0.01 for the dataset excluding values at the limit of detection. Measures depicted in the graphs above come from 133 African-American and 241 Dominican study participants, respectively, from which both personal PAH exposure and umbilical cord blood B[a]P-DNA adduct measurements were available.
The metabolic roles of each of the genes selected for this interaction study are shown in Figure 1. B[a]P, the parent compound, is primarily metabolized by cytochrome P450 (CYP) isoform 1A1, though isoforms 1A2 and 1B1 have also shown metabolic capability of the parent compound. Following the formation of the B[a]P 7,8-epoxide metabolite, the epoxide hydrolase enzyme catalyzes the hydrolysis of the epoxide to form the B[a]P 7,8-diol. A second CYP reaction results in the formation of B[a]P BPDE. This metabolite is the ultimate reactive carcinogen, which harbors an electrophilic center at the C10 position. The sterics of BPDE coupled with its electrophilicity can result in covalent adduction with the nucleophilic N2 of the deoxyguanosine base. The deoxyguanosine moiety is depicted in the black outline in Figure 1.
In addition to this central metabolic activation pathway, it is also possible for reactive B[a]P quinone metabolites to be formed from the B[a]P diols. NAD(P)H:quinone oxidoreductase (NQO) is a protective enzyme, which catalyzes the 2-electron reduction of quinones back to the diol metabolites. B[a]P metabolites can also be conjugated by glutathione S-transferase (GST) and UDP-glucuronosyl transferase enzymes, respectively, to generate readily excretable metabolites.
Here, we report the significant results on interactions between maternal PAH exposure and haplotypes (and in one instance, a SNP) in selected B[a]P metabolism genes (CYP1A1, CYP1A2, CYP1B1, GSTM3, GSTT2, NQO1 and XRCC1) on B[a]P-DNA adducts in paired umbilical cord blood samples. Per the B[a]P metabolism detailed, CYP1A1, CYP1A2 and CYP1B1 are involved in metabolizing the parent B[a]P compound to BPDE, which is involved in the formation of the B[a]P-DNA adduct. In contrast, GSTM3, GSTT2 and NQO1 are involved in shunting B[a]P metabolism so as to prevent formation of the reactive BPDE. XRCC1 is involved in the repair of B[a]P-adducted DNA.
Materials and methods
Study participants
Study subjects were selected from a longitudinal cohort study conducted in New York City; the details on study design have been published previously (13). Study subjects included African-American and Dominican women residing in the Washington Heights, Central Harlem and South Bronx neighborhoods, who were recruited through the obstetrical services of New York Presbyterian Hospital, Harlem Hospital or satellite clinics between February 1998 and February 2003. Ethnicity was self-identified by the study participants. The institutional review board of New York Presbyterian Medical Center approved the study, and informed consent was obtained from all study participants.
Pregnant women were considered eligible for the study if they were not currently smoking, were registered at a prenatal health care clinic, had lived at their present address for at least a year before the initial interview, were ≥18 years of age, had no history of illicit drug use, or pregnancy-related diabetes or hypertension and had a valid estimate of gestational age.
Maternal PAH exposure assessment
During the second or third trimesters of pregnancy, the study participants carried a backpack containing a portable personal exposure air monitor during the day and kept it near their beds at night over a consecutive 48-h period for personal PAH exposure measurements. Air extracts were analyzed at the Southwest Research Institute in San Antonio, TX, for levels of pyrene and the following eight carcinogenic PAH: benzo[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, B[a]P, indeno[1,2,3-cd]pyrene, dibenz[a,h]anthracene and benzo[g,h,i]perylene. The methodological details have been described previously (13,14). The sum of these PAH measurements was used to generate the final values for total maternal PAH exposure. Supporting the validity of the 48-h personal monitoring of PAH, sequential 2-week indoor residential monitoring (four 2-week periods from the 32nd to the 42nd weeks of pregnancy) in the Columbia Center for Children’s Environmental Health (CCCEH) cohort showed that the overall mean indoor level was significantly correlated with the individual 48-h personal monitor estimate of PAH exposure (15).
B[a]P-DNA adduct quantitation
Umbilical cord blood (30–60 ml) was collected at delivery. White blood cells (WBCs) were isolated from the blood samples and total DNA was harvested from the cells. B[a]P-DNA adducts in WBC DNA samples were measured and quantified using a high-performance liquid chromatography-fluorescence detection method described previously, which detects B[a]P tetrols specifically measuring B[a]P-DNA adducts (16). This detection method has a coefficient of variation of 12% and a lower limit of detection of 0.25 adducts per 108 nucleotides DNA. As in prior analyses, samples falling below the limit of detection were assigned a value midway between the limit of detection and 0 (0.125 adducts per 108 nucleotides DNA). In order to minimize batch effects, a standard curve was generated for each batch of samples analyzed and cross-batch comparisons were also conducted.
Genetic polymorphism and haplotype selection
A total of 24 SNPs from seven maternal and newborn genes related to PAH metabolism, detoxification and repair were selected for haplotype analyses: CYP1A1, CYP1A2, CYP1B1, GSTM3, GSTT2, NQO1 and XRCC1. The specific SNPs analyzed were selected from the SNP500Cancer resource (17). DNA sequence analysis was performed on WBC DNA from umbilical cord blood and also on WBC DNA isolated from maternal blood, which was collected (30–35 ml) within 1 day postpartum.
Haplotypes were determined by analyzing linkage disequilibrium patterns of these genes using the software Haploview, available at: http://www.broadinstitute.org/scientific-community/science/programs/medical-and-population-genetics/haploview/haploview (18). Pairwise linkage disequilibrium between any two genetic markers was measured by standardized linkage disequilibrium coefficient D′ and visualized through Haploview. Haplotype blocks were defined based on a previously described method (19), where a pair of genetic markers is defined to be in ‘strong linkage disequilibrium’ if the one-sided upper 95% confidence bound on D′ is 0.98 and the lower bound is >0.7. Haplotype blocks resulting from linkage disequilibrium analyses are shown in Supplementary Figure 1, available at Carcinogenesis Online. Haplotype frequencies of defined haplotype blocks were also estimated using Haploview.
Interaction analyses between maternal PAH exposure and haplotypes on newborn B[a]P-DNA adducts
The previously listed eight PAH measured were significantly correlated, so a composite PAH variable was computed, as done previously (11,20). As in prior studies (11), this summed measure was dichotomized at the median (3.39ng/m3 for African-Americans and 3.15ng/m3 for Dominicans) to obtain a binary PAH exposure, defined as either ‘PAH high’ or ‘PAH low’. Because haplotypes composed of multiple genetic markers can sometimes provide greater power for detection of an effect than the analysis of a single SNP (12), we examined haplotypes for six of the seven genes of interest. A single SNP in GSTM3, rather than a haplotype, was analyzed based on limited available genetic data. We modeled the probabilities of the possible haplotype pairs per subject using a generalized linear model. The haplotype analyses, adjusted for environmental covariates, were conducted with the R package ‘haplo.stats’ (21). We assessed PAH × haplotype interactions on logarithm-transformed B[a]P-DNA adduct levels.
With respect to covariates, although environmental tobacco smoke (ETS) was not significantly associated with B[a]P-DNA adducts, it was included in the model to be consistent with other studies. Other covariates, such as maternal age, dietary PAH and maternal body mass index, were not confounders of B[a]P-DNA adducts nor were they significantly associated with B[a]P-DNA adducts at the significance level of P ≤ 0.05 and so were not included in the model. Although seasonal differences in maternal PAH exposure were observed, season was not included in the model because it was not a confounder and including it as a covariate in the models did not change the statistical significance of the interactions between the haplotypes and maternal PAH exposure on cord blood adducts.
Only those maternal PAH exposure × haplotype interactions on cord blood B[a]P-DNA adducts that achieved statistical significance (P ≤ 0.05) are reported here. A further requisite for reporting the interaction was an observed haplotype frequency of ≥5%. Associations of significant interactions with either increased or decreased levels of adducts are reported. The analyses did not apply Bonferroni or another type of correction because this is an initial study and therefore exploratory in nature.
Assessment of functional implications of interaction analysis results
In order to interpret the significant interactions between maternal PAH exposure and haplotypes in terms of their impact on function of the B[a]P metabolism, detoxification and repair enzymes we assessed, we utilized interaction coefficients to determine the fold change in the original level of adducts with the variant and reference haplotypes under either high or low PAH exposure conditions. This was based on linear regressions of logarithm-transformed B[a]P-DNA adduct levels. To determine the fold change in the level of adducts formed with the reference haplotype under high PAH exposure conditions compared with the level of adducts formed under low PAH exposure conditions, we used the formula:
To determine the fold change in the level of adducts formed with the variant haplotype under high PAH exposure conditions compared with the level of adducts formed under low PAH exposure conditions, we used the formula:
Depending on the role of the protein in B[a]P metabolism and detoxification, the calculated fold change for the variant haplotype was identified as having either a ‘protective’ or ‘not protective’ effect on adduct formation based on whether the fold change was higher or lower than 1.0.
Results
The study population from which samples were collected for analysis is described in Table I. Although 247 African-American and 433 Dominican mothers in the cohort had data on maternal PAH exposure, and 133 African-American and 241 Dominican newborns in the cohort had data on cord adducts, there were 132 African-Americans and 235 Dominicans for whom we had maternal PAH exposure data, paired cord adduct data and data on one or more candidate genes. Mean maternal PAH exposure based on personal monitoring, newborn B[a]P-DNA adducts in cord blood, gestational age, relative serving frequency of dietary PAH and maternal exposure to ETS (yes/no) are shown in Table I for African-American and Dominican study participants separately. A significantly higher percentage of African-American participants reported household ETS exposure as compared with Dominican participants. There were otherwise no statistically significant differences observed between the two ethnic groups with regard to the listed characteristics.
Table I.
Exposure, biomarker and demographic characteristics of the study population with mother and/or newborn haplotypes [mean ± SD (n)]
| African-American study participants [mean ± SD (n)] | Dominican study participants [mean ± SD (n)] | |
|---|---|---|
| Maternal PAH exposure (ng/m3) | 3.39±4.19 (247) | 3.15±3.60 (433) |
| Newborn B[a]P-DNA adducts (adducts/108 nucleotides DNA) | 0.23±0.14 (133) | 0.23±0.13 (241) |
| Gestational age (weeks) | 39.04±1.59 (236) | 39.44±1.25 (422) |
| Dietary PAHa (relative serving frequency) | 47.16±10.35 (238) | 39.83±7.81 (391) |
| Maternal exposure to ETSb (% reporting household ETS) | 47 (244) | 25 (430) |
Subjects included in the present analysis are mothers with both genotype data and paired cord blood B[a]P-DNA adduct data, and newborns with both genotype data and cord blood B[a]P-DNA adduct data. No significant differences were observed between the two ethnic groups with respect to the selected demographic and exposure characteristics, except as noted.
aRelative PAH exposure from dietary consumption of smoked meat, cheese and fish. This value is a composite score from a dietary questionnaire administered to the study participants. Each of 27 questions regarding the participants’ dietary habits related to PAH were scored on consumption frequency (1: never; 2: rarely or <1 time/month; 3: 1–2 times/month; 4: 1–2 times/week; 5: >2 times/week; 6: daily), and the sum of these 27 individual scores is represented in the dietary PAH value.
bAfrican-American and Dominican groups are statistically significantly different from each other.
As previously mentioned, the impetus for conducting this interaction analysis was the observation that maternal PAH exposure levels do not correlate with paired measurements of B[a]P-DNA adducts measured in umbilical cord blood. Figure 2 shows the lack of correlation between these two parameters, as measured within each ethnic group of study participants. Adjusting for household ETS exposure did not alter this finding.
Tables II and III display the statistically significant PAH exposure × haplotype interactions identified in our analysis, as well as the haplotype frequencies and interaction coefficients. The nucleotides distinguishing the reference from the variant haplotypes are also listed. We identified two significant PAH × maternal haplotype interactions (CYP1B1 and XRCC1) and one significant PAH × maternal SNP (GSTM3) interaction on cord B[a]P-DNA adducts, and two significant PAH × newborn haplotype interactions (CYP1A2 and XRCC1) on cord B[a]P-DNA adducts in African-Americans. Our analysis also identified one significant PAH × maternal haplotype interaction (XRCC1) on cord B[a]P-DNA adducts, and one significant PAH × newborn haplotype interaction (NQO1) on cord B[a]P-DNA adducts in Dominicans. The identical XRCC1 haplotype was involved in the significant interaction for both African-American mothers and newborns but not for Dominican mothers.
Table III.
Statistically significant (P ≤ 0.05) haplotype × PAH interactions on B[a]P-DNA adducts in cord blood in Dominican participants
| Maternal gene | SNP500Cancer SNP ID | Chromosome position | Reference haplotype | Variant haplotype | High PAH exposure with reference haplotype (n) | High PAH exposure with variant haplotype (n) | Interaction coefficient (β)a | P |
|---|---|---|---|---|---|---|---|---|
| Low PAH Exposure with Reference Haplotype (n) | Low PAH Exposure with Variant Haplotype (n) | |||||||
| XRCC1 | rs25487 | 44055726 | G | A | 65 | 19 | −0.31 | 0.01 |
| rs25489 | 44056412 | G | G | 66 | 21 | |||
| Newborn gene | SNP500Cancer SNP ID | Chromosome position | Reference haplotype | Variant haplotype | High PAH exposure with reference haplotype (n) | High PAH exposure with variant haplotype (n) | Interaction coefficient (β)a | P |
| Low PAH exposure with reference haplotype (n) | Low PAH exposure with variant haplotype (n) | |||||||
| NQO1 | rs10517 | 69743760 | C | C | 59 | 6 | −0.51 | 0.03 |
| rs1800566 | 69745145 | C | C | |||||
| rs689453 | 69752373 | G | A | 53 | 6 | |||
| rs689452 | 69752464 | C | C |
aThe interaction coefficient (β) represents the increase or decrease in the effect of high PAH exposure on the log-transformed level of cord blood B[a]P-DNA adduct formation in the presence of the variant haplotype compared with the effect of high PAH exposure with the reference haplotype.
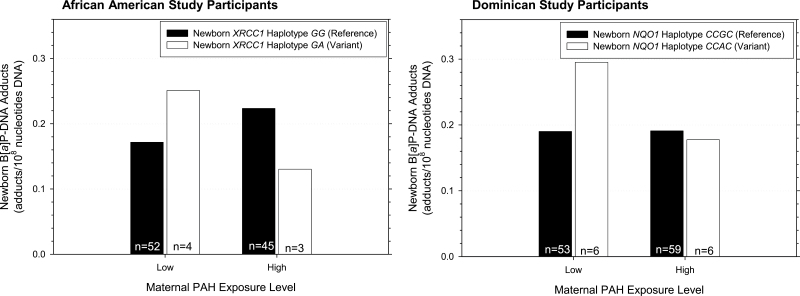
Figure 3 graphically depicts examples of significant PAH × haplotype exposure interaction effects on cord B[a]P-DNA adducts. Here, we show the significant interaction results for the XRCC1 haplotype in African-American newborns and the NQO1 haplotype in Dominican newborns. This figure illustrates that, under ‘PAH low’ conditions, cord adducts are higher in African-American newborns harboring the variant haplotype, as compared with those African-American newborns bearing the reference haplotype. Under ‘PAH high’ conditions, cord adducts are lower in African-American newborns bearing the variant haplotype as compared with African-American newborns with the reference haplotype. The same pattern is observed under ‘PAH low’ and ‘PAH high’ conditions, respectively, for the NQO1 gene in Dominican newborns. Under ‘PAH low’ conditions, cord adducts are higher in Dominican newborns harboring the variant haplotype, as compared with those Dominican newborns bearing the reference haplotype. Under ‘PAH high’ conditions, cord adducts are slightly lower in Dominican newborns bearing the variant haplotype as compared with Dominican newborns with the reference haplotype.
Fig. 3.
Interaction between maternal PAH exposure and XRCC1 and NQO1 newborn haplotypes on B[a]P-DNA adducts in cord blood. The significance of newborn XRCC1 haplotype in African-Americans and newborn NQO1 haplotype in Dominicans are shown. Cord blood adducts are higher when African-American newborns harbor XRCC1 haplotype GA (the variant haplotype) and are within the low PAH exposure group, but cord blood adducts are higher when this variant haplotype is harbored and the study participants are within the high PAH exposure group (the interaction coefficient b = −0.92, P = 0.01; n = 113). This same pattern is observed with Dominican newborns with the example of the NQO1 variant haplotype (the interaction coefficient b = −0.51, P = 0.03; n = 106).
The suggested biologic impact of the identified haplotypes involved in significant interactions with high maternal PAH exposure on cord B[a]P-DNA adducts are summarized in Table IV, based on the known function of each enzyme in B[a]P metabolism and detoxification. The information presented in Table IV offers our speculation about the effect a haplotype could be having on cord B[a]P-DNA adduct formation, under conditions of high maternal PAH exposure. We interpret our results to suggest that the CYP1B1 and XRCC1 haplotypes and GSTM3 SNP in African-American mothers are protective with regard to cord B[a]P-DNA adduct formation. The newborn CYP1A2 haplotype in African-Americans is not protective, but the XRCC1 haplotype is protective in this group. The maternal XRCC1 haplotype in Dominicans is not protective with regards to cord B[a]P-DNA adduct formation, whereas the newborn NQO1 haplotype in Dominicans is protective.
Table IV.
Functional implications of statistically significant (P ≤ 0.05) PAH exposure × haplotype interactions on cord blood B[a]P-DNA adducts
| Study participant ethnicity | Gene | Effect of high PAH exposure on B[a]P-DNA adduct formation with the reference haplotype, [e (βPAH)]a (95% confidence intervals) | Effect of high PAH exposure on B[a]P-DNA adduct formation with the variant haplotype, [e (βPAH + βinteraction)]a (95% confidence intervals) | Suggested variant haplotype function under conditions of high PAH exposure |
|---|---|---|---|---|
| African-American | Maternal CYP1B1 | 1.63 (0.89, 2.99) | 0.83 (0.50, 1.38) | Protective, decreased CYP1B1-mediated DNA adduct formation |
| Maternal XRCC1 | 1.34 (1.05, 1.71) | 0.75 (0.48, 1.19) | Protective, increased XRCC1-mediated DNA repair | |
| Maternal GSTM3 | 1.19 (0.91, 1.47) | 0.64 (0.43, 0.96) | Protective, clearance of B[a] P metabolites prior to adduct formation | |
| African-American | Newborn CYP1A2 | 0.95 (0.59, 1.52) | 1.70 (1.04, 2.77) | Not protective, increased CYP1A2-mediated DNA adduct formation |
| Newborn XRCC1 | 1.30 (1.03, 1.59) | 0.52 (0.31, 0.88) | Protective, increased XRCC1-mediated DNA repair | |
| Dominican | Maternal XRCC1 | 1.14 (0.93, 1.39) | 0.83 (0.67, 1.03) | Protective, increased XRCC1-mediated DNA repair |
| Dominican | Newborn NQO1 | 1.01 (0.80, 1.26) | 0.60 (0.39, 0.92) | Protective, decreased DNA adduct formation |
GSTM3 data are shown in italics to depict the distinction as a SNP analysis as opposed to a haplotype analysis.
aFold change of adducts formed with high PAH exposure compared with adducts formed with low PAH exposure.
Discussion
We had originally observed a lack of correlation between maternal PAH exposure and B[a]P-DNA adduct levels in paired cord blood samples, as shown in Figure 2, and this prompted us to evaluate the role of genetic variability in key maternal and newborn B[a]P metabolism genes in interacting with maternal PAH exposure. It is biologically plausible that an individual’s ability to metabolically activate and detoxify B[a]P and repair DNA damage plays a role in susceptibility to DNA adduct formation following prenatal PAH exposure. In this study, we found novel interactions between maternal PAH exposure and genetic variants in key B[a]P metabolism genes on fetal B[a]P-DNA adduct levels. We identified haplotype patterns that differ between ethnic groups and also between mothers and newborns. We observed several maternal PAH exposure × haplotype interactions on B[a]P-DNA adducts in umbilical cord blood which are displayed in Tables II and III: maternal CYP1B1, XRCC1 and GSTM3 (a PAH exposure × SNP interaction), and newborn CYP1A2 and XRCC1 in African-Americans; and maternal XRCC1 and newborn NQO1 in Dominicans.
Table II.
Statistically significant (P ≤ 0.05) haplotype × PAH interactions on B[a]P-DNA adducts in cord blood in African-American participants
| Maternal gene | SNP500Cancer SNP ID | Chromosome position | Reference haplotype | Variant haplotype | High PAH exposure with reference haplotype (n) | High PAH exposure with variant haplotype (n) | Interaction coefficient (β)a | P |
|---|---|---|---|---|---|---|---|---|
| Low PAH exposure with reference haplotype (n) | Low PAH exposure with variant haplotype (n) | |||||||
| CYP1B1 | rs162549 | 38295456 | A | A | 10 | 6 | −0.67 | 0.01 |
| rs1056837 | 38298150 | T | C | |||||
| rs1056836 | 38298203 | C | G | 13 | 6 | |||
| rs162560 | 38299515 | G | G | |||||
| rs10012 | 38302390 | G | C | |||||
| rs2617266 | 38302544 | C | T | |||||
| XRCC1 | rs25487 | 44055726 | G | G | 42 | 5 | −0.58 | 0.03 |
| rs25489 | 44056412 | G | A | 48 | 6 | |||
| GSTM3 | rs7483 | 110279701 | G | A | 38 | 13 | −0.62 | 0.01 |
| 50 | 10 | |||||||
| Newborn gene | SNP500Cancer SNP ID | Chromosome position | Reference haplotype | Variant haplotype | High PAH exposure with reference haplotype (n) | High PAH exposure with variant haplotype (n) | Interaction coefficient (β)a | P |
| Low PAH Exposure with Reference Haplotype (n) | Low PAH Exposure with Variant Haplotype (n) | |||||||
| CYP1A2 | rs4886406 | 72844256 | C | A | 13 | 5 | 0.59 | 0.02 |
| rs762551 | 75041917 | C | A | |||||
| rs2472304 | 75044238 | G | G | 22 | 5 | |||
| rs4646427 | 75045692 | T | C | |||||
| XRCC1 | rs25487 | 44055726 | G | G | 45 | 3 | −0.92 | <0.01 |
| rs25489 | 44056412 | G | A | 52 | 4 |
GSTM3 data are shown in italics to depict the distinction as a SNP analysis as opposed to a haplotype analysis.
aThe interaction coefficient (β) represents the increase or decrease in the effect of high PAH exposure on the log-transformed level of cord blood B[a]P-DNA adduct formation in the presence of the variant haplotype compared with the effect of high PAH exposure with the reference haplotype.
The precise origin of B[a]P-DNA adducts measured in cord blood remains unknown. The possibilities include maternal metabolism of B[a]P to form adducts, which then cross the placenta, the passing of B[a]P and/or its metabolites through the placenta, which are then metabolized by fetal enzymes, or some combination of the two. Our study cannot directly address this issue. However, we identified different maternal and newborn haplotypes that significantly interact with maternal PAH exposure on cord B[a]P-DNA adducts as well as one example of the same haplotype in mothers and newborns (XRCC1 in African-Americans) playing a role in a significant interaction. Our findings suggest that both maternal and fetal metabolism are involved in the generation of B[a]P-DNA adducts in cord blood though the proportion attributable to the ultimate measurement of these adducts in cord blood is unknown.
We have noted the existing uncertainty about the relative roles of maternal versus fetal genetic variation in determining the adduct levels in cord blood. There is also a lack of data regarding tissue specificity (both maternal and fetal) of gene expression, and whether extrahepatic expression of the candidate genes evaluated here is sufficient to be of relevance. To appropriately account for these kinds of variables in our model, we would require data on tissue-specific expression and inducibility of each of these genes in both the developing fetus and in the adult, as well as ethnicity-related differences in their gene expression levels. We therefore opted for model parsimony in our present study and did not attempt to account for such factors.
For those haplotypes found to be involved in significant PAH exposure × haplotype interactions, we speculate on their roles under high PAH exposure conditions (Table IV). For additional detail, we have provided Supplementary Figure 2, available at Carcinogenesis Online, depicting the chromosomal location of the SNPs involved in the significant haplotype interactions we observed. We conducted a thorough literature search on other studies evaluating the role of these SNPs and when information was available, we included it in a summary table, as shown in Supplementary Table 1, available at Carcinogenesis Online. Although there were some discrepancies among these previously reported studies, we generally found that the scientific literature supported our speculation as to the biological implications of haplotypes or SNP under high or low PAH exposure conditions. Thus, the interactions identified in our study support the hypothesis that genetic variability can contribute to susceptibility and potential cancer risk from prenatal PAH exposures.
Although our overall sample size for this study was larger than in our previous work, it should be noted that the sample sizes within each group for gene–environment interaction analyses were limited in power. Therefore, these study results and interpretations should be considered preliminary and in need of more robust replication in an independent study. Some additional limitations of our study were due to our inability to incorporate some known roles of PAH on enzyme level and activity in our model. PAH are known inducers of CYP enzymes, including CYP1A2 and CYP1B1 (22–25). At higher exposures, there may be higher baseline levels of these enzymes due to PAH induction. In the instance of the CYP1A2 haplotype in African-American newborns, we reported that the variant haplotype is associated with increased cord B[a]P-DNA adduct levels under high PAH exposure conditions. Some component of the increased adduct level may be attributable to PAH-mediated induction of CYP1A2, in addition to the role of the variant haplotype in modifying the adduct level. In the instance of the CYP1B1 haplotype in African-American mothers, we reported that the variant haplotype is associated with decreased B[a]P-DNA adduct levels under high PAH exposure conditions. In this case, the effect of the variant CYP1B1 haplotype on reduced adduct levels may be shadowed by PAH-mediated enzyme induction and thus we may be missing the full magnitude of the haplotype’s biologic effect. Similarly, while we observed a protective effect of XRCC1 haplotypes on B[a]P-DNA adduct levels in both African-American mothers and newborns and Dominican mothers (albeit with different variants of XRCC1), it is important to consider that the B[a]P-DNA adduct level measurements we used for these analyses reflect, at least in part, the effect of XRCC1-mediated DNA adduct repair. As with the examples of CYP1A2 and CYP1B1, we may therefore be underestimating the magnitude of the biologic effect of the XRCC1 haplotypes. Additionally, it is unclear at what level of PAH exposure saturation effects on relevant metabolic pathways may be seen in our study. The enzyme systems responsible for metabolism, detoxification and DNA repair are immature in the fetus so the pathway saturation levels may not necessarily be the same for mothers and newborns. Thus, it is possible that at high PAH exposure levels, some genetic effects are not captured by our analyses due to the effect of saturation on relevant metabolic pathways.
There are unfortunately no comparable studies conducted in other cohorts using the same methodologies for measuring PAH exposure and B[a]P-DNA adducts as we have used here. However, a recent publication showed that Chinese coke oven workers who were exposed to high PAH levels and who carried specific genetic variations in XPA and XPC, genes involved in nucleotide excision repair, had higher levels of DNA damage in lymphocytes as measured by the comet assay (26). Additional studies like this would be helpful for us to identify whether there are ethnic subpopulations that may be more genetically susceptible to PAH exposure based on sequence variation in key metabolism and repair genes. From our research described here, we found that there were three maternal and two newborn genes in African-Americans, which significantly interact with high PAH exposure on cord B[a]P-DNA adduct levels, but only one maternal gene and one newborn gene in Dominicans are involved in significant interactions. This suggests that there is a greater genetic contribution on adduct level in African-Americans compared with Dominicans.
This type of analysis is important in the context of understanding the risks to susceptible subpopulations from the carcinogenic effects of B[a]P and related PAH. An important implication of this study is that both maternal and fetal genetic variability in relevant genes play a role in B[a]P-DNA adduct formation in the developing fetus. Exploring the functional impact of these genetic changes in a laboratory-based experimental design will be key in confirming a mechanism for haplotype role in B[a]P-DNA adduction. Taken together, the findings from population-based studies like ours and the findings from laboratory-based research have the potential to identify specific at-risk populations who are most susceptible to the formation of procarcinogenic adducts resulting from PAH exposure.
Supplementary material
Supplementary Figures 1–3 and Table 1 can be found at http://carcin.oxfordjournals.org/
Funding
US National Institutes of Health (P01 ES009600, R01 ES008977, and training grant T32 CA009529); the US Environmental Protection Agency (R827027, RD832141, RD834509); Herbert Irving Comprehensive Cancer Center; Core grant (5P30 CA3696); New York Community Trust.
Conflict of Interest Statement: None declared.
Supplementary Material
Glossary
Abbreviations:
- B[a]P
benzo[a]pyrene
- BPDE
7,8-diol-9,10-epoxide
- CYP
cytochrome P450
- ETS
environmental tobacco smoke
- GST
glutathione S-transferase
- NQO
NAD(P)H:quinone oxidoreductase
- PAH
polycyclic aromatic hydrocarbon
- SNP
single-nucleotide polymorphism
- WBC
white blood cell.
References
- 1. World Health Organization - International Agency for Research on Cancer (1983). Polynuclear aromatic compounds, part 1, chemical, environmental and experimental data. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 32 International Agency for Research on Cancer, Lyon, pp. 1–453 [Google Scholar]
- 2. Osborne M., et al. (1987). Benzopyrenes (Cambridge Monographs on Cancer Research). Cambridge University Press, Cambridge, England, pp. 1–221 [Google Scholar]
- 3. Perera F.P., et al. (1982). A pilot project in molecular cancer epidemiology: determination of benzo[a]pyrene–DNA adducts in animal and human tissues by immunoassays. Carcinogenesis, 3, 1405–1410 [DOI] [PubMed] [Google Scholar]
- 4. Poirier M.C. (1997) DNA adducts as exposure biomarkers and indicators of cancer risk. Environ. Health Perspect., 105 (suppl. 4), 907–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization - International Agency for Research on Cancer (2010). Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 92 International Agency for Research on Cancer, Lyon, pp. 1–853 [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization - International Agency for Research on Cancer (2012). A review of human carcinogens: chemical agents and related occupations. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 100F International Agency for Research on Cancer, Lyon, pp. 111–138 [Google Scholar]
- 7. Perera F.P., et al. (2004) Biomarkers in maternal and newborn blood indicate heightened fetal susceptibility to procarcinogenic DNA damage. Environ. Health Perspect., 112, 1133–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perera F., et al. (2005). DNA damage from polycyclic aromatic hydrocarbons measured by benzo[a]pyrene-DNA adducts in mothers and newborns from Northern Manhattan, the World Trade Center Area, Poland, and China. Cancer Epidemiol. Biomarkers Prev., 14, 709–714 [DOI] [PubMed] [Google Scholar]
- 9. Rojas M., et al. (2004). High DNA damage by benzo[a]pyrene 7,8-diol-9,10-epoxide in bronchial epithelial cells from patients with lung cancer: comparison with lung parenchyma. Cancer Lett., 207, 157–163 [DOI] [PubMed] [Google Scholar]
- 10. Stowers S.J., et al. (1985) Formation and persistence of benzo(a)pyrene metabolite-DNA adducts. Environ. Health Perspect., 62, 31–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang S., et al. (2008) Assessment of interactions between PAH exposure and genetic polymorphisms on PAH-DNA adducts in African American, Dominican, and Caucasian mothers and newborns. Cancer Epidemiol. Biomarkers Prev., 17, 405–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Akey J., et al. (2001) Haplotypes vs single marker linkage disequilibrium tests: what do we gain? Eur. J. Hum. Genet., 9, 291–300 [DOI] [PubMed] [Google Scholar]
- 13. Perera F.P., et al. (2003) Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Environ. Health Perspect., 111, 201–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tonne C.C., et al. (2004) Predictors of personal polycyclic aromatic hydrocarbon exposures among pregnant minority women in New York City. Environ. Health Perspect., 112, 754–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rundle A., et al. (2012) Association of childhood obesity with maternal exposure to ambient air polycyclic aromatic hydrocarbons during pregnancy. Am. J. Epidemiol., 175, 1163–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tang D., et al. (2006). PAH-DNA adducts in cord blood and fetal and child development in a Chinese cohort. Environ. Health Perspect., 114, 1297–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Packer B.R., et al. (2006). SNP500Cancer: a public resource for sequence validation, assay development, and frequency analysis for genetic variation in candidate genes. Nucleic Acids Res., 34(Database issue), D617–D621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barrett J.C., et al. (2005). Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics, 21, 263–265 [DOI] [PubMed] [Google Scholar]
- 19. Gabriel S.B., et al. (2002) The structure of haplotype blocks in the human genome. Science, 296, 2225–2229 [DOI] [PubMed] [Google Scholar]
- 20. Wang S., et al. (2010) Effect of gene-environment Interactions on mental development in African American, Dominican, and Caucasian mothers and newborns. Ann. Hum. Genet., 74, 46–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schaid D.J. (2004) Evaluating associations of haplotypes with traits. Genet. Epidemiol., 27, 348–364 [DOI] [PubMed] [Google Scholar]
- 22. Chaloupka K., et al. (1995) Induction of Cyp1a-1 and Cyp1a-2 gene expression by a reconstituted mixture of polynuclear aromatic hydrocarbons in B6C3F1 mice. Chem. Biol. Interact., 96, 207–221 [DOI] [PubMed] [Google Scholar]
- 23. Misaki K., et al. (2007) Metabolic enzyme induction by HepG2 cells exposed to oxygenated and nonoxygenated polycyclic aromatic hydrocarbons. Chem. Res. Toxicol., 20, 277–283 [DOI] [PubMed] [Google Scholar]
- 24. Spink D.C., et al. (2008) Induction of CYP1A1 and CYP1B1 by benzo(k)fluoranthene and benzo(a)pyrene in T-47D human breast cancer cells: roles of PAH interactions and PAH metabolites. Toxicol. Appl. Pharmacol., 226, 213–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wen X., et al. (2005). Preferential induction of CYP1B1 by benzo[a]pyrene in human oral epithelial cells: impact on DNA adduct formation and prevention by polyphenols. Carcinogenesis, 26, 1774–1781 [DOI] [PubMed] [Google Scholar]
- 26. Wang F., et al. (2010) Genetic variants of nucleotide excision repair genes are associated with DNA damage in coke oven workers. Cancer Epidemiol. Biomarkers Prev., 19, 211–218 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.