Abstract
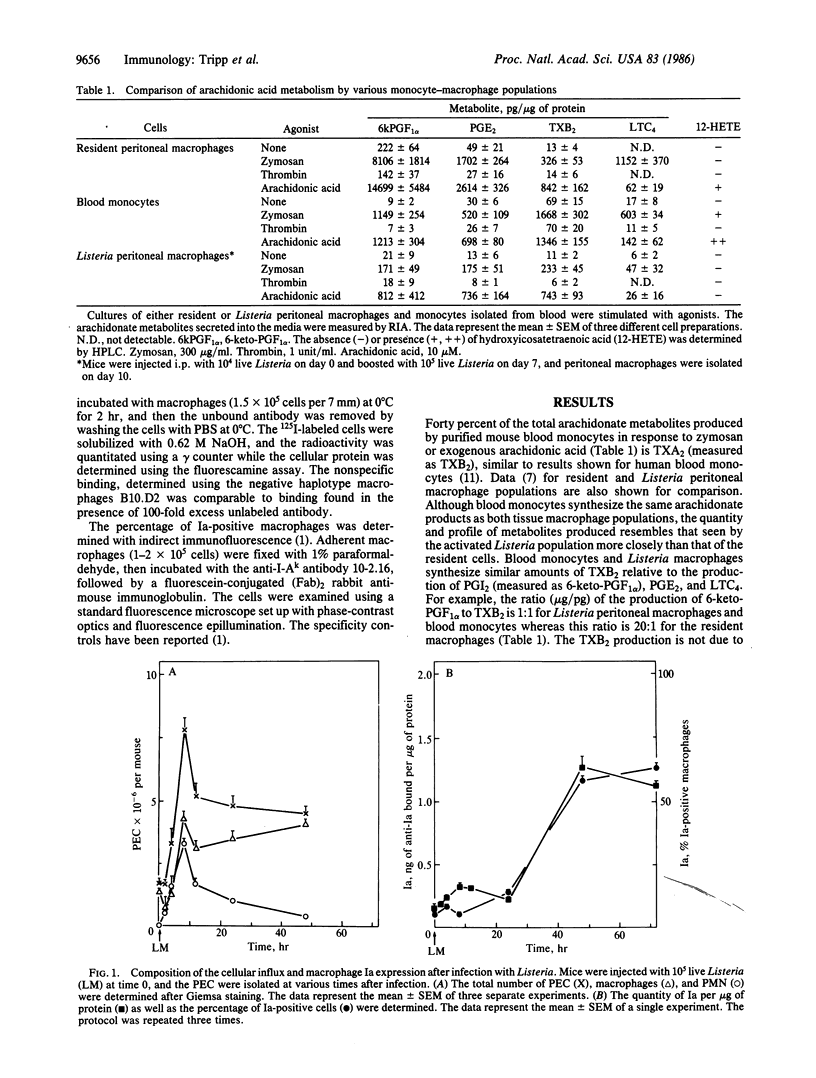
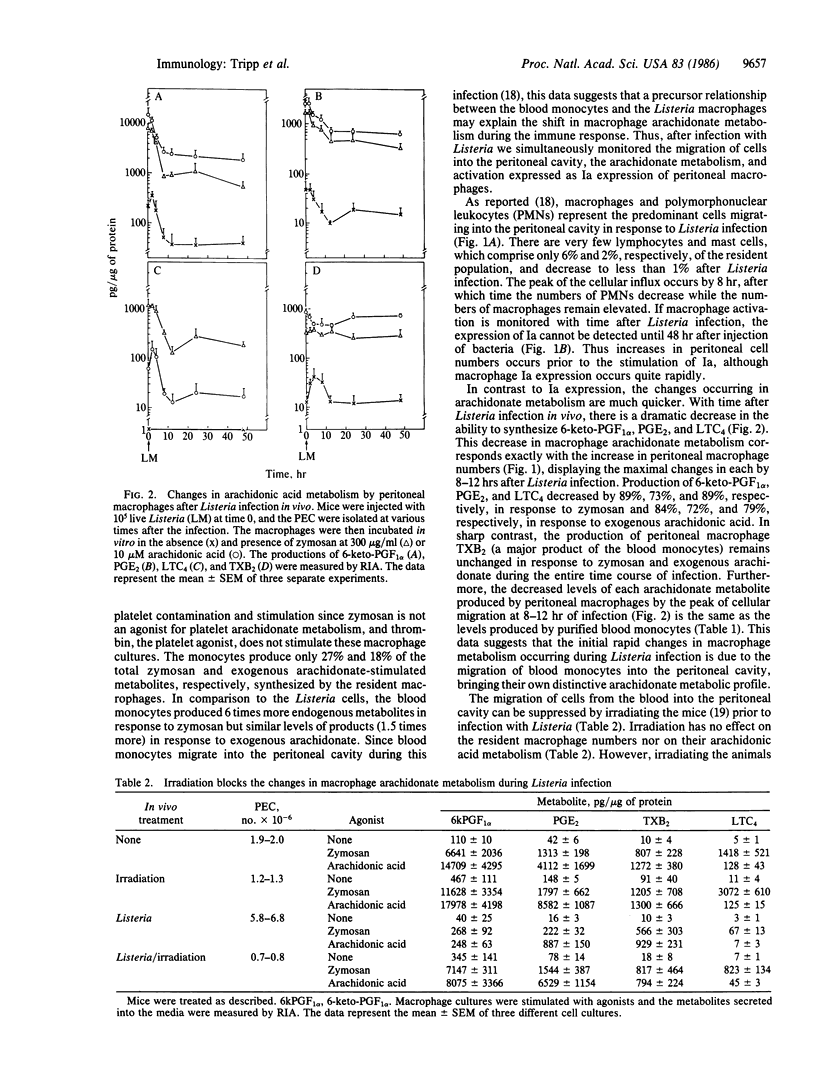
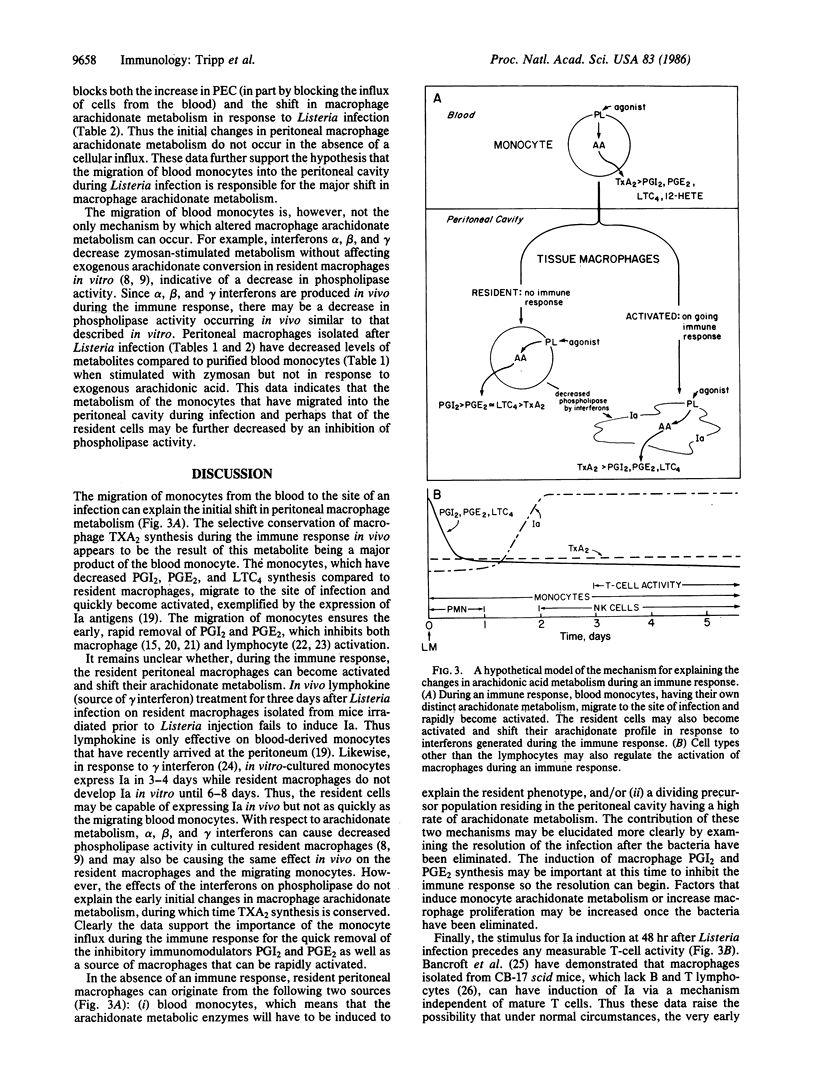
The profile of arachidonic acid metabolites in resident peritoneal macrophages is distinctly different from the profile of macrophages isolated after an acute bacterial infection. The latter produce decreased prostaglandins E2 and I2 and leukotriene C4 while conserving the synthesis of thromboxane A2. We show here that the initial changes in peritoneal macrophage arachidonate metabolism during the immune response appear to be the result of the large influx of blood monocytes, which have a characteristic metabolism distinct from resident macrophages. We demonstrate that the initial decrease in peritoneal macrophage arachidonate metabolism and the increase in macrophage numbers occur simultaneously after infection with Listeria monocytogenes. Also the macrophage arachidonate metabolism seen at the height of the peritoneal cellular influx is the same as that of purified blood monocytes. Both Listeria peritoneal macrophages and blood monocytes produce equal or greater quantities of thromboxane A2 relative to prostaglandins I2 and E2 or leukotriene C4 whereas resident cells produce 1/10 to 1/25 as much thromboxane A2 compared to the other products. Furthermore, the changes in peritoneal macrophage arachidonate metabolism in response to Listeria infection do not occur if the influx of blood monocytes is stopped by irradiating the mice prior to infection implying that the cellular influx is necessary to see the changes in arachidonate metabolism. Finally, activation of peritoneal macrophages, measured as an increase in Ia expression, occurs 36 hr after the influx of monocytes from the blood and the resultant shift in arachidonate metabolism during Listeria infection.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beller D. I., Ho K. Regulation of macrophage populations. V. Evaluation of the control of macrophage Ia expression in vitro. J Immunol. 1982 Sep;129(3):971–976. [PubMed] [Google Scholar]
- Beller D. I., Kiely J. M., Unanue E. R. Regulation of macrophage populations. I. Preferential induction of Ia-rich peritoneal exudates by immunologic stimuli. J Immunol. 1980 Mar;124(3):1426–1432. [PubMed] [Google Scholar]
- Bonney R. J., Wightman P. D., Davies P., Sadowski S. J., Kuehl F. A., Jr, Humes J. L. Regulation of prostaglandin synthesis and of the selective release of lysosomal hydrolases by mouse peritoneal macrophages. Biochem J. 1978 Nov 15;176(2):433–442. doi: 10.1042/bj1760433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boraschi D., Censini S., Bartalini M., Scapigliati G., Barbarulli G., Vicenzi E., Donati M. B., Tagliabue A. Interferon inhibits prostaglandin biosynthesis in macrophages: effects on arachidonic acid metabolism. J Immunol. 1984 Apr;132(4):1987–1992. [PubMed] [Google Scholar]
- Boraschi D., Censini S., Tagliabue A. Interferon-gamma reduces macrophage-suppressive activity by inhibiting prostaglandin E2 release and inducing interleukin 1 production. J Immunol. 1984 Aug;133(2):764–768. [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Czuprynski C. J., Henson P. M., Campbell P. A. Enhanced accumulation of inflammatory neutrophils and macrophages mediated by transfer of T cells from mice immunized with Listeria monocytogenes. J Immunol. 1985 May;134(5):3449–3454. [PubMed] [Google Scholar]
- Evers A. S., Murphree S., Saffitz J. E., Jakschik B. A., Needleman P. Effects of endogenously produced leukotrienes, thromboxane, and prostaglandins on coronary vascular resistance in rabbit myocardial infarction. J Clin Invest. 1985 Mar;75(3):992–999. doi: 10.1172/JCI111801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr A. G., Wechter W. J., Kiely J. M., Unanue E. R. Induction of cytocidal macrophages after in vitro interactions between Listeria-immune T cells and macrophages--role of H-2. J Immunol. 1979 Jun;122(6):2405–2412. [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes J. L., Burger S., Galavage M., Kuehl F. A., Jr, Wightman P. D., Dahlgren M. E., Davies P., Bonney R. J. The diminished production of arachidonic acid oxygenation products by elicited mouse peritoneal macrophages: possible mechanisms. J Immunol. 1980 May;124(5):2110–2116. [PubMed] [Google Scholar]
- Jelinek D. F., Thompson P. A., Lipsky P. E. Regulation of human B cell activation by prostaglandin E2. Suppression of the generation of immunoglobulin-secreting cells. J Clin Invest. 1985 Apr;75(4):1339–1349. doi: 10.1172/JCI111835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston A. E., Kay J. E., Ivanyi J. The effects of prostaglandin E and I analogues on lymphocyte stimulation. Int J Immunopharmacol. 1985;7(1):57–64. doi: 10.1016/0192-0561(85)90009-8. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Oppenheim J. J., Rosenstreich D. L. Characterization of lymphocyte-activating factor (LAF) produced by the macrophage cell line, P388D1. I. Enhancement of LAF production by activated T lymphocytes. J Immunol. 1978 May;120(5):1497–1503. [PubMed] [Google Scholar]
- Pawlowski N. A., Kaplan G., Hamill A. L., Cohn Z. A., Scott W. A. Arachidonic acid metabolism by human monocytes. Studies with platelet-depleted cultures. J Exp Med. 1983 Aug 1;158(2):393–412. doi: 10.1084/jem.158.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reingold D. F., Watters K., Holmberg S., Needleman P. Differential biosynthesis of prostaglandins by hydronephrotic rabbit and cat kidneys. J Pharmacol Exp Ther. 1981 Mar;216(3):510–515. [PubMed] [Google Scholar]
- Russell S. W., Pace J. L. Gamma interferon interferes with the negative regulation of macrophage activation by prostaglandin E2. Mol Immunol. 1984 Mar;21(3):249–254. doi: 10.1016/0161-5890(84)90080-4. [DOI] [PubMed] [Google Scholar]
- Scher M. G., Unanue E. R., Beller D. I. Regulation of macrophage populations. III. The immunologic induction of exudates rich in Ia-bearing macrophages is a radiosensitive process. J Immunol. 1982 Jan;128(1):447–450. [PubMed] [Google Scholar]
- Scott W. A., Pawlowski N. A., Murray H. W., Andreach M., Zrike J., Cohn Z. A. Regulation of arachidonic acid metabolism by macrophage activation. J Exp Med. 1982 Apr 1;155(4):1148–1160. doi: 10.1084/jem.155.4.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder D. S., Beller D. I., Unanue E. R. Prostaglandins modulate macrophage Ia expression. Nature. 1982 Sep 9;299(5879):163–165. doi: 10.1038/299163a0. [DOI] [PubMed] [Google Scholar]
- Tripp C. S., Leahy K. M., Needleman P. Thromboxane synthase is preferentially conserved in activated mouse peritoneal macrophages. J Clin Invest. 1985 Aug;76(2):898–901. doi: 10.1172/JCI112051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]


