Abstract
Background
Cognitive impairment (CI), highly prevalent in patients with heart failure (HF), increases risk for hospitalization, and mortality. However, the course of cognitive change in HF is not well characterized. The purpose of this systematic review was to examine the available evidence regarding longitudinal changes in cognitive function in patients with HF.
Methods and Results
A literature search of several electronic databases was performed. Studies published from January 1st, 1980 to September 30th, 2012 that used validated measures to diagnose HF and assess cognitive function two or more times in adults with HF were eligible for inclusion. Change in cognitive function was examined in the context of HF treatments applied (e.g., medication initiation, left ventricular assist device implantation), length of follow-up, and by comparison group. 15 studies met eligibility criteria. Significant decline in cognitive function was noted among patients with HF followed up for >1 year. Improvements in cognition were observed among patients with HF undergoing interventions to improve cardiac function (e.g., heart transplant) and among patients examined over short time periods (< 1 year). Studies comparing HF patient to their own baseline tended to report improvements while studies using a comparison group without HF tended to report declines or stability in cognition over time among patients with HF.
Conclusions
Patients with HF are at increased risk for cognitive decline but this risk appears to be modifiable with cardiac treatment. Further research is needed to identify the mechanisms that cause cognitive change in HF.
Keywords: heart failure, cognition, epidemiology
Background
Cognitive impairment (CI) is an important patient factor associated with poor outcomes in HF1. CI affects approximately 25–85% of patients with HF and develops earlier in patients with HF than in persons of similar age without HF2–5. Patients with HF have up to a 2-fold increased risk of impaired cognitive function compared to age-matched controls, particularly in the domains of memory, psychomotor speed, attention, and executive function2,6. Patients with HF and co-occurring CI may have poor somatic awareness and decreased ability to carry out essential self-care activities to manage their disease7,8, leading to worsening of HF, hospitalization, and mortality. However, the course of cognitive change in HF is not well understood.
Understanding the longitudinal course of cognitive function and identifying factors that influence cognition in patients with HF will guide clinicians in identifying patients at risk for poor outcomes and creating treatment plans that improve outcomes and quality of life. Although the relationship between cognitive function and heart failure has been examined in previous systematic reviews, these reviews4,9 were focused primarily on cross-sectional studies and did not report on change in cognition over time. Our objective was to conduct a systematic review of the literature evaluating the current evidence about longitudinal changes in cognition among patients with HF.
Methods
Searches to identify relevant articles were performed in Medline, Ovid, and ISI Web of Science in September of 2012. Keywords and Medical Subject Heading (MeSH) terms used in these searches included “cognitive disorder”,” cognition disorder”, “cognition”, “cognitive impairment”, “neurocognitive”,” memory”,” dementia”, “processing speed”, “attention”,” executive function”, “visuospatial”, “ heart failure”, “congestive heart failure”, “cardiovascular disease”, “longitudinal”, “time” and “follow-up”. Bibliographies of eligible articles were searched for additional references.
This review was limited to observational studies and controlled trials investigating changes in cognition over time in humans with HF. Additional inclusion criteria included: use of adult sample (>18 years old), publication date from January 1st, 1980 through September 30th, 2012, published in English, use of validated criteria to diagnose HF, and use of validated neurocognitive measures to assess cognitive function at two or more time points. Editorials, reviews, case studies, and meeting abstracts were excluded, as were studies with n<50 or those using a general sample from which data specific to participants with HF could not be obtained.
Data collection
We performed an initial review of the titles and abstracts of all articles to exclude any studies that did not meet inclusion criteria. Full review of all remaining studies was undertaken to determine eligibility for inclusion. One author (A.H.) independently abstracted data from all included studies using a standardized form. Information was abstracted for: study type; number of participants; type of comparator group used (e.g., self at baseline, healthy controls); demographic characteristics (i.e., age, gender, race/ethnicity, and education level); cardiac characteristics (e.g., mean left ventricular ejection fraction); HF diagnostic criteria (e.g., NYHA criteria); cognitive domains assessed and associated neurocognitive tests; frequency and timing of cognitive assessments; type of intervention or treatments applied (e.g., disease management program, heart transplant); and primary results for change in cognitive function.
Definition of Change in Cognitive Function
We defined significant change in cognitive function as a statistically significant (p<0.05) change between two time points. We chose this definition because of the variability in cognitive tests administered in the included studies which ranged from tests with standardized cut-points for impairment (e.g., Mini Mental Status Exam10) to domain-specific measures on which performance is measured on a linear scale. All studies reported whether there was significant change in performance over two time points, allowing for comparisons across studies by using this definition. Since many studies examined changes in a single domain of cognition (e.g., memory) using several assessment tools, cognitive change was recorded as significant if the results from any test measuring that domain were statistically significant.
Quality Assessment
The quality of each study was assessed using Downs & Black criteria11, which examine validity, bias, power and other study attributes. The Downs & Black scale, developed to assess quality in clinical trials, was modified, based on previous systematic reviews,12,13 to accommodate the characteristics of observational studies. For example, for non-randomized trials, criteria pertaining to randomization technique were removed. The checklist item about power was dichotomized into sufficient or insufficient power rather than a five-level ordinal item. A quality score for each study was calculated by dividing the total number of points received by the total number of points for which the study was eligible to receive based on its design characteristics (maximum=28 for RCTs, 21–26 for non-RCTs) and are reported in percentages. Due to the heterogeneous nature of the included studies, meta-analyses were not performed.
Results
Study selection
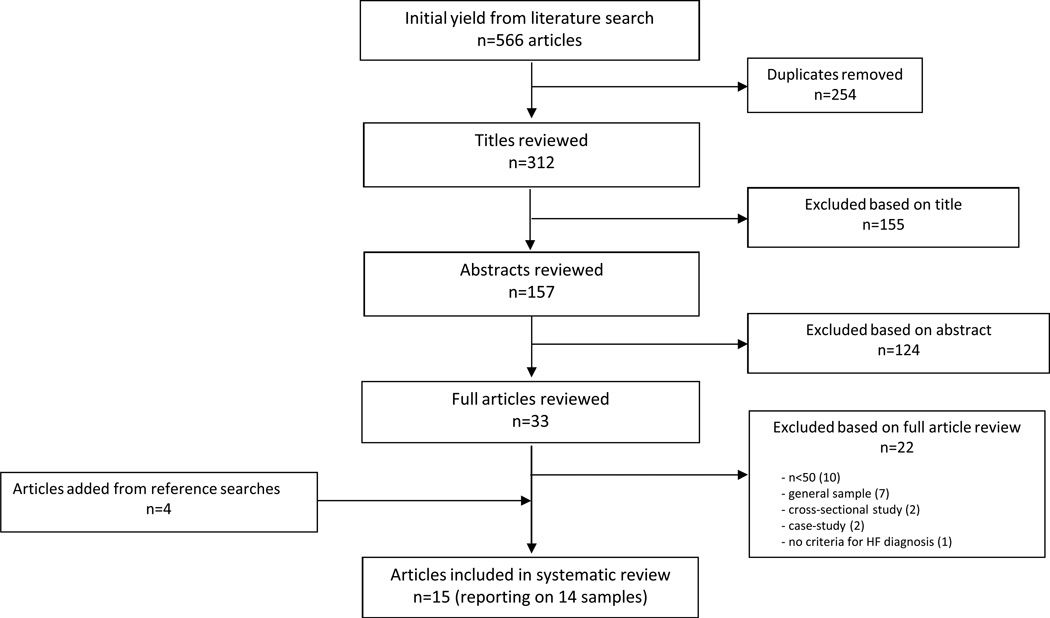
The literature search yielded 566 articles, from which 254 duplicates were removed, leaving 312 articles for review. Of these, 279 were excluded based on title and abstract review. Of the 33 full text articles reviewed, 22 were excluded, the majority due to small sample sizes (n=10) or inability to examine patterns of function in HF patients specifically (n=7). Four additional articles were identified from the references of included articles. Thus this review reports on 15 articles that report on 14 study samples (Figure 1). Characteristics of included articles are detailed in Table 1.
Figure 1.
Article Selection Process
Table 1.
Characteristics of the 16 Studies Included in the Systematic Review of Cognitive Change in Heart Failure
| First Author, Publication Year, (Reference number) |
Study Type | Sample Size and Comparison Group |
Sample Demographics |
Frequency and Timing of Cognitive Assessments |
Cognitive Domains Assessed |
Treatments/ Interventions |
Change in Cognition |
|---|---|---|---|---|---|---|---|
| Almeida, et al., 2012 (16) | Observational cohort | N=231 HF group: 77 CVD group= 73 Healthy controls=81 - HF group compared to their own, CVD group, and healthy controls at f/u |
Mean age(SD): HF group= 68.4 (10.2) CVD, no HF group= 67.8 (9.5) Healthy controls= 69.3 (11.3) |
2- baseline,2 years | Global Memory Attention |
None (standard clinical treatment assumed) | -HF patients declined in global cognition faster than healthy controls, but not compared to CVD controls - no changes in other domains |
| Almeida & Tamai, 2001 (17) | Observational cohort | N=81 HF group: 50 (31 with complete f/u)Elderly controls: 31 - Pts’ f/u scores compared to their own and controls’ baseline scores |
Mean age(SD): CHF=67.3(0.9) Controls=76.7(1.5) % Male: CHF=76.0 Controls=66.7 |
2- baseline, 6 weeks | Attention Visuospatial | None (standard clinical treatment assumed) | -HF patients improved on some attention tests -HF group had similar scores for all attention tests at f/u as controls at baseline |
| Bornstein et al, 1995 (18) | Observational cohort | N=62 (pre-operative) Intervention group: n=7 Control group: n=4 -transplant recipients compared to non-recipients |
Mean age: 44.7 (10.6) yrs 75.8% male | 2- baseline, 36-months (25-months for transplant recipients) | Global cognition Memory Reasoning/Concept Formation Mental Flexibility/Response Fluency (executive function) Attention/Concentration Other | Heart transplant | -transplanted patients showed 11.6% mean improvement in cognition, controls showed 0% change. - transplanted patients had improvements or less decline in memory, mental flexibility, and attention than non-transplanted pts |
| Ghali, et al., 2012 (27) | Randomized controlled trial | N=170 Lixivaptan group= 111 Placebo group=59 | Mean age(SD): Lixivaptan group=69.1(11.8) Placebo group= 70.6 (11.2) 64% male |
2- baseline, 4 weeks | Global Attention | Treatment with lixivaptan (vasopressin receptor antagonist) | -global function and attention improved in both groups, but difference between experimental groups NS |
| Grimm et al., 1996 (19) | Observational cohort | N=110 (baseline) - 55 heart transplant candidates - 55 healthy controls N=19 (end of f/u) -HF pts’ baseline served as own controls at f/u |
Mean age=54.8 (9.2) 92.7% male | 3- baseline, 4 and12 months post-transplant | Global cognition Other | Heart Transplant | - improvements in global cognition -improvements in cognition attenuated by in cyclosporine users |
| Hjelm et al., 2011 (14) | Observational population-based cohort | N=702 -HF=variable, 66–147 -no-HF=variable, 301–511 -HF pts compared to no-HF pts and their own baseline data at f/u |
Mean age, yrs: -HF=84.3 (4.1) -no-HF=83.3 (2.9) % male: -HF=67 -no-HF=67 100% White |
5- baseline, 2, 4, 6 and 8 years | Memory Processing speed Visuospatial | None (standard treatment assumed) | - HF pts consistently performed lower on almost all cognitive tests -HF pts declined faster than no-HF pts in memory -no-HF pts cognitively “caught up” with HF patients by end of study |
| Karlsson et al., 2005 (28) | Randomized clinical trial | N=208 (randomized) N=146 (baseline) -intervention=72 -control=74 N=90 (f/u) -pts compared to controls and their own baseline data at f/u |
Mean age=76 (7.5) % male=56 |
2- baselin, 6 months | Global | Nurse-based HF management program | -no significant improvement in cognition found in intervention group vs. control group -% impaired decreased over time (12% to 4%) |
| Kindermann, et al., 2012 (20) | Observational cohort | N at baseline=70 N at f/u=60 Decompensated HF group=20 Stable HF group=20 Healthy controls=20 - decompensated HF group compared to stable HF and healthy control groups at f/u |
Mean age (SD): Decompensated HF group= 60.4(16.4) Stable HF group= 60.6 (16.6) Healthy controls=61.6 (15.4) 75% male |
2- baseline, two weeks (14±7 days) | Memory Processing speed Executive function | Standard clinical treatment | -memory, processing speed, executive function improved in decompensated HF group to level of stable HF group, but not to level of healthy controls |
| Petrucci et al., 2009 (21) | Observational cohort | N=93 (baseline) N=28 (complete data at end of f/u) -pts’ f/u scores compared to baseline |
Mean age=50 (14) yrs % male=81 |
3-1-month (served as baseline), 3and 6 months | Memory Visuospatial Language Processing Speed Executive Function | Left Ventricular Assist Device (LVAD) implantation | -visuospatial perception, memory, and processing speed improved in pts with complete data |
| Qiu et al., 2006 (15) | Observational population-based cohort | N=1301 -HF=205 -no-HF=1096 -HF pts compared to no-HF pts and their own baseline data at f/u |
Mean age, yrs: -HF=83.3 (5.4) -no-HF=81.2 (4.8) % male: -HF=20 -no-HF=74 |
4- baseline, 4, 6, and 9 years (mean f/u= 5.02 years) | Global cognition (dementia diagnosis) | None (standard treatment assumed) | -HF at baseline associated with HR of 1.84 for dementia at f/u compared to no-HF pts -use of antihypertensives attenuated dementia risk |
| Riegel, et al., 2012 (22) | Observational cohort | N=279 Average processing speed group=114 Below average processing speed group=165 | Mean age (SD): Average PS group=56.1(12.1) Below average PS group=66.3(11.9) 64% male |
3- baseline, 3 and 6 months | Global Memory Attention Processing speed | None (standard treatment assumed) | - no significant changes changes in cognition |
| Schall, et al., 1989 (23) | Observational cohort | N=54 (pre-transplant) N=20 (post-transplant) -patients served as own controls |
Mean age, yrs =46 (11) %male= 81.5% |
2- baseline, 7.7 months (range=3–15) |
Global cognition Memory Visuospatial Other |
Heart Transplant | -No change in cognition (except “other”) |
| Stanek, et al., 2009 (24) | Observational cohort | N=70 (complete cases) -HF=40 -no-HF=35 -HF pts’ scores compared to no-HF patients’ scores and their own baseline |
Mean age, yrs=70.7 (7.7) % male= 63 |
2- baseline and 12 months | Global cognition Memory Attention Verbal fluency Visuospatial Planning/reasoning |
None (standard clinical treatment assumed) | -HF pts’ global cognition, attention, verbal fluency, planning/reasoning improved at f/u |
| Zuccala, et al., 2005 (25) | Observational cohort | N=1511 -HF pts compared at f/u according to their “normalization” status on clinical tests |
Mean age, yrs: -impaired=82 (8) -not impaired=76 (10) % male: -impaired=39 -not impaired=50 |
2- baseline and immediately before hospital discharge (mean=15 +/− 10 days) | Global cognition | None (standard clinical treatment) | -Normalization of hypo/hyperglycemia, hypo/hyperkalemia, and anemia associated with improved global cognition |
| Zuccala, et al., 2005 (26) | Observational cohort | N=1220 -ACI-inhibitor (ACEI) group=446 -no-ACEI group=774 -pts on (ACEI) compared to patients not on ACEI |
Mean age, yrs: -ACEI=79(9) -no-ACEI=79 (9) % male: -ACEI=43 -no-ACEI=47 |
2- baseline and immediately before hospital discharge [mean=13 (IQR=8–20) days] | Global cognition | Standard treatment with ACEI | -ACEI pts experienced greater improvement in global cognition than no-ACEI pts |
Study Design & Recruitment
The majority of included studies were observational cohort studies (n=13). Two of these cohort studies14,15 included population-based cohorts in which residents of entire municipalities or countries were eligible for enrollment and eleven studies16–26 reported on samples recruited from inpatient or outpatient care settings. Two non-randomized trials27,28 were included.
Six studies recruited individuals without HF to serve as comparison groups. Individuals without HF were recruited from ambulatory or outpatient care settings16,17,20,24, voluntarily responded to a media campaign16, or were recruited as part of a population-based cohort14,15. Individuals without HF were recruited at the same general location (i.e., same study sites) and during the same time period as individuals with HF.
Several exclusion criteria that may impact the association between HF and cognitive change were consistently noted in included studies. A number of studies reported excluding participants based on comorbidities of HF that increase with age and may also be associated with cognitive function such as history of stroke, cerebrovascular disease, or severe neurologic disease16–18,24–27, dementia or other cognitive impairments15,16,22,24–26, recent myocardial infarction16,17,27, illiteracy16,17 and depression or other psychiatric disorder16,20,22,24.
Quality Ratings
Quality ratings, based on modified Downs & Black criteria, ranged from 67.9% to 91.3% (out of a possible 100%); the average score was 84.3.
Sample Characteristics
Sample sizes ranged from 54 to 1511 participants. Six studies17,18,20,21,23,24 had less than one hundred participants, six studies14,16,19,22,27,28 had 100–999 participants, and three studies15,25,26 had ≥1,000 participants.
Comparison groups ranged widely among the 15 studies. Four studies19,21–23 did not use a comparison group but rather compared participants’ cognitive performance at follow-up to their baseline scores. Eleven studies made formal comparisons to control groups9,14–16,18,20,24–28 and compared treatment/intervention groups to control participants without HF,14–17,20,24 stable HF patients,20 HF patients who did not undergo the treatment/intervention under study,18,26–28, or HF patients who did not respond to the treatment/intervention under study25. Eight studies17,18,20,24–28 compared change in cognition across multiple comparison groups, including one study20 that compared cognitive change among participants with decompensated HF, participants with stable HF, and healthy controls. Length of follow-up ranged from thirteen days26 to nine years15. The follow-up period was less than six months in over half of the studies17,20–23,25–28, 6–11 months in two studies19,24,and ≥1 year in four studies14–16,18. Eight studies17–19,21–23,27,28 reported on loss to follow-up. Rates of loss to follow-up (excluding loss to follow-up due to death, which is appreciable in patients with HF) varied widely according to the characteristics of the study sample and length of follow-up time and ranged from less than 1%22 to 66%28; the majority of studies17–19,21,23,28 experienced attrition rates greater than 30%.
Mean sample ages ranged from 44.7 ±10.618 to 84.3 ±4.114 years; studies examining cognition in the context of standard treatment practices tended to have older participants, while studies examining cognition in the context of invasive treatments (e.g., heart transplant) tended to have younger participants, reflecting the patient population typically eligible for such treatments. Cardiovascular disease risk factors common in HF that may contribute to cognitive decline including hypertension, diabetes, depression, and smoking history were accounted for in twelve14,15,17,19,20,21,22,24,25,26,27,28, seven14,15,20,24,25,26,28, five14,16,17,18,20, and three14,17,20 studies, respectively.
Cognitive Assessments & Outcomes
The majority of studies (n=10)16–18,20,23–28 included two cognitive assessments, while others had three19,21,22, four15 or five14 assessments;. The cognitive domains most often assessed were: global cognition15,16,18,19,22–28, memory14,16,18,20–24, attention16–18,22,24,27, visuospatial ability14,17,21,23,24, processing speed14,20–22, executive function18,20,21, language21,24, and reasoning18,24. Other domains tested less frequently (e.g., praxis) were not examined in this review. The studies used a wide variety of neurocognitive tests to assess cognition, with some studies using multiple tests to measure cognition in a single domain. Most studies did not report whether alternative versions of the neurocognitive tests were used to limit the influence of practice effects associated with repeated assessments.
Changes in Cognition According to Comparison Group
Results for change in cognitive function among patients with heart failure differed according to the comparison group to which they were evaluated (Table 2). Studies that compared patients’ follow-up scores on cognitive tests to their baseline scores tended to report significant improvements in performance over time17–19,21,23–26, although several of these studies reported no significant change20,23,27,28. Conversely, studies that compared change in function to comparison groups of patients without HF were more likely to report significant declines in cognition over time14–16,20. Results of studies that compared changes in cognition among decompensated heart failure patients to other heart failure patients differed according to whether the comparison group had stable or decompensated HF. Cognition tended to improve in patients with unstable HF undergoing a treatment or intervention when compared to patients with decompensated HF not receiving or responding to the treatment18,25,26, although this was not observed in one study27. When stable HF patients were used as a comparator group20, cognition in decompensated HF patients was lower than the comparison group at baseline but often improved up to the level of stable HF patients upon compensation.
Table 2.
Change in Cognition According to Comparison Group
| Comparison Group Lead Author(reference #) |
n | Cognitive Change over Time |
|---|---|---|
| Self at Baseline | ||
| Almeida & Tamai17 | 81 | Improvement |
| Bornstein, et al.18 | 62 | Improvement |
| Ghali, et al.27 | 170 | Stable |
| Grimm, et al.19 | 62 | Improvement |
| Karlsson, et al.28 | 146 | Stable |
| Kindermann, et al.20 | 70 | Stable |
| Petrucci, et al.21 | 93 | Improvement |
| Riegel, et al.22 | 279 | Stable |
| Schall, et al.23 | 54 | Stable |
| Stanek, et al.24 | 70 | Improvement |
| Zuccala, et al.25 | 1151 | Improvement |
| Zuccala, et al.26 | 1220 | Improvement |
| HF Control | ||
| Bornstein, et al.¶18 | 62 | Improvement |
| Ghali, et al.27 | 170 | Stable |
| Karlsson, et al.28 | 146 | Stable |
| Kindermann, et al.20 | 70 | Stable |
| Zuccala, et al.25 | 1151 | Improvement |
| Zuccala, et al.26 | 1220 | Improvement |
| Controls without HF | ||
| Almeida, et al.16 | 231 | Decline |
| Almeida & Tamai17 | 81 | Stable |
| Hjelm, et al.14 | 702 | Decline |
| Kindermann, et al.20 | 70 | Decline |
| Qiu, et al.15 | 1301 | Decline |
| Stanek, et al.24 | 70 | Stable |
statistical significance of comparisons not reported
Changes in Cognition According to Length of Follow-up
Cognitive changes in patients with HF were also related to length of study follow-up time (Table 3). Three14–16 out of four studies that followed participants for one year or more reported cognitive decline among HF patients, while only one20 of eleven studies that followed patients for less than one year reported cognitive decline. One study14 which followed patients for eight years reported that cognitive decline occurred faster and at a younger age in patients with HF than those without HF. Studies that examined cognitive change over shorter time periods (≤12months)17,19–28 tended to report improvements17,19,21,25–27 or no change22–24,28 in cognition. This finding implies that cognition remains stable over short periods and may even improve, particularly among HF patients whose baseline assessments were during hospitalization for HF decompensation. However, over longer periods of follow-up (>12 months) cognitive function tends to decline.
Table 3.
Change in Cognition According to Length of Follow-up
| Length of Follow-up Lead Author(reference #) |
n | Cognitive Change over Time |
|---|---|---|
| ≤ 6 months | ||
| Almeida & Tamai17 | 81 | Improvement†*, Stable‡ |
| Ghali, et al.27 | 170 | Improvement†*, Stable† |
| Karlsson, et al.28 | 146 | Stable*† |
| Kindermann, et al.20 | 70 | Improvement†*, Stable†, Decline‡ |
| Petrucci, et al.21 | 93 | Improvement†* |
| Riegel, et al.22 | 279 | Stable* |
| Schall, et al.23 | 54 | Stable* |
| Zuccala, et al.25 | 1151 | Improvement†*† |
| Zuccala, et al.26 | 1220 | Improvement†*† |
| 6 to ≤12 months | ||
| Grimm, et al.19 | 110 | Improvement†* |
| Stanek, et al.24 | 70 | Improvement†*, Stable‡ |
| >12 months | ||
| Almeida, et al.16 | 231 | Decline‡ |
| Bornstein, et al.¶18 | 62 | Improvement†*† |
| Hjelm, et al.14 | 702 | Decline‡ |
| Qiu, et al.15 | 1301 | Decline‡ |
compared to self at baseline
compared to a control group of HF patients who did not receive the treatment/intervention under study, did not respond to an intervention under study, or stable HF patients for which the intervention under study was not indicated
compared to a control group of participants without HF
statistical significance of comparisons not reported
Changes in Cognition According to Treatment
Most studies included in this review examined the effects of treatments and interventions such as heart transplant18,19,23, left ventricular assist device (LVAD) implantation21 , a nurse-led HF management program28, various medications26,27 and standard in-hospital treatments17,20,25 on cognitive change in patients with HF. Five studies14–16,22,24 examined the natural course of cognitive change among patients with HF. Evidence from included studies suggests that the direction of cognitive change varied according to the type of treatment or intervention applied (Table 4). Studies assessing the influence of invasive surgeries18,19,21,23 tended to report improvements in cognition among patients who had severe heart failure at baseline. One study28 reporting on change following a non-invasive intervention (i.e., disease management program) found no significant improvements in cognitive function over time but did report that the proportion of the study sample with cognitive impairment (defined as a Mini Mental State Examination score of ≤24) decreased from 12% at baseline to 4% at the end of six-months of follow-up. Studies examining the influence of compensation of HF among acutely decompensated patients through standard in-hospital treatments17,20,25 reported improvements in cognition that brought them up similar to scores of patients with stable HF, with the exception of one randomized controlled trial assessing the influence of a vasopressin receptor antagonist among HF patients27 that found no significant change in cognition over time despite improvements in edema. The majority (3 of 5)14–16 of studies assessing the natural course of cognition if HF documented declines. Findings from the only study24 to report improvements in cognitive function in the natural course of HF must be interpreted with caution due to potential bias introduced by lack of information on patients’ treatments, the exclusion of patients with cognitive impairment at baseline, and the use of complete case analysis.
Table 4.
Change in Cognition According to Treatment or Intervention
| Intervention Lead Author(reference #) |
n | Change in Cognition |
| Heart Transplant | ||
| Bornstein, et al.¶18 | 62 | Improvement*† |
| Grimm, et al.19 | 110 | Improvement* |
| Schall, et al.23 | 54 | Stable* |
| LVAD Implantation | ||
| Petrucci, et al.21 | 93 | Improvement* |
| Disease Management Program | ||
| Karlsson, et al.28 | 146 | Stable*† |
| Clinical/Pharmacological Treatment | ||
| Almeida & Tamai17 | 61 | Improvement*, Stable‡ |
| Ghali, et al.27 | 170 | Improvement*, Stable† |
| Kindermann, et al.20 | 70 | Improvement*, Stable†, Decline‡ |
| Zuccala, et al.25 | 1151 | Improvement*† |
| Zuccala, et al.26 | 1220 | Improvement*† |
| No Treatment Specified (Standard Treatment Assumed) | ||
| Almeida, et al.16 | 231 | Decline‡ |
| Hjelm, et al.14 | 702 | Decline‡ |
| Qiu, et al.15 | 1301 | Decline‡ |
| Riegel, et al.22 | 279 | Stable* |
| Stanek, et al.24 | 70 | Improvement*, Stable‡ |
compared to self at baseline
compared to control group of HF patients who did not receive the treatment/intervention under study, did not respond to intervention under study, or stable HF patients
compared to a control group without HF
Association of Changes in Clinical Parameters and Cognitive Function
Eight16–19,25–28 of fifteen studies included information on changes in cardiac function concomitant with changes in cognitive function. Five studies16,18,19,25,27 reported that changes in clinical parameters at least partially correlated with changes in cognitive function. Three studies17,26,28 reported that improvements in LVEF and blood pressure did not correlate with changes in cognitive function. These mixed findings and the absence of reports on correlations between changes in clinical and cognitive parameters in almost half of the studies in this review makes it difficult to draw conclusions about the association between changes in physical and cognitive health among patients with HF.
Discussion
In this review, we found evidence for significant decline in cognitive function in patients with HF followed for 11 months or more. We also found that over shorter periods of follow-up and in the setting of interventions aimed at improving cardiac function, cognition can improve in patients with HF. Our findings are consistent with those of a previous systematic review4 that suggested cognitive impairment could be reversed in patients with heart failure through heart transplantation, although the data available at the time was too sparse to draw any firm conclusions. Furthermore, this previous review only examined changes in cognitive function in HF in the context of treatments and did not include studies that examined the natural course of cognition in HF. The current review expands our understanding of longitudinal changes in cognitive function in patients with HF by examining this relationship in comparison to cognitive changes observed in samples without HF and in relation to length of study follow-up time and various treatments.
Proposed Mechanisms of Change in Cognition in HF
Several included studies posited mechanisms by which heart failure influences cognition: (1) via decreased cerebral blood perfusion, resulting in microvascular changes in the brain, and (2) cerebral emboli, both attributable to cardiac insufficiency29. As evidenced by the handful of studies that reported change in cardiac function during follow-up, the development of HF or worsening of HF severity may be associated with declines in cognition. Conversely, improvements in cardiac parameters (e.g., ejection fraction, cardiac index) as a result of treatments or interventions may improve cognition; this association was most apparent in studies documenting dramatic improvements in cardiac function, such as those evaluating heart transplant recipients. However, not all studies examining this association, even in the context of heart transplant, reported significant correlations between changes in clinical and cognitive parameters, so the link between cardiac and cognitive function cannot be confirmed. Future studies that measure changes in cardiac and cerebrovascular function of HF patients are necessary to clarify how cardiac function, especially factors related to systemic circulation, may influence cognition in the HF population. In addition, information on cardiovascular disease risk factors (e.g.,hypertension, diabetes, depression, smoking) that are common among patients with HF and are known to impact cognition should be collected and accounted for in statistical models to determine the independent effect of HF on cognitive change over time.
Comparison Group
Changes in cognition among HF patients varied according to the groups to which these changes were compared. Generally, cognitive scores tended to decline more or remain lower among patients with HF when compared with changes in samples without HF that were matched or adjusted for age, suggesting that HF patients are at greater risk for cognitive decline than their healthy counterparts. However, studies comparing cognitive change in patients with decompensated HF to those with stable HF found that patients with decompensated HF had lower cognitive scores than those with stable HF at baseline and that compensation of HF among decompensated patients was associated with improvements in cognition up to the level of stable HF patients. This finding suggests that there is room for improvement in cognition among patients with decompensated or poorly controlled HF, although the potential for improvement is probably not large enough to bring HF patients’ cognition up to the level of age-matched people without HF. Studies assessing changes in cognition in HF patients in comparison to samples with other types of cardiovascular disease did not find significant differences, suggesting that these cognitive changes may not be specific to HF but rather general deficits in cardiovascular function. This is further corroborated by the inconsistent results found between changes in measures of HF disease severity (such as left ventricular ejection fraction) and changes in cognition. Studies that did not use comparison groups to evaluate changes in cognitive function often reported improvements in cognition. However, the absence of a control group in these studies may have introduced bias by not accounting for changes in cognitive scores attributable to Hawthorne, placebo, or practice effects. These threats to validity may be appreciable especially when we consider that, with one exception19, studies did not use alternative forms of neurocognitive tests to limit bias from learning effects. Inclusion of comparison groups and the use of alternative test forms may increase the quality of future studies and make drawing conclusions about the causes of cognitive change over time easier.
Study Follow-up Time
Cognitive decline among patients with HF was reported in studies that assessed changes in cognition over long periods of time (i.e., longer than one year), while improvements or stability in cognitive scores were more often reported in studies that followed patients for shorter periods of time. Improvements in cognition were especially apparent in studies that followed patients for less than one month, such as those that followed patients admitted to a hospital for acute HF decompensation until discharge; some of these studies found improvement in patients who were quite physically and cognitively ill at baseline whose cognitive scores improved up to the level of stable HF patients by time of hospital discharge. These findings suggest that cognition may be amenable to improvement in the short-term among patients with HF, but that these improvements are not sustainable and HF patients remain at risk for long-term decline, especially as HF severity progresses. However, heterogeneity of the relatively small number of studies with long follow-up time precludes making broad conclusions about the temporality of cognitive decline in HF; more studies examining longitudinal trajectories of cognition in HF patients over long periods of time are warranted to confirm our findings.
Treatments
The majority of studies included in this review examined the association of cognition with some type of treatment or intervention for HF and generally found that patients who underwent these treatments experienced improvements in cognition. Conversely, studies that examined the natural course (i.e, no treatments or interventions specified) of cognitive change in patients with HF were more likely to report declines. These findings provide promising evidence that changes in cardiac or overall health brought on by these treatments may improve cognition. However, the use of studies14–16,22,24 characterized as “no treatment specified” (i.e., not receiving treatment) as the “reference” group for the purposes of this examination presents some problems. Realistically, we must assume that the vast majority, if not all, patients involved in these observational studies received some type of treatment for their HF, but these studies provided little or no information about the medications or other treatments patients received. It is feasible that many participants involved in these non-interventional observational studies received treatments similar to those reported to positively influence cognition in other studies, such as ACE inhibitors26, which may affect our characterization of cognitive change among these patients. However, if the “no treatment specified” group of studies did encounter this contamination from treatment, it would likely bias our results toward the null, so our findings for decline in the “no treatment specified” group of HF patients may actually be more conservative than what occurs among HF patients not receiving any treatment for their disease. Therefore, it is important for future non-interventional observational studies to transparently document any treatments being received by the study participants so that we can gain a more comprehensive understanding of how these treatments and interventions affect cognition in HF.
Research Implications
Measurement of cognitive function in older and acutely ill patients is difficult and often global tests of cognitive function are used. These tests may not be sensitive to subtle changes in cognitive function or may not adequately measure domains of cognition known to decline in cardiovascular disease, such as executive function. Domain-specific measures, when included, were not standardized across studies, limiting the ability to compare results across studies. Recently, Hachinski, et al., in collaboration with the National Institute for Neurological Disorders and Stroke and the Canadian Stroke Network, encouraged the use of standardized 5-, 30-, and 60-minute cognitive testing protocols consisting of well-validated and standardized measures that assess multiple domains of cognitive function known to be affected in patients with cardiovascular disease30. Most of the recommended measures have standardized cut-off scores for cognitive impairment that allow for easy interpretation of test scores. The consistent use of these recommended measures in future research will help to facilitate comparisons across studies and inform the development of brief assessments of cognitive function in clinical settings by identifying which measures are most sensitive to changes in cognition among patients with HF.
Loss to follow-up and attrition rates were appreciable in a number of studies, and not all studies reported information about attrition rates, reasons for attrition, or baseline characteristics of patients who were lost to follow-up. Large rates of loss to follow-up and omission of information about follow-up may introduce selection bias or healthy survivor bias, as it is reasonable to believe that patients who dropped out due to these reasons may have had poorer physical and cognitive health than those who persisted in the study. Understandably, research in HF patients is difficult and there is bound to be significant attrition due to death and disability. Future work should explicitly report loss to follow-up and examine differences between initial and follow-up samples to improve the validity of studies and make study findings easier to interpret.
Lastly, since the mechanisms of cognitive change in HF remain poorly understood future studies should aim to recruit diverse samples of patients that traverse the spectra of cardiac disease severity and cognitive ability and track changes to patients’ cardiac and cognitive function over time. Uncovering correlations between change in cardiac function and change in cognitive function may help to improve our understanding the mechanisms by which heart failure affects cognition and provide clinicians with opportunities to improve cognition through cardiac treatments.
Clinical Implications
Based on the findings of this review, change in cognitive function is common among patients with HF. Clinicians should routinely assess cognition in HF patients using the aforementioned recommended batteries30, especially during crucial times when changes in cognition may occur due to changes in cardiac function, such as at diagnosis, during hospitalization, and during initiation or discontinuation of treatment. Longitudinal follow-up of patients’ cognitive status may provide clues about changes in the patient’s ability to adequately perform self-care, which is essential for maintaining disease stability and preventing poor outcomes. Also, patients’ and family members’ perspectives regarding treatment options may be positively influenced by the possibility of cognitive improvement. It is known that treatments such as surgery31, lifestyle maintenance programs32, and medications33 improve physical function in patients with heart failure; findings from this review suggest these treatments may also improve cognitive function, which may make them more attractive to patients and their families.
Limitations
This systematic review had a number of limitations. This review was limited to studies published in English, potentially introducing publication bias. Also, due to heterogeneity of study designs and treatment methods, a quantitative meta-analysis could not be performed.
Conclusions
Short-term (≤12 months) stabilization or improvement in cognition is common with treatment in HF; however, patients with HF remain at risk of greater cognitive decline over the long-term compared to patients without HF. Clinical interventions that improve cardiac function may also improve cognitive function, which has implications for the health and quality of life of patients and their families. Cognition should be assessed regularly along with cardiac function in HF patients to identify patients at risk for poor health outcomes and those whose physical and cognitive health may benefit from optimal disease management and treatments. Future investigations with non-HF comparator groups, long follow-up periods and clear descriptions of treatments are warranted to further clarify the association between HF and changes in cognition over time.
What is known
Cognitive impairment, common among patients with heart failure, increases risk for poor outcomes such as hospitalization and mortality.
Understanding the course of cognitive change in patients with heart failure is important for identifying patients at risk for poor outcomes and developing treatments that may preserve cognitive function. The course of cognitive change in heart failure over time has not been systematically examined.
What this article adds
Findings from the 15 studies examined in this systematic review indicate that patients with HF are at higher risk of cognitive decline over time than same age peers without HF.
Following interventions to improve cardiac function (e.g., medications, cardiac resynchronization), cognitive function can stabilize or improve in patients with HF over the short-term (i.e., up to a year).
Studies that examine the natural course of cognition in HF for more than one year uniformly show declines among patients with heart failure.
Acknowledgments
Funding Source
NIH/NHLBI U01HL105268–01, NIH/NCRR U54RR026088, and NIA K01AG033643
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
References
- 1.Zuccala G, Pedone C, Cesari M, et al. The effects of cognitive impairment on mortality among hospitalized patients with heart failure. Am J Med. 2003;115:97–103. doi: 10.1016/s0002-9343(03)00264-x. [DOI] [PubMed] [Google Scholar]
- 2.Athilingam P, King KB. Heart and brain matters in heart failure: A literature review. J N Y State Nurses Assoc. 2007;38:13–19. [PubMed] [Google Scholar]
- 3.Sila CA. Cognitive impairment in chronic heart failure. Cleve Clin J Med. 2007;74(Suppl 1):S132–7. doi: 10.3949/ccjm.74.suppl_1.s132. [DOI] [PubMed] [Google Scholar]
- 4.Vogels RL, Scheltens P, Schroeder-Tanka JM, Weinstein HC. Cognitive impairment in heart failure: A systematic review of the literature. Eur J Heart Fail. 2007;9:440–449. doi: 10.1016/j.ejheart.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Harkness K, Demers C, Heckman GA, McKelvie RS. Screening for cognitive deficits using the montreal cognitive assessment tool in outpatients >=65 years of age with heart failure. Am J Cardiol. 2011;107:1203–1207. doi: 10.1016/j.amjcard.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 6.Cacciatore F, Abete P, Ferrara N, et al. Congestive heart failure and cognitive impairment in an older population osservatorio geriatrico campano study group. J Am Geriatr Soc. 1998;46:1343–1348. doi: 10.1111/j.1532-5415.1998.tb05999.x. [DOI] [PubMed] [Google Scholar]
- 7.Dickson VV, Tkacs N, Riegel B. Cognitive influences on self-care decision making in persons with heart failure. Am Heart J. 2007;154:424–431. doi: 10.1016/j.ahj.2007.04.058. [DOI] [PubMed] [Google Scholar]
- 8.Cameron J, Worrall-Carter L, Page K, Riegel B, Lo SK, Stewart S. Does cognitive impairment predict poor self-care in patients with heart failure? Eur J Heart Fail. 2010;12:508–515. doi: 10.1093/eurjhf/hfq042. [DOI] [PubMed] [Google Scholar]
- 9.Almeida OP, Flicker L. The mind of a failing heart: A systematic review of the association between congestive heart failure and cognitive functioning. Intern Med J. 2001;31:290–295. doi: 10.1046/j.1445-5994.2001.00067.x. [DOI] [PubMed] [Google Scholar]
- 10.Folstein MF, Folstein SE, McHugh PR“Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 11.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen HL, Saczynski JS, Gore JM, Goldberg RJ. Age and sex differences in duration of prehospital delay in patients with acute myocardial infarction: A systematic review. Circ Cardiovasc Qual Outcomes. 2010;3:82–92. doi: 10.1161/CIRCOUTCOMES.109.884361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Placzek H, Madoff LC. The use of immunization registry-based data in vaccine effectiveness studies. Vaccine. 2011;29:399–411. doi: 10.1016/j.vaccine.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Hjelm C, Dahl A, Brostrom A, Martensson J, Johansson B, Stromberg A. The influence of heart failure on longitudinal changes in cognition among individuals 80 years of age and older. J Clin Nurs. 2011 doi: 10.1111/j.1365-2702.2011.03817.x. [DOI] [PubMed] [Google Scholar]
- 15.Qiu C, Winblad B, Marengoni A, Klarin I, Fastbom J, Fratiglioni L. Heart failure and risk of dementia and alzheimer disease: A population-based cohort study. Arch Intern Med. 2006;166:1003–1008. doi: 10.1001/archinte.166.9.1003. [DOI] [PubMed] [Google Scholar]
- 16.Almeida OP, Beer C, Lautenschlager NT, Arnolda L, Alfonso H, Flicker L. Two-year course of cognitive function and mood in adults with congestive heart failure and coronary artery disease: The heart-mind study. Int Psychogeriatr. 2012;24:38–47. doi: 10.1017/S1041610211001657. [DOI] [PubMed] [Google Scholar]
- 17.Almeida OP, Tamai S. Clinical treatment reverses attentional deficits in congestive heart failure. BMC Geriatr. 2001;1:2. doi: 10.1186/1471-2318-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bornstein RA, Starling RC, Myerowitz PD, Haas GJ. Neuropsychological function in patients with end-stage heart failure before and after cardiac transplantation. Acta Neurol Scand. 1995;91:260–265. doi: 10.1111/j.1600-0404.1995.tb07001.x. [DOI] [PubMed] [Google Scholar]
- 19.Grimm M, Yeganehfar W, Laufer G, et al. Cyclosporine may affect improvement of cognitive brain function after successful cardiac transplantation. Circulation. 1996;94:1339–1345. doi: 10.1161/01.cir.94.6.1339. [DOI] [PubMed] [Google Scholar]
- 20.Kindermann I, Fischer D, Karbach J, et al. Cognitive function in patients with decompensated heart failure: The cognitive impairment in heart failure (CogImpair-HF) study. Eur J Heart Fail. 2012;14:404–413. doi: 10.1093/eurjhf/hfs015. [DOI] [PubMed] [Google Scholar]
- 21.Petrucci RJ, Wright S, Naka Y, et al. Neurocognitive assessments in advanced heart failure patients receiving continuous-flow left ventricular assist devices. J Heart Lung Transplant. 2009;28:542–549. doi: 10.1016/j.healun.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Riegel B, Lee CS, Glaser D, Moelter ST. Patterns of change in cognitive function over six months in adults with chronic heart failure. Cardiol Res Pract. 2012;2012:631075. doi: 10.1155/2012/631075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schall RR, Petrucci RJ, Brozena SC, Cavarocchi NC, Jessup M. Cognitive function in patients with symptomatic dilated cardiomyopathy before and after cardiac transplantation. J Am Coll Cardiol. 1989;14:1666–1672. doi: 10.1016/0735-1097(89)90013-2. [DOI] [PubMed] [Google Scholar]
- 24.Stanek KM, Gunstad J, Paul RH, Poppas A, Jefferson AL, Sweet LH, Hoth KF, Haley AP, Forman DE, Cohen RA. Longitudinal cognitive performance in older adults with cardiovascular disease: Evidence for improvement in heart failure. J Cardiovasc Nurs. 2009;24:192–197. doi: 10.1097/JCN.0b013e31819b54de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zuccala G, Marzetti E, Cesari M, et al. Correlates of cognitive impairment among patients with heart failure: Results of a multicenter survey. Am J Med. 2005;118:496–502. doi: 10.1016/j.amjmed.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 26.Zuccala G, Onder G, Marzetti E, et al. Use of angiotensin-converting enzyme inhibitors and variations in cognitive performance among patients with heart failure. Eur Heart J. 2005;26:226–233. doi: 10.1093/eurheartj/ehi058. [DOI] [PubMed] [Google Scholar]
- 27.Ghali JK, Orlandi C, Abraham WT. CK-LX2401 Study Investigators The efficacy and safety of lixivaptan in outpatients with heart failure and volume overload: Results of a multicentre, randomized, double-blind, placebo-controlled, parallel-group study. Eur J Heart Fail. 2012;14:642–651. doi: 10.1093/eurjhf/hfs051. [DOI] [PubMed] [Google Scholar]
- 28.Karlsson MR, Edner M, Henriksson P, Mejhert M, Persson H, Grut M, Billing E. A nurse-based management program in heart failure patients affects females and persons with cognitive dysfunction most. Patient Educ Couns. 2005;58:146–153. doi: 10.1016/j.pec.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Pressler SJ. Cognitive functioning and chronic heart failure: A review of the literature (2002-july 2007) J Cardiovasc Nurs. 2008;23:239–249. doi: 10.1097/01.JCN.0000305096.09710.ec. [DOI] [PubMed] [Google Scholar]
- 30.Hachinski V, Iadecola C, Petersen RC, et al. National institute of neurological disorders and stroke-canadian stroke network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 31.McAlister FA, Ezekowitz JA, Wiebe N, et al. Systematic review: Cardiac resynchronization in patients with symptomatic heart failure. Ann Intern Med. 2004;141:381–390. doi: 10.7326/0003-4819-141-5-200409070-00101. [DOI] [PubMed] [Google Scholar]
- 32.Prescott E, Hjardem-Hansen R, Dela F, Orkild B, Teisner AS, Nielsen H. Effects of a 14-month low-cost maintenance training program in patients with chronic systolic heart failure: A randomized study. Eur J Cardiovasc Prev Rehabil. 2009;16:430–437. doi: 10.1097/HJR.0b013e32831e94f8. [DOI] [PubMed] [Google Scholar]
- 33.Mayer B, Holmer SR, Hengstenberg C, Lieb W, Pfeifer M, Schunkert H. Functional improvement in heart failure patients treated with beta-blockers is associated with a decline of cytokine levels. Int J Cardiol. 2005;103:182–186. doi: 10.1016/j.ijcard.2004.08.053. [DOI] [PubMed] [Google Scholar]