Abstract
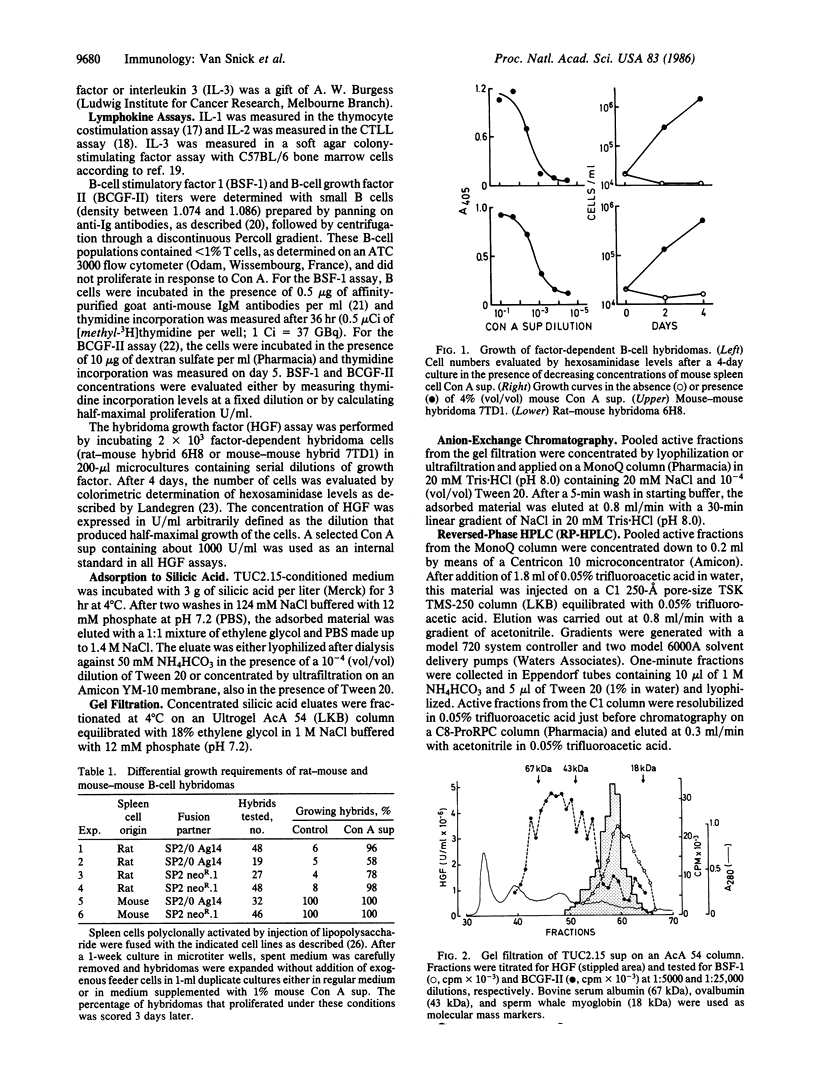
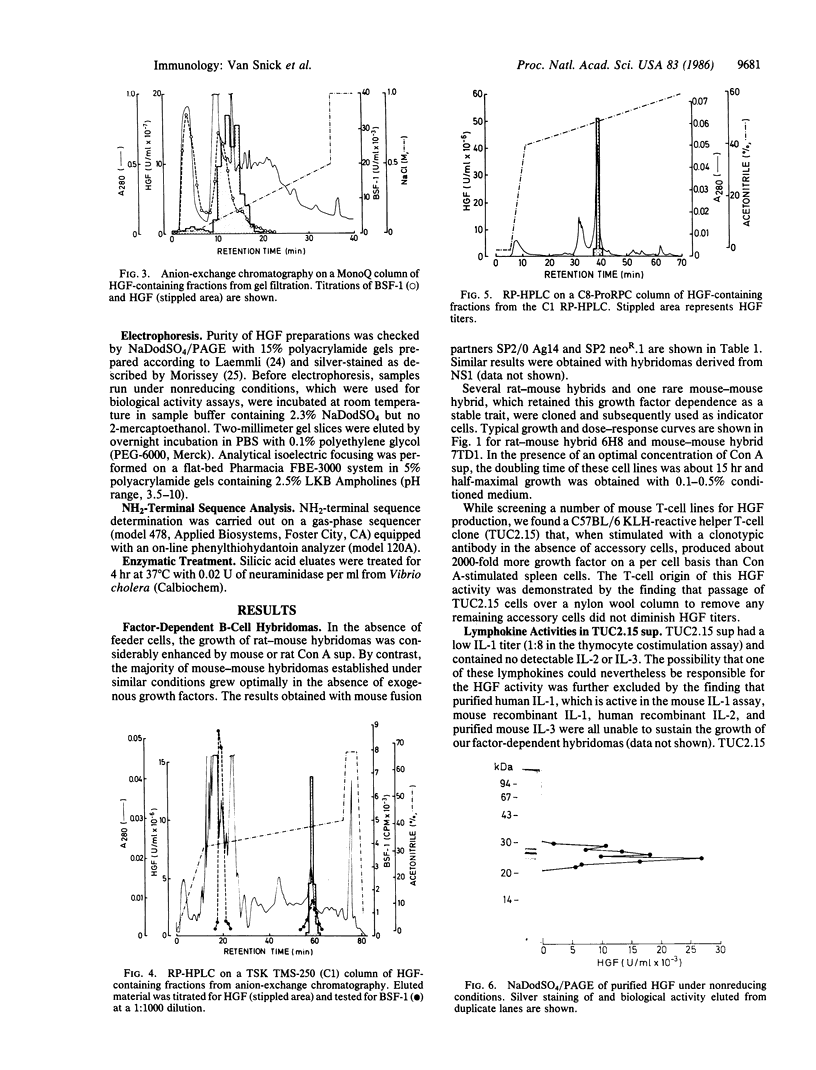
A T-cell-derived lymphokine was identified by its ability to support the growth of a subset of B-cell hybridomas. Hybrids that failed to survive in the absence of this molecule represented a major proportion of rat-mouse hybridomas but were very rare among mouse-mouse B-cell hybrids. Stable factor-dependent B-cell hybridomas were used to monitor the purification of the growth factor from the supernatant of a clonotypically stimulated mouse helper T-cell clone. Sequential fractionation using gel filtration, anion-exchange chromatography, and reversed-phase HPLC resolved the factor from other B-cell growth factors and yielded a single-chain protein characterized by a major charge (pI = 5-7) and molecular mass (22- to 29-kDa) heterogeneity, probably due to variations in glycosylation. The NH2-terminal amino acid sequence of this protein, which is active on B-cell hybridomas in the 0.1 pM range, showed no significant homology with that of known lymphokines. Because the purified factor also supported the growth and survival in vitro of murine plasmacytomas (to be published elsewhere), it was provisionally designated interleukin-HP1 (where H stands for hybridoma and P stands for plasmacytoma).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astaldi G. C., Janssen M. C., Lansdorp P., Willems C., Zeijlemaker W. P., Oosterhof F. Human endothelial culture supernatant (HECS): a growth factor for hybridomas. J Immunol. 1980 Oct;125(4):1411–1414. [PubMed] [Google Scholar]
- Brodsky F. M., Parham P., Barnstable C. J., Crumpton M. J., Bodmer W. F. Monoclonal antibodies for analysis of the HLA system. Immunol Rev. 1979;47:3–61. doi: 10.1111/j.1600-065x.1979.tb00288.x. [DOI] [PubMed] [Google Scholar]
- Chatelain C., Burstein S. A., Harker L. A. Lithium enhancement of megakaryocytopoiesis in culture: mediation via accessory marrow cells. Blood. 1983 Jul;62(1):172–176. [PubMed] [Google Scholar]
- Claflin L., Williams K. Mouse myeloma--spleen cell hybrids: Enhanced hybridization frequencies and rapid screening procedures. Curr Top Microbiol Immunol. 1978;81:107–109. doi: 10.1007/978-3-642-67448-8_16. [DOI] [PubMed] [Google Scholar]
- Corradin G., Etlinger H. M., Chiller J. M. Lymphocyte specificity to protein antigens. I. Characterization of the antigen-induced in vitro T cell-dependent proliferative response with lymph node cells from primed mice. J Immunol. 1977 Sep;119(3):1048–1053. [PubMed] [Google Scholar]
- Devos R., Plaetinck G., Cheroutre H., Simons G., Degrave W., Tavernier J., Remaut E., Fiers W. Molecular cloning of human interleukin 2 cDNA and its expression in E. coli. Nucleic Acids Res. 1983 Jul 11;11(13):4307–4323. doi: 10.1093/nar/11.13.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Howard M., Farrar J., Hilfiker M., Johnson B., Takatsu K., Hamaoka T., Paul W. E. Identification of a T cell-derived b cell growth factor distinct from interleukin 2. J Exp Med. 1982 Mar 1;155(3):914–923. doi: 10.1084/jem.155.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Howe S. C., Milstein C. Fusion between immunoglobulin-secreting and nonsecreting myeloma cell lines. Eur J Immunol. 1976 Apr;6(4):292–295. doi: 10.1002/eji.1830060411. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976 Jul;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landegren U. Measurement of cell numbers by means of the endogenous enzyme hexosaminidase. Applications to detection of lymphokines and cell surface antigens. J Immunol Methods. 1984 Mar 16;67(2):379–388. doi: 10.1016/0022-1759(84)90477-0. [DOI] [PubMed] [Google Scholar]
- Lomedico P. T., Gubler U., Hellmann C. P., Dukovich M., Giri J. G., Pan Y. C., Collier K., Semionow R., Chua A. O., Mizel S. B. Cloning and expression of murine interleukin-1 cDNA in Escherichia coli. 1984 Nov 29-Dec 5Nature. 312(5993):458–462. doi: 10.1038/312458a0. [DOI] [PubMed] [Google Scholar]
- Long W. J., McGuire W., Palombo A., Emini E. A. Enhancing the establishment efficiency of hybridoma cells. Use of irradiated human diploid fibroblast feeder layers. J Immunol Methods. 1986 Jan 22;86(1):89–93. doi: 10.1016/0022-1759(86)90269-3. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Oppenheim J. J., Rosenstreich D. L. Characterization of lymphocyte-activating factor (LAF) produced by the macrophage cell line, P388D1. I. Enhancement of LAF production by activated T lymphocytes. J Immunol. 1978 May;120(5):1497–1503. [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Spiess P. J., Rosenberg S. A. A simplified method for the production of murine T-cell growth factor free of lectin. J Immunol Methods. 1981;42(2):213–222. doi: 10.1016/0022-1759(81)90151-4. [DOI] [PubMed] [Google Scholar]
- Swain S. L., Howard M., Kappler J., Marrack P., Watson J., Booth R., Wetzel G. D., Dutton R. W. Evidence for two distinct classes of murine B cell growth factors with activities in different functional assays. J Exp Med. 1983 Sep 1;158(3):822–835. doi: 10.1084/jem.158.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme J., De Ley M., Opdenakker G., Billiau A., De Somer P., Van Beeumen J. Homogeneous interferon-inducing 22K factor is related to endogenous pyrogen and interleukin-1. Nature. 1985 Mar 21;314(6008):266–268. doi: 10.1038/314266a0. [DOI] [PubMed] [Google Scholar]
- Van Snick J., De Plaen E., Boon T. A neomycin-resistant cell line for improved production of monoclonal antibodies to cell surface antigens. Eur J Immunol. 1985 Nov;15(11):1151–1153. doi: 10.1002/eji.1830151116. [DOI] [PubMed] [Google Scholar]
- Walker K. Z., Gibson J., Axiak S. M., Prentice R. L. Potentiation of hybridoma production by the use of mouse fibroblast conditioned media. J Immunol Methods. 1986 Apr 3;88(1):75–81. doi: 10.1016/0022-1759(86)90053-0. [DOI] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de StGroth S. F., Scheidegger D. Production of monoclonal antibodies: strategy and tactics. J Immunol Methods. 1980;35(1-2):1–21. doi: 10.1016/0022-1759(80)90146-5. [DOI] [PubMed] [Google Scholar]



