SUMMARY
The murine Six gene family, homologous to Drosophila sine oculis (so) which encodes a homeodomain transcription factor, is composed of six members (Six1-6). Among the six members, only the Six2 gene has been previously shown to be expressed early in kidney development, but its function is unknown. We have recently found that the Six1 gene is also expressed in the kidney. In the developing kidney, Six1 is expressed in the uninduced metanephric mesenchyme at E10.5 and in the induced mesenchyme around the ureteric bud at E11.5. At E17.5 to P0, Six1 expression became restricted to a subpopulation of collecting tubule epithelial cells. To study its in vivo function, we have recently generated Six1 mutant mice. Loss of Six1 leads to a failure of ureteric bud invasion into the mesenchyme and subsequent apoptosis of the mesenchyme. These results indicate that Six1 plays an essential role in early kidney development. In Six1−/− kidney development, we have found that Pax2, Six2 and Sall1 expression was markedly reduced in the metanephric mesenchyme at E10.5, indicating that Six1 is required for the expression of these genes in the metanephric mesenchyme. In contrast, Eya1 expression was unaffected in Six1−/− metanephric mesenchyme at E10.5, indicating that Eya1 may function upstream of Six1. Moreover, our results show that both Eya1 and Six1 expression in the metanephric mesenchyme is preserved in Pax2−/− embryos at E10.5, further indicating that Pax2 functions downstream of Eya1 and Six1 in the metanephric mesenchyme. Thus, the epistatic relationship between Pax, Eya and Six genes in the metanephric mesenchyme during early kidney development is distinct from a genetic pathway elucidated in the Drosophila eye imaginal disc. Finally, our results show that Eya1 and Six1 genetically interact during mammalian kidney development, because most compound heterozygous embryos show hypoplastic kidneys. These analyses establish a role for Six1 in the initial inductive step for metanephric development.
Keywords: Six1, Kidney development, Eya1, Pax2, Six2, Sall1, Metanephric mesenchyme, Apoptosis, Gdnf, Mouse
INTRODUCTION
The development of permanent kidney starts at around embryonic day (E) 11 in the mouse from the metanephric mesenchyme and the ureteric bud, which both derive from the intermediate mesoderm via inductive interactions between both tissues (Lechner and Dressler, 1997; Kuure et al., 2000; Schedl and Hastie, 2000). The ureteric bud is an epithelial tube produced from the Wolffian duct and it invades the metanephric mesenchyme or blastema. Once the bud and the mesenchyme meet, a series of reciprocal inductive events take place; as a result, the ureteric bud grows and branches to form the urinary collecting system, and the mesenchyme proliferates and differentiates into nephrons. This interactive process continues until a mature kidney is formed. Although it is still unclear whether the metanephric mesenchyme initiates organogenesis by inducing the formation of the ureteric bud, or whether the initial signals derive from the Wolffian duct before budding the ureteric bud, recent genetic and molecular studies have indicated that the metanephric mesenchyme might be specified first and that a mesenchymal signal might promote ureteric bud formation (reviewed by Vainio and Lin, 2002). However, it remains unclear which genes determine the origin of the metanephric kidney and which actual molecules control the establishment of metanephric mesenchyme.
It has been shown that without the metanephric mesenchyme, neither the collecting system nor the nephrons can form (Ashley and Mostofi, 1960). Thus, the formation of a functional metanephric mesenchyme is required for normal renal development. Gene inactivation and in situ hybridization experiments have recently implicated several transcription factors in a role in mediating the formation of the metanephric mesenchyme. The Foxc1 gene, which encodes a winged helix protein, has been shown to play a role in positioning the mesenchyme, because in Foxc1−/− mice, the metanephric mesenchymes form unusually far anteriorly, which causes the ureter to grow too anteriorly or to form more than one ureter (Kume et al., 2000). The homeobox gene Lim1 is expressed in the intermediate mesoderm from its inception and has been shown to be required for all kidneys (Tsang et al., 2000). The paired box gene Pax2 is expressed in the intermediate mesoderm from E8.5 and in the metanephric mesenchyme, Wolffian duct and ureteric bud at E10.5 (Torres et al., 1995). Pax2−/− mice fail to form any kidneys and there is no ureteric bud, although the metanephric mesenchyme can be observed morphologically (Torres et al., 1995; Brophy et al., 2001). Recent studies have shown that the absence of Pax2 causes Gdnf expression to be lost from the metanephric mesenchyme, and Pax2 regulates Gdnf transcription in vitro (Brophy et al., 2001). The eyes absent 1 (Eya1) gene, which encodes a transcriptional coactivator, is only expressed in the metanephric mesenchyme and Eya1−/− mice show renal agenesis and their posterior intermediate mesoderm fails to produce Gdnf (Xu et al., 1999; Buller et al., 2001). Sall1, which encodes a zinc finger protein, is also expressed in the metanephric mesenchyme and Sall1−/− mice show the failure of tubule formation because of the incomplete ureteric bud outgrowth (Nishinakamura et al., 2001). The transcription factor Wt1 is first expressed in the metanephric mesenchyme before induction, and in Wt1-knockout mice the ureteric bud fails to grow out of the Wolffian duct and the metanephric mesenchyme subsequently apoptoses, leading to a complete failure of kidney development (Kreidberg et al., 1993). However, how these regulatory genes function and whether they interact during early metanephric induction is unclear. In addition, the molecular pathway controlling the formation of metanephric mesenchyme is not established.
The glial-derived neurotrophic factor (Gdnf) has been identified as a mesenchyme-derived signal that acts on the receptor tyrosine kinase (Ret) and Gfrα1 coreceptor which are distributed in the ureteric epithelium and induces it to produce a ureteric bud which invades the metanephric mesenchyme (Sainio et al., 1997; Saarma and Sariola, 1999). Indeed, the null mutants of Gdnf, c-Ret and Gfrα1 show similar perturbation of ureteric bud outgrowth (Schuchardt et al., 1994; Moore et al., 1996; Pichel et al., 1996; Sanchez et al., 1996; Cacalano et al., 1998). Despite the importance of Gdnf and its receptors c-Ret and Gfrα1 as inductive signals in early kidney morphogenesis, exactly how this signal transduction pathway regulates the development of the ureteric bud and the mechanisms controlling the expression of Gdnf in the mesenchyme are not well understood.
The Six1 gene is homologous to Drosophila sine oculis (so) gene, an early regulator for Drosophila eye formation (Cheyette et al., 1994; Serikaku and O'Tousa, 1994). In Drosophila, so functions synergistically with the fly Pax6 gene eyeless (ey), eyes absent (eya) and dachshund (dac) to regulate the eye morphogenesis (reviewed by Treisman, 1999). The mammalian Six gene family consists of six members (Six1-6) which share two highly conserved domains, a homeodomain (HD) and a specific Six-domain (SD) crucial for protein-protein interaction (Kawakami et al., 1996; Chen et al., 1997; Pignoni et al., 1997). Besides the eye, the Six genes are widely coexpressed with Pax, Eya and Dach (the mammalian Dachshund) genes in many tissues during mammalian organogenesis, suggesting possible interaction between their gene products and the existence of a conserved Pax-Eya-Six regulatory hierarchy (Oliver et al., 1995a; Oliver et al., 1995b; Xu et al., 1997a; Xu et al., 1997b; Xu et al., 1999; Xu et al., 2002). In early mammalian kidney development, Six2 is expressed in the metanephric mesenchyme before and after induction of kidney organogenesis and its expression in the metanephric mesenchyme is Eya1-dependent (Xu et al., 1999). Similarly, we have recently found that Six1 is also expressed in the metanephric mesenchyme before and after induction. However, the function of Six genes during kidney development has not been established.
We have recently generated Six1 null mutant mice and the mice die at birth because of malformations in several organs (Xu et al., 2002; Laclef et al., 2003). We have now examined the role of Six1 during early kidney development. Six1 is expressed in the uninduced and induced metanephric mesenchyme and Six1−/− embryos lack kidneys because of a failure of metanephric induction. Our analyses show that the epistatic relationship between Pax, Eya and Six in the metanephric mesenchyme during early kidney development is distinct from a genetic pathway elucidated in the Drosophila eye imaginal disc. Furthermore, our results show that Six1 is also required for the expression of Six2 and Sall1 in the metanephric mesenchyme. These analyses indicate that Pax2, Eya1, Six1, Six2 and Sall1 function in a molecular and genetic pathway during early kidney development, suggesting a role for Six1 in the establishment of the inductive capacity of the metanephric mesenchyme.
MATERIALS AND METHODS
Animals and genotyping
The Six1 null mutant allele was created by replacement of the endogenous start codon as well as the exon 1 with a promoterless E. coli ATG-lacZ-poly(A) cassette and the PGK-neo gene (Laclef et al., 2003). Mutant mice carrying Six1 mutant allele, Six1lacZ, were obtained using gene targeting technology. Eya1/Six1 double heterozygous mutant mice were generated by crossing mice carrying mutant alleles of Eya1 and Six1 (Six1lacZ). Mice heterozygous for a targeted disruption of the Eya1 or Pax2 gene were intercrossed to produce embryos of all three possible genotypes, respectively.
Genotyping of mice and embryos was performed as described (Torres et al., 1995; Xu et al., 1999; Xu et al., 2002).
Phenotype analyses and in situ hybridization
Embryos for histology and in situ hybridization were dissected out in PBS and fixed with 4% paraformaldehyde at 4°C overnight. Embryonic membranes were saved in DNA isolation buffer for genotyping. Histology was performed as described (Xu et al., 1999). To visualize Six1lacZ expression, mutant embryos were stained with X-gal and sectioned as described (Xu et al., 2002).
For in situ hybridization, we used four wild type or mutant embryos at each stage for each probe as described (Xu et al., 1997a).
TUNEL analysis
We performed TUNEL assay for detecting apoptotic cell death using the ApopTag detection kit (Intergen). We used six wild type or mutant embryos for this assay.
RESULTS
Six1 is required for kidney development
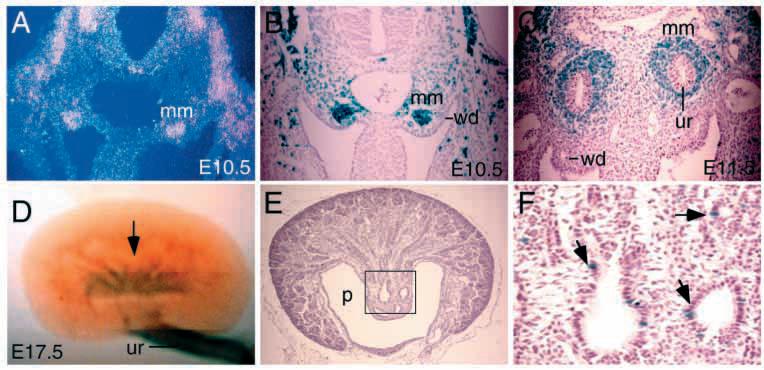
Six1 is strongly expressed in the metanephric mesenchyme and its expression was not detected in the Wolffian duct or the ureteric bud epithelium at E10.5 (Fig. 1A). To further confirm our observation, we next determined the expression of Six1 using X-gal staining for Six1lacZ. X-gal staining of heterozygous Six1lacZ embryos at E10.5 recapitulated the Six1-expression pattern obtained by RNA in situ hybridization studies (Fig. 1B). At E11.5, strong X-gal staining was observed in the induced mesenchyme around the ureteric bud (Fig. 1C). From E13.5 to P0, Six1 expression in the developing kidney was observed in the metanephric tubules as detected by X-gal staining (Fig. 1D and data not shown). Histological sections of X-gal stained E17.5 kidneys revealed that the lacZ-positive cells are localized in the collecting tubules (Fig. 1E,F). To study whether Six1 plays any role during the formation of kidney, we next examined the kidney development in Six1−/− mice. Among the 40 Six1−/− (Six1lacZ/lacZ) newborn mice analyzed so far, 39 animals showed renal agenesis (97.5%). Only one animal showed severely hypoplastic or dysplastic kidney rudiments on both sides (data not shown). Other organs that are derived from the embryonic urogenital intermediate mesoderm, including the pro- and mesonephros, the adrenal glands and the genital tracts, appeared normal (Fig. 2A-D and data not shown). Histological examination of Six1−/− mice at E12.5, when metanephric kidney forms, demonstrated the presence of a Wolffian duct and sometime the ureter-like structure (Fig. 2B,D).
Fig. 1.
Expression of Six1 during kidney development analyzed by in situ and X-gal staining of heterozygous Six1lacZ embryos for the Six1lacZ allele. (A) In situ hybridization showing Six1 expression in metanephric mesenchyme (mm) at E10.5. (B) X-gal staining of Six1lacZ heterozygous embryos showing strong Six1lacZ expression at E10.5, similar to that observed by in situ hybridization (A). (C) At E11.5, Six1 is expressed in the induced mesenchyme (mm) around the ureteric bud epithelium (ur). (D) X-gal staining of E17.5 Six1lacZ heterozygous kidneys showing Six1lacZ expression in collecting tubules (arrow). Six1lacZ is also expressed in the muscles surrounding the ureter (ur). (E,F) Transverse sections of X-gal-stained Six1lacZ heterozygous kidneys at E17.5 revealed that the Six1lacZ-expressing cells are localized in the collecting tubules (arrows). F is higher magnification of the boxed area in E. p, renal pelvis; wd, Wolffian duct.
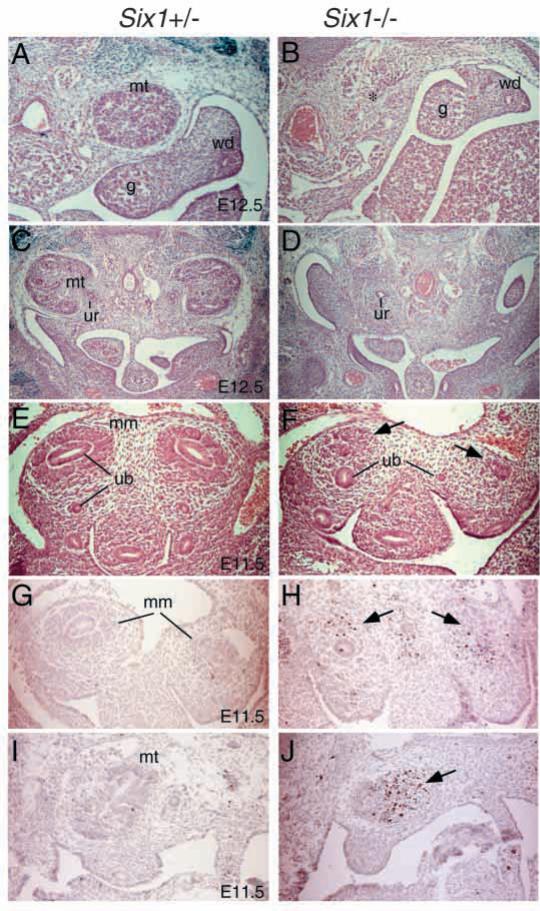
Fig. 2.
Kidney development in Six1-deficient mice. (A-D) Metanephros (mt) in Six1+/− (A,C) and Six1−/− (B,D) mice at E12.5. Kidneys are absent in Six1−/− mice (*), but ureter (ur) was observed in the left primordium (D). The genital tracts appeared to be normal both in males and females. (E) In Six1+/− embryos of E11.5, ureteric bud (ub) grows out from Wolffian duct and invades the mesenchyme and the metanephric mesenchyme (mm) are condensed around the bulging ureteric bud. (F) In Six1−/− embryos, although the ureteric buds grow out from Wolffian ducts, they fail to invade the mesenchyme (arrows) completely. (G-J) TUNEL analysis of metanephros in Six1+/− and Six1−/− embryos at E11.5. Note the apoptotic cells were markedly increased in Six1−/− metanephric mesenchyme (arrows). g, gonad; wd, Wolffian duct.
To determine whether Six1 plays a direct role in early metanephric induction, we next analyzed the kidney development in Six1−/− embryos at E10.5-11.5. At E11.5, the ureteric bud invades into the metanephric mesenchyme (Fig. 2E) and subsequent reciprocal interaction between these two tissues leads to the development of a metanephric kidney. In Six1−/− embryos, the metanephric mesenchyme morphologically distinct from the surrounding mesenchyme has formed, albeit reduced in size (Fig. 2F). The ureteric bud also formed but failed to invade the metanephric mesenchyme completely in Six1−/− embryos at E11.5 (Fig. 2F). Subsequent mesenchymal condensation and ureteric branching within the mesenchyme did not occur on either side (100%, n=20). By TUNEL analysis, apoptotic cells were increased in the mesenchyme of Six1−/− embryos at E11.5 (Fig. 2G-J). Thus, loss of Six1 leads to a failure of ureteric bud invasion into the mesenchyme and subsequent apoptosis of the mesenchyme. These results indicate that Six1 plays an essential role during early kidney morphogenesis.
Six1 is required for the expression of Pax2 and Six2 in the metanephric mesenchyme
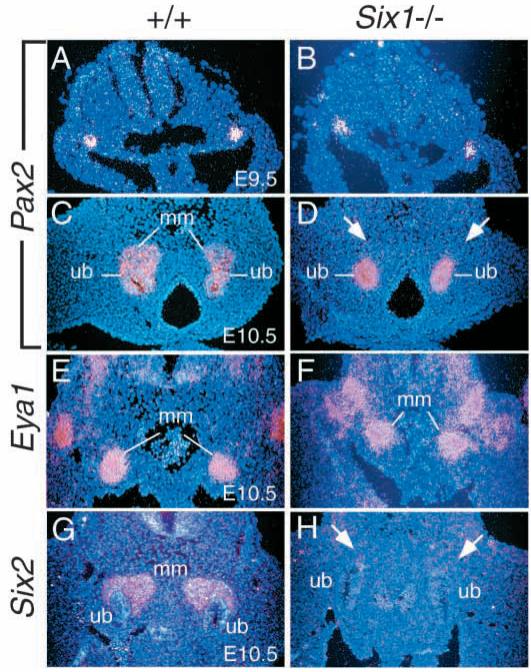
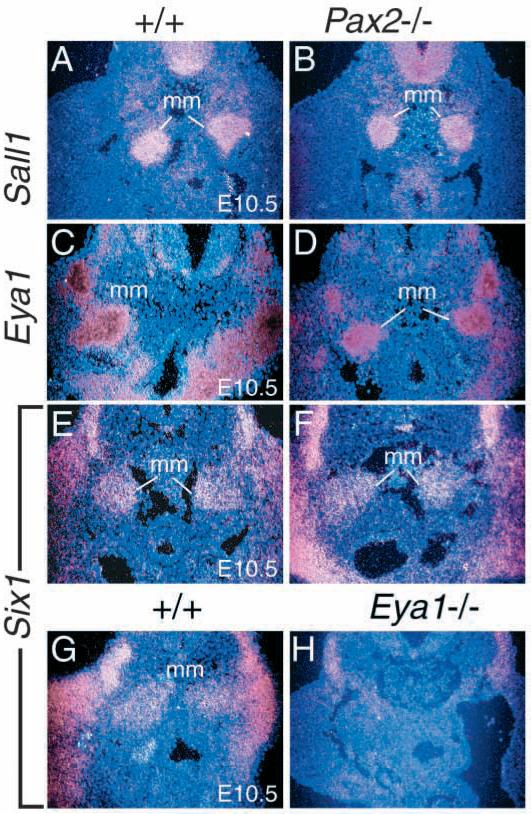
To determine the molecular defects in early kidney development of Six1−/− animals, we first examined whether the expression of the Pax and Eya gene families depends upon Six1. Studies in Drosophila indicate that eya is epistatic to so and both genes reside within the same genetic and molecular pathway downstream of the Pax6 gene ey (Halder et al., 1998). In the kidney, Pax2, Eya1 and Six1 expression overlaps in the metanephric mesenchyme and all three mutants lack kidney formation (Torres et al., 1995; Xu et al., 1999). To determine whether the Drosophila Pax-Eya-Six regulatory hierarchy is conserved during mammalian kidney development, we analyzed whether the expression of Pax2 or Eya1 is Six1-independent. The paired box gene Pax2 is normally expressed in the intermediate mesoderm before the formation of metanephric mesenchyme, in uninduced and induced metanephric mesenchyme, Wolffian duct and ureteric epithelium (Torres et al., 1995; Brophy et al., 2001). In Six1−/− embryos, no significant difference of Pax2 expression in the intermediate mesoderm, Wolffian duct and ureteric epithelium was observed at E9.0-10.5 (Fig. 3A-D). However, Pax2 expression was absent from Six1−/− metanephric mesenchyme at E10.5 (arrows, Fig. 3D). Eya1 is normally expressed in the metanephric mesenchyme before and after induction (Xu et al., 1999). In Six1−/− embryos at E10.5, the expression of Eya1 in the metanephric mesenchyme was observed at normal levels (Fig. 3E,F). Because recent studies demonstrated that Pax2 expression in the uninduced mesenchyme is independent of induction by the ureteric bud (Brophy et al., 2001), these results indicate that Six1 is required for the expression of Pax2, but not Eya1 in the metanephric mesenchyme before induction.
Fig. 3.
Six1 is required for the expression of Pax2 and Six2 but not Eya1 in the metanephric mesenchyme (mm) at E10.5. (A-D) Pax2 is normally expressed in the intermediate mesoderm (A,B), the metanephric mesenchyme before and after induction and in the ureteric epithelium (ub). In Six1−/− embryos, however, its expression in the metanephric mesenchyme at E10.5 is undetectable (arrows in D). (E,F) Eya1 is expressed in the metanephric mesenchyme before and after induction and its expression is not affected in Six1−/− mesenchyme at E10.5. (G,H) Six2 is also expressed in the metanephric mesenchyme before and after induction and its expression is undetectable in Six1−/− mesenchyme at E10.5 (arrows).
Six2, another member of the Six gene family, is also expressed in the uninduced and induced metanephric mesenchyme (Fig. 3G), and its expression was unaffected in the mesenchyme of Pax2−/− embryos (Torres et al., 1995). To address whether Six2 functions redundantly with Six1 in the mesenchyme during early kidney development, we analyzed the expression of Six2 in Six1−/− metanephric mesenchyme at E10.5-11.5. Interestingly, the expression of Six2 in the metanephric mesenchyme was markedly reduced in Six1−/− embryos at E10.5-11.5 (Fig. 3H), indicating that Six1 is required for normal expression of Six2 in the metanephric mesenchyme during early kidney development.
Six1 is also required for the expression of Sall1 in the metanephric mesenchyme
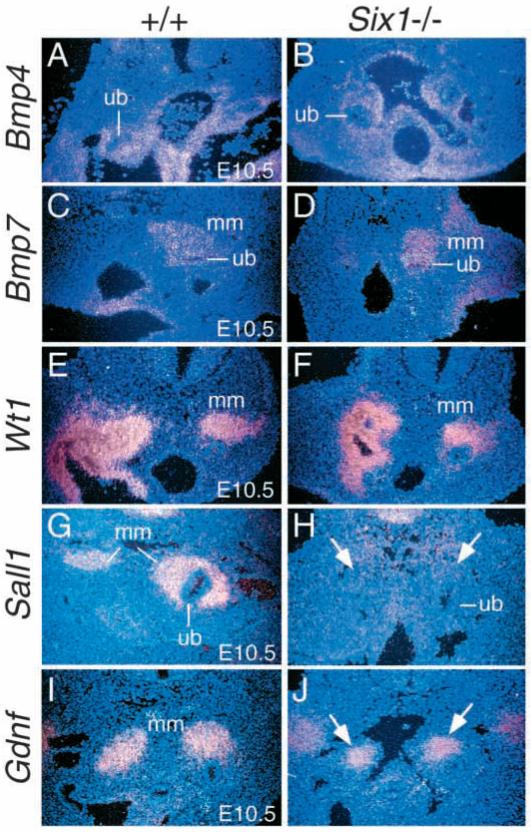
We next analyzed the expression of several other well-characterized molecular markers in metanephric mesenchyme at E10.5 and 11.5. Bmp4, a member of the Tgfβ superfamily of secreted signals, is expressed in the mesenchymal cells surrounding the Wolffian duct and ureteric stalk (Fig. 4A) and has been implicated in regulating ureteric bud growth and branching (Miyazaki et al., 2000; Raatikainen-Ahokas et al., 2000). Bmp4+/− mutant mice show kidney defects that are caused by the misregulated development of the ureteric bud (Miyazaki et al., 2000). Bmp4 protein has also been shown to regulate genes that are expressed by both the ureteric bud and the mesenchyme, including Gdnf in organ culture (Miyazaki et al., 2000; Raatikainen-Ahokas et al., 2000). No significant difference of Bmp4 expression was observed between wild type and Six1−/− mesenchyme at E10.5 (Fig. 4A,B), indicating that Six1 is not required for the expression of Bmp4 during early kidney development. Bmp7, another member of the Tgfβ superfamily, has been proposed to function as a survival signal that prevents mesenchymal cells from undergoing apoptosis during kidney development (Dudley et al., 1999; Reddi, 2000; Al-Awqati and Oliver, 2002). Bmp7 is normally expressed in the metanephric mesenchyme and ureteric epithelium and its expression level was unaffected in both structures in Six1−/− embryos at E10.5 (Fig. 4C,D). However, its expression domain in Six1−/− metanephric mesenchyme is reduced in size (Fig. 4D). Wt1 is expressed in the metanephric mesenchyme and its absence leads to failure of mesenchymal induction (Kreidberg et al., 1993). In E10.5 Six1−/− embryos, although the expression level of Wt1 in the mesenchyme is normal, its expression domain became smaller than that in wild-type embryos (Fig. 4E,F). Sall1, which encodes a zinc finger protein, is expressed in the kidney mesenchyme (Fig. 4G) and its inactivation in mice leads to incomplete ureteric bud growth and failure of tubule formation (Nishinakamura et al., 2001), similar to that seen in Six1−/− animals. Interestingly, Sall1 expression in Six1−/− metanephric mesenchyme was reduced to background level at E10.5-11.5 (arrows, Fig. 4H), indicating that Sall1 expression in the mesenchyme is Six1-dependent.
Fig. 4.
Six1 is required for the expression of Sall1 in the metanephric mesenchyme (mm). (A,B) Bmp4 is normally expressed in the mesenchyme around the ureteric stalk (ub) and its expression was not affected in Six1−/− embryos at E10.5. (C,D) Bmp7 is normally expressed in the ureteric bud (ub) and metanephric mesenchyme at E10.5 and its expression levels are normal in both structures in Six1−/− embryos at E10.5. However, its expression domain is reduced in size in Six1−/− metanephric mesenchyme (D). (E,F) Wt1 is widely expressed in the mesenchyme and urogenital ridge region during kidney development and its expression level is not affected in Six1−/− embryos at E10.5. However, its expression domain in the metanephric mesenchyme also appears to be reduced in size in E10.5 Six1−/− embryos. (G,H) Sall1 is expressed in the metanephric mesenchyme before and after induction, however its expression was undetectable in the mesenchyme in Six1−/− embryos at E10.5 (arrows). (I,J) Gdnf is expressed in the metanephric mesenchyme and its expression levels are normal in Six1−/− embryos at E10.5. However, its expression domain appears to be reduced in size in Six1−/− embryos at E10.5 (arrows).
Gdnf has been shown to act as a mesenchymal signal regulating ureteric bud outgrowth through its receptors c-Ret and Gfrα1 expressed in the ureteric epithelium (Vega et al., 1996; Sainio et al., 1997). The null embryos of Gdnf lack induction of the ureteric bud, resulting in the complete absence of the metanephric kidney, and Gdnf-soaked beads can ectopically induce budding of the ureter from the Wolffian duct (Pichel et al., 1996; Sainio et al., 1997). No significant difference of Gdnf expression level in the metanephric mesenchyme was observed between wild type and Six1−/− embryos at E10.5 (Fig. 4I,J). However, its expression domain is also reduced in size when compared to that in wild-type embryos (arrows in Fig. 4J). This result is consistent with the observation that the ureteric bud outgrows from Wolffian duct, but fails to invade mesenchyme completely in Six1−/− embryos (Fig. 2). In summary, our results show that Six1 is required for the expression of Pax2, Six2 and Sall1 in the mesenchyme at E10.5-11.5. In addition, our data show that Six1 inactivation led to size reduction of Bmp7, Wt1 and Gdnf expression domains in the mesenchyme.
Our results also show that both Pax2 and Bmp7 expression in the ureteric epithelium was unaffected in Six1−/− embryos (Fig. 3C,D and Fig. 4C,D). To determine whether the failure of kidney development in Six1−/− mice is also caused by a defect in the ureteric epithelium, we next examined several other epithelial factors that are known to be important for early kidney formation, including c-Ret, Gfrα1 and Lim1. Our results show that the expression of these markers in the ureteric bud epithelium was also unaffected in the absence of Six1 (data not shown).
Eya1, Six1 and Sall1 expression in the metanephric mesenchyme is Pax2-independent
To further clarify the genetic relationship between Pax, Eya, Six and Sall1 in the metanephric mesenchyme during early kidney development, we next examined the expression of Sall1, Eya1 and Six1 in Pax2−/− embryos. Pax2 mutant mice do not have a ureteric bud, however the metanephric mesenchyme can be observed morphologically (Torres et al., 1995; Brophy et al., 2001). As shown in Fig. 5, the expression levels of all three genes in the metanephric mesenchyme were unaffected in Pax2−/− embryos at E10.5. This result is consistent with previous observation that Six2 expression was also unaffected in Pax2−/− mesenchyme at E10.5 (Torres et al., 1995). In addition, similar to the expression of Six2 in Eya1−/− embryos at E10.5 (Xu et al., 1999), Six1 expression was also reduced to background level in Eya1−/− mesenchyme at E10.5 (Fig. 5G,H). These results together with previous observations further indicate that Eya1, Six1 and Six2 function upstream of Pax2 in the metanephric mesenchyme during early kidney development. Therefore, the genetic relationship between these genes in the metanephric mesenchyme before induction differs from that observed in Drosophila eye imaginal disc.
Fig. 5.
Sall1, Eya1 and Six1 expression is not affected in Pax2−/− metanephric mesenchyme (mm). (A,B) Sall1 is expressed in the metanephric mesenchyme and its expression is not affected in Pax2−/− mesenchyme at E10.5. (C,D) Eya1 is expressed in the metanephric mesenchyme and its expression level is unaffected in Pax2−/− embryos at E10.5. (E,F) Six1 is expressed in the metanephric mesenchyme and its expression level is also unaffected in Pax2−/− embryos at E10.5. (G,H) However, Six1 expression in Eya1−/− mesenchyme is significantly reduced when compared to that in wild-type embryos at E10.5. Its expression in the limb bud and somites is also significantly reduced. Six homozygous embryos were used for each probe.
Eya1 and Six1 genetically interact during kidney development
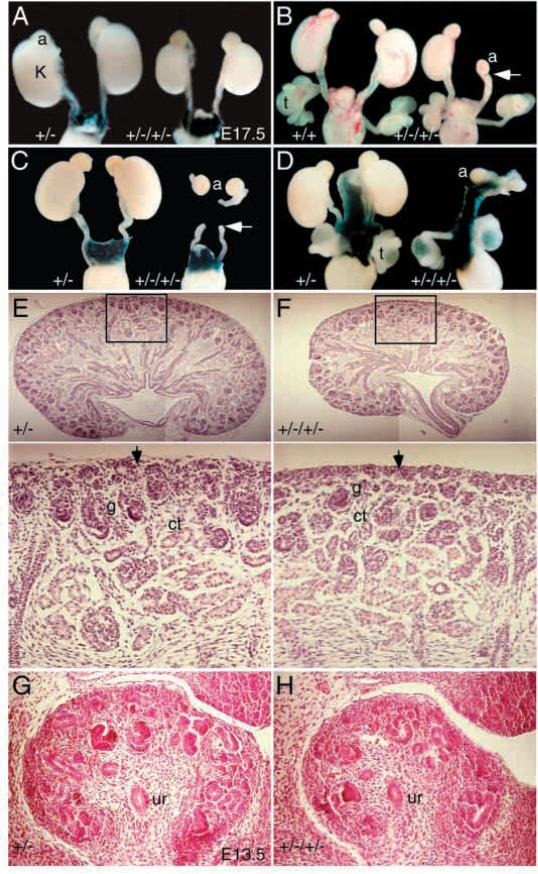
Because Eya1 and Six1 expression overlaps in the metanephric mesenchyme in the developing kidney and Eya1 and Six1 physically interact in vitro and in cultured cells (Buller et al., 2001), to further test whether these genes interact in a molecular pathway during mammalian kidney development, we examined the kidneys of newborn compound heterozygotes of Eya1+/−/Six1+/− (Table 1 and Fig. 6). On 129 background, 15 of 21 (15/21) compound heterozygous mice had smaller than normal kidneys (Table 1 and Fig. 6A). Hypoplastic kidneys were either unilateral (6/15) or bilateral (9/15). In severe cases, complete absence of the kidney (agenesis) was observed (28.6%). Similar observation was obtained in C57BL6 background (Table 1). Renal agenesis was either unilateral or bilateral (Table 1 and Fig. 6B-D). In some compound heterozygous animals that show renal agenesis, ureters that end blindly were observed (arrow, Fig. 6B,C). In contrast, each single heterozygote alone had no or mild kidney abnormalities (Table 1). These data suggest that there is a genetic interaction between Eya1 and Six1 during mammalian kidney development.
Table 1.
Kidney abnormalities in newborn compound heterozygotes of Eya1 and Six1
| Genotype | n | Small kidney | No kidney |
|---|---|---|---|
| Wild type 129 | 13 | 0 | 0 |
| Eya1+/− 129 | 17 | 3* | 0 |
| Six1+/− 129 | 19 | 2† | One bilateral |
| Eya1+/−/Six1+/− 129 | 21 | 15‡ | Five bilateral, one unilateral |
| Wild type C57BL6 | 16 | 0 | One unilateral |
| Eya1+/− C57BL6 | 13 | 1§ | 0 |
| Six1+/− C57BL6 | 12 | 0 | 0 |
| Eya1+/−/Six1+/− C57BL6 | 14 | 10¶ | Four unilateral |
n, number of animals
Three Eya1+/− 129 animals showed unilaterally smaller kidney with reduction of ~10% in weight.
Two Six1+/− 129 animals showed smaller kidneys on one side, with reduction of ~10-15% in weight.
Fifteen Eya1/Six1 129 compound heterozygotes showed small kidneys either bilaterally (n=9) or unilaterally (n=6), with reduction of ~75-22% in weight.
One Eya1+/− C57BL6 animal showed smaller kidney on one side, with reduction of ~15% in weight.
Ten Eya1/Six1 C57BL6 compound heterozygotes showed small kidneys either unilaterally (n=6) or bilaterally (n=4). Weight reduction was from 20 to 30%, less severe than that observed in 129 background.
Fig. 6.
Renal hypoplasia or agenesis in Eya1/Six1 double heterozygous animals. (A-D) E17.5 wild type, Six1+/− or Eya1+/−/Six1+/− kidneys. Samples shown in A, C and D were X-gal stained for Six1lacZ and it is expressed in the testis (t) and muscles surrounding the ureters and kidneys (D). Eya1+/−/Six1+/− animals show either smaller kidneys bilaterally (A), a small kidney on one side and no kidney on the other side (B), or complete absence of kidneys (C,D). In some Eya1+/−/Six1+/− animals that show renal agenesis, ureters that end blindly are observed (arrow in B,C). Adrenal glands and the genital tracts appeared to be normal in all compound heterozygous animals analyzed so far. (E,F) Histological analysis of kidneys of E17.5 Six1+/− and Eya1+/−/Six1+/− animals. The number of nephrons was markedly reduced in the double heterozygous kidneys, but normal developing structures are present. The lower panels are higher magnification of the boxed areas. In Six1+/− animals, the differentiating metanephric cap tissue (vesicles) in the peripheral nephrogenic zone, in which ureteric bud branching and induction of new nephrons takes place, are morphologically apparent (arrow in E). However, in the hypoplastic Eya1+/−/Six1+/− kidney, the differentiating metanephric vesicles in the peripheral nephrogenic zone were largely reduced in number (arrow in F). (G,H) A reduced number of ureteric bud branches is detected in the compound heterozygous animals at E13.5. ct, convoluting tubule; g, glomerulus; ur, ureter.
To analyze the developmental basis of the renal hypoplasia associated with Eya1+/−/Six1+/− heterozygotes, we compared histological sections of Eya1+/−/Six1+/− heterozygous and control kidneys at various stages. Transverse or longitudinal sections of E17.5 Eya1+/−/Six1+/− hypoplastic kidneys confirmed that the volume of the renal parenchyma is reduced and there are fewer nephrons, but that normal developing structures are present (Fig. 6E,F). In Six1+/− kidneys, the differentiating metanephric cap tissues (vesicles) in the peripheral nephrogenic zone, in which ureteric bud branching and induction of new nephrons takes place, are morphologically apparent (arrow, lower panel of Fig. 6E). However, in the hypoplastic Eya1+/−/Six1+/− kidneys, the differentiating metanephric vesicles in the peripheral nephrogenic zone were largely reduced in number (arrow, lower panel of Fig. 6F). Therefore, the reduction of nephrons in the hypoplastic kidneys may result from a reduced induction between the ureteric bud and the metanephric cap tissue in the peripheral nephrogenic zone. To analyze the onset of the phenotype during development, we analyzed the kidneys at earlier stages. Although the first stages of metanephric kidney development, including evagination of the ureteric bud and its initial branching between E10.5 and 12.5 appeared to occur normally in all Eya1+/−/Six1+/− embryos (n=12), a reduction in the number of ureteric bud branches was first observed at E13.5 (Fig. 6G,H). Taken together, the results suggest that kidney hypoplasia observed in Eya1+/−/Six1+/− animals resulted from abnormal nephrogenesis during late stages of embryogenesis.
Six1−/− metanephric mesenchyme is incompetent for tubulogenesis in organ culture
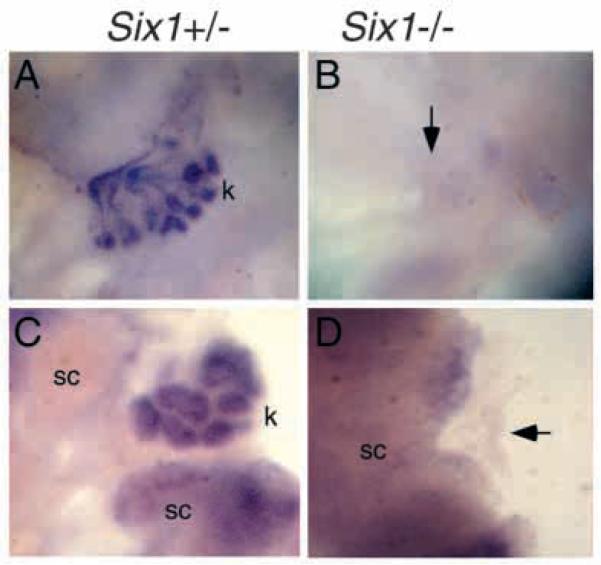
To further demonstrate that kidney development is arrested at the initial step in Six1−/− embryos, kidney rudiments were isolated from Six1−/− embryos at E11.0 and cultured in vitro. Five days after culture, all wild type or heterozygous rudiments developed into a fully branched kidney structure showing strong Pax2 expression (n=5 and n=10, respectively; Fig. 7A). In contrast, Six1−/− kidney rudiments formed no kidneys (n=6, Fig. 7B). We next examined whether Six1 mutant mesenchyme could respond to inductive signals by culturing E11.0 Six1−/− mesenchyme with wild type or heterozygous spinal cord. Five days after culture, 100% (11/11) of the Six1+/− mesenchymal cultures exhibited characteristic tubules showing Pax2 mRNA expression (Fig. 7C). In contrast, none of the Six1−/− mesenchymes (0/6) exhibited any sign of tubule formation (Fig. 7D). The Six1−/− mesenchyme left in the cultures showed no expression of Pax2 (arrow, Fig. 7D). Pax2 mRNA expression was detected in the spinal cord, which was used as a heterologous inducer. Thus, Six1 mutant mesenchyme was unresponsive to inductive signals.
Fig. 7.
Six1 mutant mesenchyme is unresponsive to induction. (A) E11.0 Six1+/− kidney rudiments cultured for 5 days and stained with Pax2 in situ probe. After 5 days of culture, they developed into a fully branched kidney structure (k) showing Pax2 expression in the collecting tubules and in the nephrons. (B) E11.0 Six1−/− metanephric rudiments cultured for 5 days and stained with Pax2 in situ probe. No kidney formation was observed (arrow). (C) E11.0 Six1+/− metanephric mesenchyme cultured with spinal cord (sc) for 5 days and stained with Pax2 in situ probe. The Six1+/− mesenchymal cultures exhibited characteristic tubules (k) showing Pax2 expression. (D) E11.0 Six1−/− metanephric mesenchyme cultured with hetorozygous spinal cord for 5 days and stained with Pax2 in situ probe. None of the Six1−/− mesenchymes exhibited any sign of tubule formation. Note the disappearance of Six1 mutant mesenchyme (arrow), which shows no Pax2 expression.
DISCUSSION
Despite exciting progress in elucidating important genes involved in inductive events during early kidney development, the molecular mechanisms governing the inductive processes of kidney organogenesis remain largely unknown. We show here that the homeobox gene Six1 is expressed in the metanephric mesenchyme before and after metanephric induction and inactivation of this gene results in renal agenesis. Moreover, we show that Eya1, Six1, Six2, Pax2 and Sall1 may function in a molecular pathway and provide evidence for a genetic interaction between Eya1 and Six1 in kidney development.
The formation of mammalian kidney involves three distinct processes: first, establishment of the metanephric mesenchyme from posterior intermediate mesoderm; second, outgrowth and branching of the ureteric bud; and third, transformation and differentiation of the metanephric mesenchyme to renal epithelial cells. Our data indicate that in the absence of Six1, kidney development was arrested at the second step of these three processes. Although the ureteric bud is present in Six1−/− embryos, it fails to invade the mesenchyme completely and the mesenchymal cells undergo abnormal apoptosis from E11.5. Subsequent branching morphogenesis of the ureteric bud and tubule differentiation in the mesenchyme do not occur. It is known that Gdnf and its receptors, c-Ret and Gfrα1, are essential for normal growth and branching morphogenesis of the ureteric bud during kidney development. Indeed, Gdnf can function as a chemoattractant for Ret-expressing epithelial cells and stimulate branching morphogenesis of the ureteric bud (Vega et al., 1996; Tang et al., 1998). Consistent with the observation that the ureteric bud has formed in Six1−/− animals, we have detected Gdnf expression in Six1−/− metanephric mesenchyme. This result demonstrates that the initial expression of Gdnf at mRNA level does not require Six1. Although we were unable to directly determine whether GDNF protein is produced by Six1−/− metanephric mesenchyme, our results demonstrate that whatever amount is made in Six1−/− embryos, is insufficient to ensure invasion of the ureteric bud into the metanephric mesenchyme. This evidence also suggests that some other factors that are under the control of a Six1-regulatory pathway may be important for fully supporting ureteric bud invasion of the metanephric mesenchyme. They could be, for example, cell atrix components that mediate interaction between the epithelium and mesenchyme. Further expression studies in Six1 mutant embryos are required to test this hypothesis.
In the mammalian kidney, Pax2, Eya1, Six1 and Six2 expression overlaps in the metanephric mesenchyme and the null mutants of Pax2, Eya1 and Six1 lack kidney formation (Torres et al., 1995; Xu et al., 1999). Because Pax2 expression in the intermediate mesoderm was unaffected in Eya1−/− embryos at E9.5 and Six2 expression was lost in Eya1−/− metanephric mesenchyme at E10.5, we previously suggested that the Drosophila Pax-Eya-Six regulatory hierarchy has been conserved in mammalian kidney development (Xu et al., 1999). Although we previously did not detect Pax2 expression in Eya1−/− metanephric mesenchyme at E10.5, we concluded that it was because of deficient ureteric bud outgrowth and failure of metanephric induction. This interpretation was based on previous analyses in Danforths’ Short tail (Sd) mutants suggesting that Pax2 expression in the metanephric mesenchyme requires inductive interaction between the mesenchyme and the ureteric bud (Phelps and Dressler, 1993). However, recent expression studies in Ret mutants have demonstrated that Pax2 is expressed in the metanephric mesenchyme before induction and its expression in the mesenchyme is independent of ureteric bud outgrowth (Brophy et al., 2001). Here we show that during mouse kidney development, Pax2 expression in the metanephric mesenchyme before induction is Eya1- and Six1-dependent. Consistent with our observation, it has been previously shown that Six2 expression is also preserved in Pax2−/− mesenchyme (Torres et al., 1995). In contrast, we have found that Six1 expression in the mesenchyme was lost in Eya1−/− embryos, similar to that of Six2 (Xu et al., 1999). Interestingly, we have found that Six2 expression in the metanephric mesenchyme is also Six1-dependent. Therefore, our results together with previous observations suggest that there is an Eya1-Six-Pax2 regulatory hierarchy controlling early mammalian kidney development, distinct from the Pax-Eya-Six regulatory pathway elucidated in Drosophila eye imaginal disc. Detailed examination of kidneys in Pax2/Six1 or Eya1/Six1/Pax2 compound knockouts will enhance our understanding of the possible molecular and genetic interactions between these transcription factors during early mammalian kidney morphogenesis.
Pax2 has recently been proposed to be a direct positive regulator of Gdnf, because Pax2−/− embryos do not express Gdnf in the uninduced mesenchyme and Pax2 regulates the expression of Gdnf in vitro (Brophy et al., 2001). However, our result shows that Pax2 is not required for the expression of Gdnf in the metanephric mesenchyme. We propose two hypotheses to explain these observations. First, because Pax2 expression in the intermediate mesoderm was unaffected in Six1−/− embryos, we hypothesize that Pax2 expression in the posterior intermediate mesoderm is required for the initiation of Gdnf expression during the specification of metanephric mesenchyme. Once Gdnf is turned on in the mesenchyme, Pax2 expression in the mesenchyme may not be required for the maintenance of Gdnf expression as metanephric development proceeds. This could explain why Gdnf expression was absent in Pax2−/− embryos. Consistent with this hypothesis, Gdnf expression was also observed in Wt1−/− metanephric mesenchymes which do not express Pax2 protein, although Pax2 mRNA expression was observed in Wt1−/− metanephric mesenchyme (Kreidberg et al., 1993; Donovan et al., 1999). Second, because Pax2 is expressed normally in the Wolffian duct and ureteric bud in Six1−/− embryos, it is possible that the expression of Pax2 in the Wolffian duct and ureteric bud epithelium is required for the maintenance of Gdnf expression in the mesenchyme. This could also explain the reduction of Gdnf expression observed in Pax2−/− metanephric mesenchyme. In support of this hypothesis, a greatly reduced level of Gdnf mRNA in the metanephric mesenchyme at E11.5 has also been seen in mice defective for Emx2, a homeobox gene expressed primarily in the ureteric bud, whose disruption inhibits ureteric bud growth and branching (Miyamoto et al., 1997). Interestingly, Pax2 expression was also significantly reduced in Emx2−/− ureteric bud at E11.5, whereas its expression in Emx2−/− metanephric mesenchyme was apparently normal at this stage (Miyamoto et al., 1997).
Our results also show that Sall1 functions downstream of Six1. Sall1 is a mammalian homolog of the Drosophila region-specific homeotic gene spalt (sal). Inactivation of murine Sall1 results in renal agenesis or severe dysgenesis because of incomplete ureteric bud outgrowth and the failure of tubule formation, similar to that seen in Six1−/− embryos. It has been shown previously that Gdnf, Eya1, Pax2 and Wt1 are expressed in Sall1−/− metanephric mesenchyme at E10.5, indicating that Sall1 may function downstream of or independent from these genes. Because our results show that Sall1 expression is also unaffected in E10.5 Pax2−/− metanephric mesenchyme, it is possible that Sall1 and Pax2 function in parallel during early kidney development. Heterozygous mutations in the human SALL1 lead to Townes-Brocks syndrome, which shows phenotypic overlap with Branchio-Oto-Renal (BOR) syndrome, a deficiency for the human EYA1 gene. Interestingly, Sall1 expression was also undetectable in Eya1−/− mesenchyme (data not shown). Therefore, it is probable that Eya1, Six1, Six2, Pax2 and Sall1 function in a genetic and molecular pathway in the metanephric mesenchyme during early kidney morphogenesis.
Wt1 is also expressed in the metanephric mesenchyme and its absence leads to failure of ureteric bud outgrowth and apoptosis of the mesenchyme. Our results show that Six1 is not required for the expression of Wt1. It has been shown previously that Six2 is expressed in Wt1−/− metanephric mesenchyme (Donovan et al., 1999) and Wt1 is expressed in Eya1−/− mesenchyme (Xu et al., 1999). Thus, it is possible that Wt1 functions in a pathway independent from Eya1 and Six genes for metanephric development. It is also possible that Wt1 functions in parallel or synergistically with Eya1 and Six genes for metanephric development.
Finally, it should be noted that during late embryonic mouse kidney development, Six1 expression was only observed in collecting tubules, but not in renal epithelia which are derived from metanephric mesenchyme. Although it is generally accepted that metanephric mesenchyme is committed to differentiating into nephrons whereas the ureteric bud is restricted to forming the renal collecting system, several in vitro cell fate studies demonstrated that metanephric mesenchyme differentiates into portions of the renal collecting system, in addition to nephron epithelia (Koseki et al., 1991; Herzlinger et al., 1992; Qiao et al., 1995). The observation of Six1 expression in a subpopulation of collecting tubule epithelial cells during kidney development is consistent with this finding. Therefore, it is possible that the Six1-expressing metanephric mesenchymal cells at E11.5 are pluripotent renal epithelial stem cells and a subpopulation of those cells are recruited into collecting tubule epithelia during renal collecting system morphogenesis. Our results indicate that in addition to its early function in the initiation of mammalian kidney development, Six1 may also play a role in the morphogenesis of the renal collecting system.
Acknowledgments
We thank P. Gruss for the Pax2 mutant mice, R. Nishinakamura for the Sall1 probe, B. Tang for technical assistance and L. Ross for helpful comments. Photomicroscopy and image analysis was made possible by equipment purchased with a grant from the M. J. Murdock Charitable Trust. This work was supported by NIH P20RR 12345-02 (to P.-X.X.).
REFERENCES
- Al-Awqati Q, Oliver JA. Stem cells in the kidney. Kidney Int. 2002;61:387–395. doi: 10.1046/j.1523-1755.2002.00164.x. [DOI] [PubMed] [Google Scholar]
- Ashley DJB, Mostofi FK. Renal agenesis and dysgenesis. J. Urol. 1960;83:211–230. doi: 10.1016/S0022-5347(17)65695-7. [DOI] [PubMed] [Google Scholar]
- Brophy PD, Ostrom L, Lang KM, Dressler GR. Regulation of ureteric bud outgrowth by Pax2-dependent activation of the glial derived neurotrophic factor gene. Development. 2001;128:4747–4756. doi: 10.1242/dev.128.23.4747. [DOI] [PubMed] [Google Scholar]
- Buller C, Xu X, Marquis V, Schwanke R, Xu P-X. Molecular effects of Eya1 domain mutations causing organ defects in BOR syndrome. Hum. Mol. Genet. 2001;10:2775–2781. doi: 10.1093/hmg/10.24.2775. [DOI] [PubMed] [Google Scholar]
- Cacalano G, Farinas I, Wang LC, Hagler K, Forgie A, Moore M, Armanini M, Phillips H, Ryan AM, Reichardt LF, et al. GFRalpha1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron. 1998;21:53–62. doi: 10.1016/s0896-6273(00)80514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Amoui M, Zhang Z, Mardon G. Dachshund and eyes absent proteins form a complex and function synergistically to induce ectopic eye formation in Drosophila. Cell. 1997;91:893–903. doi: 10.1016/s0092-8674(00)80481-x. [DOI] [PubMed] [Google Scholar]
- Cheyette BN, Green PJ, Martin K, Garren H, Hartenstein V, Zipursky SL. The Drosophila sine oculis locus encodes a homeodomain-containing protein required for the development of the entire visual system. Neuron. 1994;12:977–996. doi: 10.1016/0896-6273(94)90308-5. [DOI] [PubMed] [Google Scholar]
- Donovan MJ, Natoli TA, Sainio K, Amstutz A, Jaenisch R, Sariola H, Kreidberg JA. Initial differentiation of the metanephric mesenchyme is independent of Wt1 and the ureteric bud. Dev. Genet. 1999;24:252–262. doi: 10.1002/(SICI)1520-6408(1999)24:3/4<252::AID-DVG8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Halder G, Callaerts P, Flister S, Walldorf U, Kloter U, Gehring WJ. Eyeless initiates the expression of both sine oculis and eyes absent during Drosophila compound eye development. Development. 1998;125:2181–2191. doi: 10.1242/dev.125.12.2181. [DOI] [PubMed] [Google Scholar]
- Herzlinger D, Koseki C, Mikawa T, Al-Awqati Q. Metanephric mesenchyme contains multipotent stem cells whose fate is restricted after induction. Development. 1992;114:565–572. doi: 10.1242/dev.114.3.565. [DOI] [PubMed] [Google Scholar]
- Kawakami K, Ohto H, Takizawa T, Saito T. Identification and expression of six family genes in mouse retina. FEBS Lett. 1996;393:259–263. doi: 10.1016/0014-5793(96)00899-x. [DOI] [PubMed] [Google Scholar]
- Koseki C, Herzlinger D, Al-Awqati Q. Integration of embryonic nephrogenic cells carrying a reporter gene into functioning nephrons. Am. J. Physiol. 1991;261:C550–C554. doi: 10.1152/ajpcell.1991.261.3.C550. [DOI] [PubMed] [Google Scholar]
- Kreidberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, Housman D, Jaenisch R. WT-1 is required for early kidney development. Cell. 1993;74:679–691. doi: 10.1016/0092-8674(93)90515-r. [DOI] [PubMed] [Google Scholar]
- Kume T, Deng K, Hogan BL. Murine forkhead/winged helix genes Foxc1 (Mf1) and Foxc2 (Mfh1) are required for the early organogenesis of the kidney and urinary tract. Development. 2000;127:1387–1395. doi: 10.1242/dev.127.7.1387. [DOI] [PubMed] [Google Scholar]
- Kuure S, Vuolteenaho R, Vainio S. Kidney morphogenesis: cellular and molecular regulation. Mech. Dev. 2000;92:31–45. doi: 10.1016/s0925-4773(99)00323-8. [DOI] [PubMed] [Google Scholar]
- Laclef C, Hamard G, Demignon J, Souil E, Houbron C, Maire P. Altered myogenesis in Six1 deficient mice. Development. 2003;130:2239–2252. doi: 10.1242/dev.00440. [DOI] [PubMed] [Google Scholar]
- Lechner MS, Dressler GR. The molecular basis of embryonic kidney development. Mech. Dev. 1997;62:105–120. doi: 10.1016/s0925-4773(97)00667-9. [DOI] [PubMed] [Google Scholar]
- Miyamoto N, Yoshida M, Kuratani S, Matsuo I, Aizawa S. Defects of urogenital development in mice lacking Emx2. Development. 1997;124:1653–1664. doi: 10.1242/dev.124.9.1653. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, Oshima K, Fogo A, Hogan BL, Ichikawa I. Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J. Clin. Invest. 2000;105:863–873. doi: 10.1172/JCI8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MW, Klein RD, Farinas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryan AM, Carver-Moore K, Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382:76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- Nishinakamura R, Matsumoto Y, Nakao K, Nakamura K, Sato A, Copeland NG, Gilbert DJ, Jenkins NA, Scully S, Lacey DL, et al. Murine homolog of SALL1 is essential for ureteric bud invasion in kidney development. Development. 2001;128:3105–3115. doi: 10.1242/dev.128.16.3105. [DOI] [PubMed] [Google Scholar]
- Oliver G, Wehr R, Jenkins NA, Copeland NG, Cheyette BNR, Hartenstein V, Zipursky SL, Gruss P. Homeobox genes and connective tissue patterning. Development. 1995a;121:693–705. doi: 10.1242/dev.121.3.693. [DOI] [PubMed] [Google Scholar]
- Oliver G, Mailhos A, Wehr R, Copeland NG, Jenkins NA, Gruss P. Six3, a murine homologue of the sine oculis gene, demarcates the most anterior border of the developing neural plate and is expressed during eye development. Development. 1995b;121:4045–4055. doi: 10.1242/dev.121.12.4045. [DOI] [PubMed] [Google Scholar]
- Phelps DE, Dressler GR. Aberrant expression of Pax-2 in Danforth's short tail (Sd) mice. Dev. Biol. 1993;157:251–258. doi: 10.1006/dbio.1993.1129. [DOI] [PubMed] [Google Scholar]
- Pichel JG, Shen L, Sheng HZ, Granholm AC, Drago J, Grinberg A, Lee EJ, Huang SP, Saarma M, Hoffer BJ, et al. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382:73–76. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- Pignoni F, Hu B, Zavitz KH, Xiao J, Garrity PA, Zipursky SL. The eye-specification proteins So and Eya form a complex and regulate multiple steps in Drosophila eye development. Cell. 1997;91:881–891. doi: 10.1016/s0092-8674(00)80480-8. [DOI] [PubMed] [Google Scholar]
- Qiao J, Cohen D, Herzlinger D. The metanephric blastema differentiates into collecting system and nephron epithelia in vitro. Development. 1995;121:3207–3214. doi: 10.1242/dev.121.10.3207. [DOI] [PubMed] [Google Scholar]
- Raatikainen-Ahokas A, Hytonen M, Tenhunen A, Sainio K, Sario H. Bmp4 affects the differentiation of metanephric mesenchyme and reveals an early anterior-posterior axis of the embryonic kidney. Dev. Dyn. 2000;217:146–158. doi: 10.1002/(SICI)1097-0177(200002)217:2<146::AID-DVDY2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Reddi AH. Bone morphogenetic proteins: from basic science to clinical applications. J. Bone Jt Surg. Ser. A Suppl. 1. 2000;83:S1–6. doi: 10.2106/00004623-200100001-00001. [DOI] [PubMed] [Google Scholar]
- Saarma M, Sariola H. Other neurotrophic factors: glial cell line-derived neurotrophic factor (GDNF). Microsc. Res. Tech. 1999;45:292–302. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<292::AID-JEMT13>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Sainio K, Suvanto P, Davies J, Wartiovaara J, Wartiovaara K, Saarma M, Arumae U, Meng X, Lindahl M, Pachnis V, et al. Glial-cell-line-derived neurotrophic factor is required for bud initiation from ureteric epithelium. Development. 1997;124:4077–4087. doi: 10.1242/dev.124.20.4077. [DOI] [PubMed] [Google Scholar]
- Sanchez MP, Silos-Santiago I, Frisen J, He B, Lira SA, Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382:70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- Schedl A, Hastie ND. Cross-talk in kidney development. Curr. Opin. Genet. Dev. 2000;10:543–549. doi: 10.1016/s0959-437x(00)00125-8. [DOI] [PubMed] [Google Scholar]
- Schuchardt A, D'Agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- Serikaku MA, O'Tousa JE. sine oculis is a homeobox gene required for Drosophila visual system development. Genetics. 1994;138:1137–1150. doi: 10.1093/genetics/138.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang MJ, Worley D, Sanicola M, Dressler GR. The RET-glial cell-derived neurotrophic factor (GDNF) pathway stimulates migration and chemoattraction of epithelial cells. J. Cell Biol. 1998;142:1337–1345. doi: 10.1083/jcb.142.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres M, Gomez-Pardo E, Dressler GR, Gruss P. Pax-2 controls multiple steps of urogenital development. Development. 1995;121:4057–4065. doi: 10.1242/dev.121.12.4057. [DOI] [PubMed] [Google Scholar]
- Treisman JE. A conserved blueprint for the eye? BioEssays. 1999;21:843–850. doi: 10.1002/(SICI)1521-1878(199910)21:10<843::AID-BIES6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Tsang TE, Shawlot W, Kinder SJ, Kobayashi A, Kwan KM, Schughart K, Kania A, Jessell TM, Behringer RR, Tam PP. Lim1 activity is required for intermediate mesoderm differentiation in the mouse embryos. Dev. Biol. 2000;223:77–90. doi: 10.1006/dbio.2000.9733. [DOI] [PubMed] [Google Scholar]
- Vainio S, Lin Y. Coordinating early kidney development: lessons from gene targeting. Nat. Rev. Genet. 2002;3:533–543. doi: 10.1038/nrg842. [DOI] [PubMed] [Google Scholar]
- Vega QC, Worby CA, Lechner MS, Dixon JE, Dressler GR. Glial cell line-derived neurotrophic factor activates the receptor tyrosine kinase RET and promotes kidney morphogenesis. Proc. Natl. Acad. Sci. USA. 1996;93:10657–10661. doi: 10.1073/pnas.93.20.10657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P-X, Woo I, Her H, Beier DR, Maas RL. Mouse Eya homologues of the Drosophila eyes absent gene require Pax6 for expression in lens and nasal placode. Development. 1997a;124:219–231. doi: 10.1242/dev.124.1.219. [DOI] [PubMed] [Google Scholar]
- Xu P-X, Cheng J, Epstein JA, Maas R. Mouse Eya genes are expressed during limb tendon development and encode a transcriptional activation function. Proc. Natl. Acad. Sci. USA. 1997b;94:11974–11979. doi: 10.1073/pnas.94.22.11974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P-X, Adams J, Peters H, Brown MC, Heaney S, Maas RL. Multiple organogenesis defects in Eya1-deficient mice. Nat. Genet. 1999;23:113–117. doi: 10.1038/12722. [DOI] [PubMed] [Google Scholar]
- Xu P-X, Zheng W, Laclef C, Maire P, Maas RL, Peters H, Xu X. Eya1 is required for the morphogenesis of mammalian thymus, parathyroid and thyroid. Development. 2002;129:3033–3044. doi: 10.1242/dev.129.13.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]