Abstract
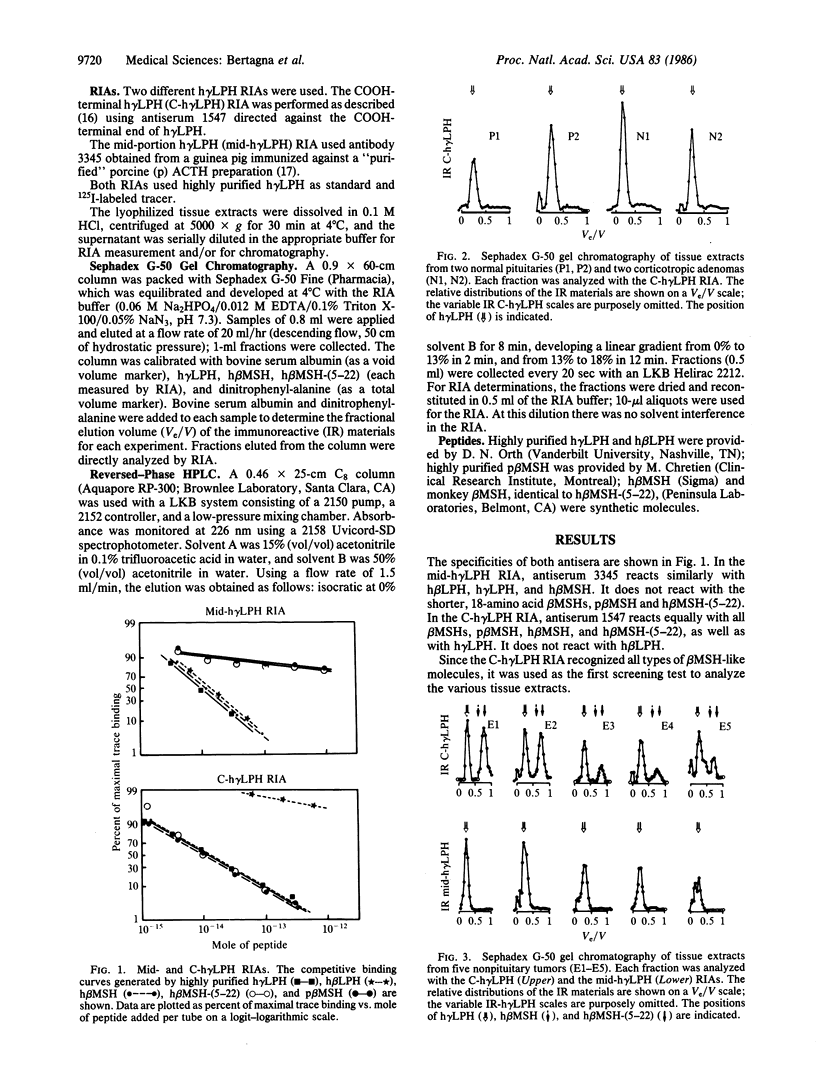
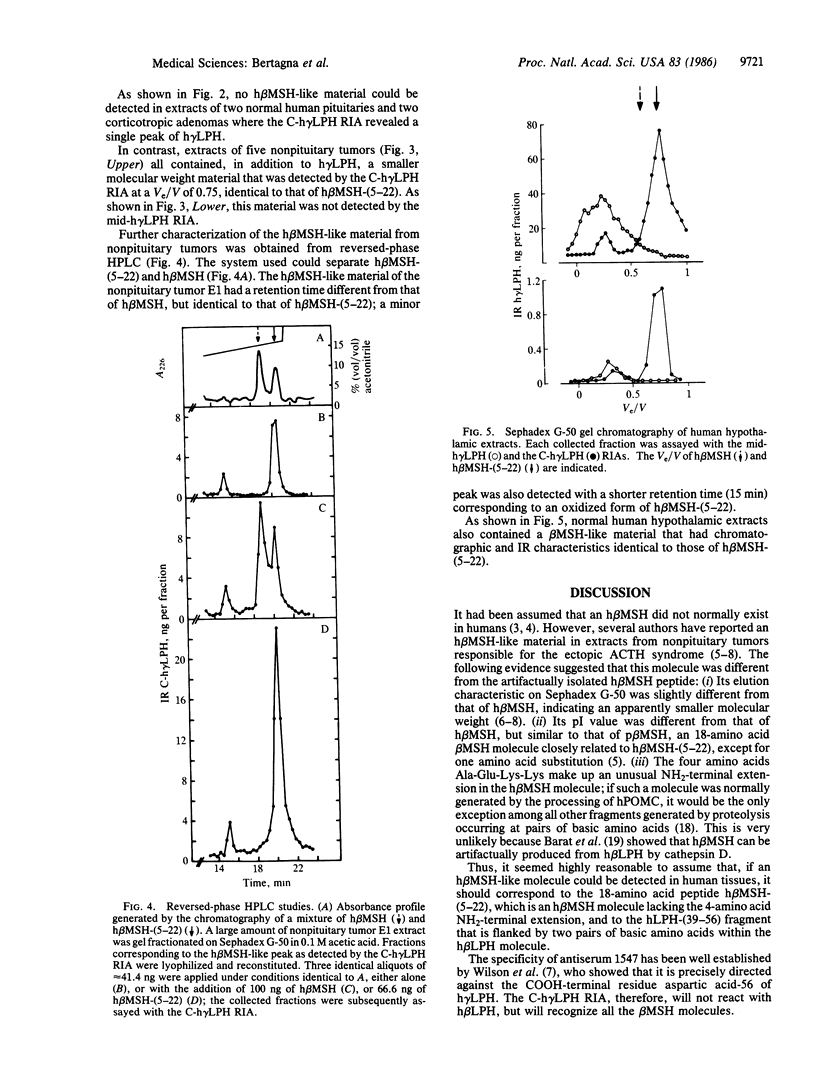
It is generally accepted that human beta-melanocyte-stimulating hormone (h beta MSH) does not normally exist in humans but was merely an artifactually generated 22-amino acid peptide corresponding to a lipotropin (LPH) fragment (residues 35-56). We examined whether the shorter 18-amino acid peptide h beta MSH-(5-22) could be detected in some human tissues. Normal human pituitaries and hypothalami as well as corticotropin-secreting pituitary and nonpituitary tumors were extracted and chromatographed on Sephadex G-50, and the fractions were measured with two radioimmunoassays using either a COOH-terminal human gamma LPH (h gamma LPH) antiserum that recognized equally h gamma LPH, h beta MSH, and h beta MSH-(5-22) or a mid-portion h gamma LPH antiserum that recognized h gamma LPH and h beta MSH but not h beta MSH-(5-22). Normal pituitaries and pituitary tumors contained a single immunoreactive material coeluting with h gamma LPH. The hypothalami and the nonpituitary tumors all contained h gamma LPH and a smaller molecular weight material that was only detected in the COOH-terminal h gamma LPH radioimmunoassay; its elution volume (Ve/V, 0.75) was identical to that of h beta MSH-(5-22) but different from that of h beta MSH (Ve/V, 0.60); on reversed-phase HPLC, it coeluted with synthetic h beta MSH-(5-22) with a retention time different from that of h beta MSH. It is concluded that h beta MSH-(5-22) that corresponds to the 18-amino acid peptide h beta LPH-(39-56), flanked by two pairs of basic amino acids within the h beta LPH molecule, is a normal maturation product of proopiomelanocortin in human nonpituitary tissues.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barát E., Patthy A., Gráf L. Action of cathepsin D on human beta-lipotropin: a possible source of human "beta-melanotropin". Proc Natl Acad Sci U S A. 1979 Dec;76(12):6120–6123. doi: 10.1073/pnas.76.12.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertagna X. Y., Nicholson W. E., Sorenson G. D., Pettengill O. S., Mount C. D., Orth D. N. Corticotropin, lipotropin, and beta-endorphin production by a human nonpituitary tumor in culture: evidence for a common precursor. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5160–5164. doi: 10.1073/pnas.75.10.5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield G. A., Scott A. P., Lowry P. J., Gilkes J. J., Rees L. H. A reappraisal of human beta MSH. Nature. 1974 Dec 6;252(5483):492–493. doi: 10.1038/252492a0. [DOI] [PubMed] [Google Scholar]
- Chrétien M., Li C. H. Isolation, purification, and characterization of gamma-lipotropic hormone from sheep pituitary glands. Can J Biochem. 1967 Jul;45(7):1163–1174. doi: 10.1139/o67-133. [DOI] [PubMed] [Google Scholar]
- DIXON H. B. Chromatographic isolations of pig and human melanocyte-stimulating hormones. Biochim Biophys Acta. 1960 Jan 1;37:38–42. doi: 10.1016/0006-3002(60)90075-5. [DOI] [PubMed] [Google Scholar]
- Donnadieu M., Sevaux D. Radioimmunoassay of melanocytes-stimulating hormone (beta-MSH) in human plasma. Biomedicine. 1973 Jun 20;19(6):272–274. [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E. Structure and biosynthesis of pro-adrenocorticotropin/endorphin and related peptides. Endocr Rev. 1980 Winter;1(1):1–27. doi: 10.1210/edrv-1-1-1. [DOI] [PubMed] [Google Scholar]
- Hsi K. L., Seidah N. G., Lu C. L., Chrétien M. Reinvestigation of the N-terminal amino acid sequence of beta-lipotropin from human pituitary glands. Biochem Biophys Res Commun. 1981 Dec 31;103(4):1329–1335. doi: 10.1016/0006-291x(81)90268-0. [DOI] [PubMed] [Google Scholar]
- Li C. H., Barnafi L., Chrétien M., Chung D. Isolation and amino-acid sequence of beta-LPH from sheep pituitary glands. Nature. 1965 Dec 11;208(5015):1093–1094. doi: 10.1038/2081093b0. [DOI] [PubMed] [Google Scholar]
- McLoughlin L., Lowry P. J., Ratter S., Besser G. M., Rees L. H. beta-Endorphin and beta-MSH in human plasma. Clin Endocrinol (Oxf) 1980 Mar;12(3):287–292. doi: 10.1111/j.1365-2265.1980.tb02712.x. [DOI] [PubMed] [Google Scholar]
- Pique L., Jegou S., Bertagna X., Javoy-Agid F., Seurin D., Proeschel M. F., Girard F., Agid Y., Vaudry H., Luton J. P. Pro-opiomelanocortin peptides in the human hypothalamus: comparative study between normal subjects and Parkinson patients. Neurosci Lett. 1985 Mar 15;54(2-3):141–146. doi: 10.1016/s0304-3940(85)80069-0. [DOI] [PubMed] [Google Scholar]
- Scott A. P., Lowry P. J. Adrenocorticotrophic and melanocyte-stimulating peptides in the human pituitary. Biochem J. 1974 Jun;139(3):593–602. doi: 10.1042/bj1390593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiess J., Mount C. D., Nicholson W. E., Orth D. N. NH2-terminal amino acid sequence and peptide mapping of purified human beta-lipotropin: comparison with previously proposed sequences. Proc Natl Acad Sci U S A. 1982 Aug;79(16):5071–5075. doi: 10.1073/pnas.79.16.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner D. F., Oyer P. E. The biosynthesis of insulin and a probable precursor of insulin by a human islet cell adenoma. Proc Natl Acad Sci U S A. 1967 Feb;57(2):473–480. doi: 10.1073/pnas.57.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner D. F., Quinn P. S., Chan S. J., Marsh J., Tager H. S. Processing mechanisms in the biosynthesis of proteins. Ann N Y Acad Sci. 1980;343:1–16. doi: 10.1111/j.1749-6632.1980.tb47238.x. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Nicholson W. E., Orth D. N. The nature of the immunoreactive lipotropins in human plasma and tissue extracts. J Clin Invest. 1978 Jul;62(1):94–104. doi: 10.1172/JCI109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda M., Takeuchi T., Abe K., Miyakawa S., Ohnami S., Yanaihara N. beta-Melanocyte-stimulating hormone immunoreactivity in human pituitaries and ectopic adrenocorticotropin-producing tumors. J Clin Endocrinol Metab. 1980 Mar;50(3):550–556. doi: 10.1210/jcem-50-3-550. [DOI] [PubMed] [Google Scholar]
- Whitfeld P. L., Seeburg P. H., Shine J. The human pro-opiomelanocortin gene: organization, sequence, and interspersion with repetitive DNA. DNA. 1982;1(2):133–143. doi: 10.1089/dna.1.1982.1.133. [DOI] [PubMed] [Google Scholar]
- Wilson R. E., Orth D. N., Nicholson W. E., Mount C. D., Bertagna X. Y. Human gamma-lipotropin radioimmunoassay: identification of immunoreactive gamma-lipotropin in human plasma and tissue. J Clin Endocrinol Metab. 1981 Jul;53(1):1–9. doi: 10.1210/jcem-53-1-1. [DOI] [PubMed] [Google Scholar]
- de Keyzer Y., Bertagna X., Lenne F., Girard F., Luton J. P., Kahn A. Altered proopiomelanocortin gene expression in adrenocorticotropin-producing nonpituitary tumors. Comparative studies with corticotropic adenomas and normal pituitaries. J Clin Invest. 1985 Nov;76(5):1892–1898. doi: 10.1172/JCI112184. [DOI] [PMC free article] [PubMed] [Google Scholar]


