Abstract
We used in situ hybridization histochemistry with synthetic oligodeoxyribonucleotide probes to identify cells that synthesize mRNAs encoding tyrosine hydroxylase in the mesencephalon and substance P, enkephalin, and dynorphin in the rat forebrain. Dopaminergic cells in the mesencephalon project to the forebrain and influence neuropeptide levels. We examined the effect of unilateral 6-hydroxydopamine lesions (which eliminated tyrosine hydroxylase mRNA-containing cells in the mesencephalon) on substance P, enkephalin, and dynorphin mRNA levels. Substance P mRNA levels were depressed, whereas enkephalin mRNA levels were elevated in consecutive sections from striatal areas in all animals. The effects of the lesions on dynorphin mRNA levels were less robust, and considerable variation between animals was observed. Changes were evident in the levels of message in individual cells but not in the numbers of labeled cells. These effects were not uniform throughout the dopamine-innervated areas, suggesting degrees of control not apparent with RNA blot-hybridization or dot-blot analyses.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aft R. L., Mueller G. C. Hemin-mediated oxidative degradation of proteins. J Biol Chem. 1984 Jan 10;259(1):301–305. [PubMed] [Google Scholar]
- Altar C. A., O'Neil S., Walter R. J., Jr, Marshall J. F. Brain dopamine and serotonin receptor sites revealed by digital subtraction autoradiography. Science. 1985 May 3;228(4699):597–600. doi: 10.1126/science.2580352. [DOI] [PubMed] [Google Scholar]
- Bannon M. J., Lee J. M., Giraud P., Young A., Affolter H. U., Bonner T. I. Dopamine antagonist haloperidol decreases substance P, substance K, and preprotachykinin mRNAs in rat striatonigral neurons. J Biol Chem. 1986 May 25;261(15):6640–6642. [PubMed] [Google Scholar]
- Beckstead R. M., Domesick V. B., Nauta W. J. Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Res. 1979 Oct 19;175(2):191–217. doi: 10.1016/0006-8993(79)91001-1. [DOI] [PubMed] [Google Scholar]
- Beckstead R. M., Kersey K. S. Immunohistochemical demonstration of differential substance P-, met-enkephalin-, and glutamic-acid-decarboxylase-containing cell body and axon distributions in the corpus striatum of the cat. J Comp Neurol. 1985 Feb 22;232(4):481–498. doi: 10.1002/cne.902320406. [DOI] [PubMed] [Google Scholar]
- Brann M. R., Emson P. C. Microiontophoretic injection of fluorescent tracer combined with simultaneous immunofluorescent histochemistry for the demonstration of efferents from the caudate-putamen projecting to the globus pallidus. Neurosci Lett. 1980 Jan;16(1):61–65. doi: 10.1016/0304-3940(80)90101-9. [DOI] [PubMed] [Google Scholar]
- Brownstein M. J., Mroz E. A., Tappaz M. L., Leeman S. E. On the origin of substance P and glutamic acid decarboxylase (GAD) in the substantia nigra. Brain Res. 1977 Oct 28;135(2):315–323. doi: 10.1016/0006-8993(77)91034-4. [DOI] [PubMed] [Google Scholar]
- Casey J., Davidson N. Rates of formation and thermal stabilities of RNA:DNA and DNA:DNA duplexes at high concentrations of formamide. Nucleic Acids Res. 1977;4(5):1539–1552. doi: 10.1093/nar/4.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J., Tang J., Yang H. Y., Costa E. Increase of striatal Met5-enkephalin-Arg6-Phe7 (YGGFMRF) content elicited by long-term treatment with haloperidol. J Pharmacol Exp Ther. 1984 Apr;229(1):171–174. [PubMed] [Google Scholar]
- Civelli O., Douglass J., Goldstein A., Herbert E. Sequence and expression of the rat prodynorphin gene. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4291–4295. doi: 10.1073/pnas.82.12.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa F. M., Innis R. B., Hester L. D., Snyder S. H. Diffuse enkephalin innervation from caudate to globus pallidus. Neurosci Lett. 1981 Aug 7;25(1):63–68. doi: 10.1016/0304-3940(81)90102-6. [DOI] [PubMed] [Google Scholar]
- Cuello A. C., Paxinos G. Evidence for a long Leu-enkephalin striopallidal pathway in rat brain. Nature. 1978 Jan 12;271(5641):178–180. doi: 10.1038/271178a0. [DOI] [PubMed] [Google Scholar]
- Del Fiacco M., Paxinos G., Cuello A. C. Neostriatal enkephalin-immunoreactive neurones project to the globus pallidus. Brain Res. 1982 Jan 7;231(1):1–17. doi: 10.1016/0006-8993(82)90003-8. [DOI] [PubMed] [Google Scholar]
- Dray A. The striatum and substantia nigra: a commentary on their relationships. Neuroscience. 1979;4(10):1407–1439. doi: 10.1016/0306-4522(79)90048-4. [DOI] [PubMed] [Google Scholar]
- Fallon J. H., Leslie F. M. Distribution of dynorphin and enkephalin peptides in the rat brain. J Comp Neurol. 1986 Jul 15;249(3):293–336. doi: 10.1002/cne.902490302. [DOI] [PubMed] [Google Scholar]
- Fallon J. H., Loughlin S. E., Ribak C. E. The islands of Calleja complex of rat basal forebrain. III. Histochemical evidence for a striatopallidal system. J Comp Neurol. 1983 Jul 20;218(1):91–120. doi: 10.1002/cne.902180106. [DOI] [PubMed] [Google Scholar]
- Gerfen C. R. The neostriatal mosaic. I. Compartmental organization of projections from the striatum to the substantia nigra in the rat. J Comp Neurol. 1985 Jun 22;236(4):454–476. doi: 10.1002/cne.902360404. [DOI] [PubMed] [Google Scholar]
- Grafe M. R., Forno L. S., Eng L. F. Immunocytochemical studies of substance P and Met-enkephalin in the basal ganglia and substantia nigra in Huntington's, Parkinson's and Alzheimer's diseases. J Neuropathol Exp Neurol. 1985 Jan;44(1):47–59. doi: 10.1097/00005072-198501000-00004. [DOI] [PubMed] [Google Scholar]
- Grima B., Lamouroux A., Blanot F., Biguet N. F., Mallet J. Complete coding sequence of rat tyrosine hydroxylase mRNA. Proc Natl Acad Sci U S A. 1985 Jan;82(2):617–621. doi: 10.1073/pnas.82.2.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S. N., Nauta W. J. Ramifications of the globus pallidus in the rat as indicated by patterns of immunohistochemistry. Neuroscience. 1983 Jun;9(2):245–260. doi: 10.1016/0306-4522(83)90291-9. [DOI] [PubMed] [Google Scholar]
- Hanson G. R., Alphs L., Wolf W., Levine R., Lovenberg W. Haloperidol-induced reduction of nigral substance P-like immunoreactivity: a probe for the interactions between dopamine and substance P neuronal systems. J Pharmacol Exp Ther. 1981 Aug;218(2):568–574. [PubMed] [Google Scholar]
- Hong J. S., Yang H. T., Costa E. Substance P content of substantia nigra after chronic treatment with antischizophrenic drugs. Neuropharmacology. 1978 Jan;17(1):83–85. doi: 10.1016/0028-3908(78)90178-8. [DOI] [PubMed] [Google Scholar]
- Hong J. S., Yang H. Y., Fratta W., Costa E. Rat striatal methionine-enkephalin content after chronic treatment with cataleptogenic and noncataleptogenic antischizophrenic drugs. J Pharmacol Exp Ther. 1978 Apr;205(1):141–147. [PubMed] [Google Scholar]
- Hong J. S., Yang H. Y., Gillin J. C., Di Giulio A. M., Fratta W., Costa E. Chronic treatment with haloperidol accelerates the biosynthesis of enkephalins in rat striatum. Brain Res. 1979 Jan 5;160(1):192–195. doi: 10.1016/0006-8993(79)90618-8. [DOI] [PubMed] [Google Scholar]
- Hong J. S., Yang H. Y., Racagni G., Costa E. Projections of substance P containing neurons from neostriatum to substantia nigra. Brain Res. 1977 Feb 25;122(3):541–544. doi: 10.1016/0006-8993(77)90464-4. [DOI] [PubMed] [Google Scholar]
- Jingami H., Matsukura S., Numa S., Imura H. Effects of adrenalectomy and dexamethasone administration on the level of prepro-corticotropin-releasing factor messenger ribonucleic acid (mRNA) in the hypothalamus and adrenocorticotropin/beta-lipotropin precursor mRNA in the pituitary in rats. Endocrinology. 1985 Oct;117(4):1314–1320. doi: 10.1210/endo-117-4-1314. [DOI] [PubMed] [Google Scholar]
- Kanazawa I., Emson P. C., Cuello A. C. Evidence for the existence of substance P-containing fibres in striato-nigral and pallido-nigral pathways in rat brain. Brain Res. 1977 Jan 7;119(2):447–453. doi: 10.1016/0006-8993(77)90323-7. [DOI] [PubMed] [Google Scholar]
- Khachaturian H., Lewis M. E., Hollt V., Watson S. J. Telencephalic enkephalinergic systems in the rat brain. J Neurosci. 1983 Apr;3(4):844–855. doi: 10.1523/JNEUROSCI.03-04-00844.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
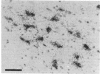
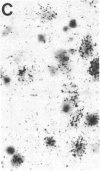
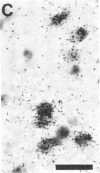
- Kohno J., Shiosaka S., Shinoda K., Inagaki S., Tohyama M. Two distinct strio-nigral substance P pathways in the rat: an experimental immunohistochemical study. Brain Res. 1984 Aug 13;308(2):309–317. doi: 10.1016/0006-8993(84)91070-9. [DOI] [PubMed] [Google Scholar]
- Kubota Y., Inagaki S., Kito S. Innervation of substance P neurons by catecholaminergic terminals in the neostriatum. Brain Res. 1986 Jun 4;375(1):163–167. doi: 10.1016/0006-8993(86)90969-8. [DOI] [PubMed] [Google Scholar]
- Kubota Y., Inagaki S., Kito S., Takagi H., Smith A. D. Ultrastructural evidence of dopaminergic input to enkephalinergic neurons in rat neostriatum. Brain Res. 1986 Mar 5;367(1-2):374–378. doi: 10.1016/0006-8993(86)91622-7. [DOI] [PubMed] [Google Scholar]
- Lathe R. Synthetic oligonucleotide probes deduced from amino acid sequence data. Theoretical and practical considerations. J Mol Biol. 1985 May 5;183(1):1–12. doi: 10.1016/0022-2836(85)90276-1. [DOI] [PubMed] [Google Scholar]
- Ljungdahl A., Hökfelt T., Nilsson G. Distribution of substance P-like immunoreactivity in the central nervous system of the rat--I. Cell bodies and nerve terminals. Neuroscience. 1978;3(10):861–943. doi: 10.1016/0306-4522(78)90116-1. [DOI] [PubMed] [Google Scholar]
- Mauborgne A., Javoy-Agid F., Legrand J. C., Agid Y., Cesselin F. Decrease of substance P-like immunoreactivity in the substantia nigra and pallidum of parkinsonian brains. Brain Res. 1983 May 23;268(1):167–170. doi: 10.1016/0006-8993(83)90403-1. [DOI] [PubMed] [Google Scholar]
- NAGATSU T., LEVITT M., UDENFRIEND S. TYROSINE HYDROXYLASE. THE INITIAL STEP IN NOREPINEPHRINE BIOSYNTHESIS. J Biol Chem. 1964 Sep;239:2910–2917. [PubMed] [Google Scholar]
- Palacios J. M., Niehoff D. L., Kuhar M. J. [3H]Spiperone binding sites in brain: autoradiographic localization of multiple receptors. Brain Res. 1981 Jun 1;213(2):277–289. doi: 10.1016/0006-8993(81)90234-1. [DOI] [PubMed] [Google Scholar]
- Peterson M. R., Robertson H. A. Effect of dopaminergic agents on levels of dynorphin1-8 in rat striatum. Prog Neuropsychopharmacol Biol Psychiatry. 1984;8(4-6):725–728. doi: 10.1016/0278-5846(84)90046-0. [DOI] [PubMed] [Google Scholar]
- Quirion R., Gaudreau P., Martel J. C., St-Pierre S., Zamir N. Possible interactions between dynorphin and dopaminergic systems in rat basal ganglia and substantia nigra. Brain Res. 1985 Apr 8;331(2):358–362. doi: 10.1016/0006-8993(85)91563-x. [DOI] [PubMed] [Google Scholar]
- Sabol S. L., Yoshikawa K., Hong J. S. Regulation of methionine-enkephalin precursor messenger RNA in rat striatum by haloperidol and lithium. Biochem Biophys Res Commun. 1983 Jun 15;113(2):391–399. doi: 10.1016/0006-291x(83)91739-4. [DOI] [PubMed] [Google Scholar]
- Sokoloff P., Brann M., Redouane K., Martres M. P., Schwartz J. C., Bouthenet M. L., Sales N., Mann A., Hamdi P., Wermuth C. G. The use of [3H](-)-DO 710 as selective dopaminergic ligand for binding and autoradiographic studies. Eur J Pharmacol. 1985 Jan 2;107(2):243–251. doi: 10.1016/0014-2999(85)90064-0. [DOI] [PubMed] [Google Scholar]
- Tang F., Costa E., Schwartz J. P. Increase of proenkephalin mRNA and enkephalin content of rat striatum after daily injection of haloperidol for 2 to 3 weeks. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3841–3844. doi: 10.1073/pnas.80.12.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taquet H., Javoy-Agid F., Hamon M., Legrand J. C., Agid Y., Cesselin F. Parkinson's disease affects differently Met5- and Leu5-enkephalin in the human brain. Brain Res. 1983 Dec 5;280(2):379–382. doi: 10.1016/0006-8993(83)90071-9. [DOI] [PubMed] [Google Scholar]
- Tenovuo O., Rinne U. K., Viljanen M. K. Substance P immunoreactivity in the post-mortem parkinsonian brain. Brain Res. 1984 Jun 11;303(1):113–116. doi: 10.1016/0006-8993(84)90217-8. [DOI] [PubMed] [Google Scholar]
- Thal L. J., Sharpless N. S., Hirschhorn I. D., Horowitz S. G., Makman M. H. Striatal met-enkephalin concentration increases following nigrostriatal denervation. Biochem Pharmacol. 1983 Nov 15;32(22):3297–3301. doi: 10.1016/0006-2952(83)90353-2. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Postsynaptic supersensitivity after 6-hydroxy-dopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand Suppl. 1971;367:69–93. doi: 10.1111/j.1365-201x.1971.tb11000.x. [DOI] [PubMed] [Google Scholar]
- Vincent S. R., Hökfelt T., Christensson I., Terenius L. Dynorphin-immunoreactive neurons in the central nervous system of the rat. Neurosci Lett. 1982 Nov 30;33(2):185–190. doi: 10.1016/0304-3940(82)90249-x. [DOI] [PubMed] [Google Scholar]
- Vincent S., Hökfelt T., Christensson I., Terenius L. Immunohistochemical evidence for a dynorphin immunoreactive striato-nigral pathway. Eur J Pharmacol. 1982 Nov 19;85(2):251–252. doi: 10.1016/0014-2999(82)90477-0. [DOI] [PubMed] [Google Scholar]
- Wolfson B., Manning R. W., Davis L. G., Arentzen R., Baldino F., Jr Co-localization of corticotropin releasing factor and vasopressin mRNA in neurones after adrenalectomy. Nature. 1985 May 2;315(6014):59–61. doi: 10.1038/315059a0. [DOI] [PubMed] [Google Scholar]
- Young W. S., 3rd, Mezey E., Siegel R. E. Quantitative in situ hybridization histochemistry reveals increased levels of corticotropin-releasing factor mRNA after adrenalectomy in rats. Neurosci Lett. 1986 Oct 8;70(2):198–203. doi: 10.1016/0304-3940(86)90463-5. [DOI] [PubMed] [Google Scholar]