Abstract
Cannabinoids both increase urine output and decrease urinary frequency in human subjects. However, these effects have not been systematically evaluated in intact mice, a species commonly used to evaluate the effects of novel cannabinoids. The present studies investigated whether cannabinoid agonists reliably produce diuresis in mice at doses comparable to those that produce other cannabinoid effects and, further, identified the receptors that may mediate these effects. Diuretic effects were measured in male mice over 6 h. In some studies, urine was collected and analyzed for electrolyte measurements. In other studies, agonist injections were preceded by pretreatment with cannabinoid CB1 or CB2 selective antagonists, including a peripherally constrained CB1 antagonist. Companion studies evaluated the antinociceptive effects of the cannabinoid agonists in a warm-water tail-withdrawal assay. Direct-acting cannabinoid CB1 agonists Δ9- tetrahydrocannabinol (THC), WIN 55,212, AM7418 and AM4054, had biphasic effects on diuresis, with peak diuretic effects occurring at lower doses than peak antinociceptive effects. Cannabinoid diuresis was similar to κ-opioid agonist–induced diuresis in terms of maximum effects with only moderate loss of Na+. Antagonism studies indicate that the diuretic effects of cannabinoids are CB1-receptor mediated, with both central and peripheral components. These findings suggest that mice may provide a model for understanding the mixed effects of marijuana on urine output, as described in clinical studies, and aid in the development of targeted cannabinoid based therapies for bladder dysfunction.
Keywords: cannabinoid, diuresis, Δ9-tetrahydrocannabinol, THC, mice, bladder dysfunction
1. Introduction
The principal psychoactive constituent of marijuana, Δ9-tetrahydrocannabinol (THC), was identified and synthesized in the early 1960’s (Mechoulam, 1970). Since then, many synthetic analogues of THC have been discovered and their behavioral and physiological effects have been characterized in laboratory animals using various in vivo procedures. Among these different effects, diuretic responses to THC have been anecdotally reported but rarely been systematically evaluated (Pryor et al., 1977). One early clinical study by Ames (1958) reported a 3-fold increase in the magnitude of urine output after THC administration and a more thorough investigation in rats suggested that THC-evoked diuretic responses were greater than those produced by thiazide diuretics (Sofia et al., 1977). A recent report from our laboratory confirmed these early findings in rats, and further, demonstrated that cannabinoid-induced diuresis in rats is mediated by cannabinoid CB1 receptors (Paronis et al., 2013).
Although our previous results indicate that cannabinoid agonists produce their diuretic effects in rats primarily by actions at cannabinoid CB1 receptors, more specific roles for centrally or peripherally located CB1 receptors were not explored (Paronis et al., 2013). Cannabinoid CB1 receptors are found throughout the body, including within the central nervous system (CNS) as well as in the lower urinary tract of humans, mice and other commonly used laboratory animals (Pertwee and Fernando, 1996; Walczak et al., 2009) and early studies suggested that THC increased urine output by actions both in the CNS as well as in peripheral systems (Barry et al., 1973; Sofia et al., 1977). In contrast to the increase in urine output observed in awake, un-instrumented animals, more recent cystometry studies in anesthetized rats have reported that WIN 55,212 increased micturition thresholds and decreased bladder motility, suggesting a role for peripheral cannabinoid CB1 receptors in potentially decreasing diuresis (Dmitrieva and Berkley, 2002). These studies have identified a potential role for peripheral cannabinoid CB1 receptors in the urinary tract of unconscious rodents or in isolated bladder tissue (Walczak et al., 2009) yet their function in either micturition or diuresis in intact mice has, to the best of our knowledge, not been evaluated previously. Indeed, diuresis has not been identified as a quantitative or qualitative measure of cannabinoid effect in mice. Hence, the studies described here characterize the diuretic effects of cannabinoid agonists in mice and, further, identify roles for both central and peripheral cannabinoid CB1 receptors in mediating these effects.
Our results extend previous reports in rats and humans by showing that cannabinoid agonists produce diuresis in intact mice. Our results further uniquely demonstrate that these effects are biphasic for all cannabinoid agonists tested, and suggest that the increases in urine output produced after administration of low to moderate cannabinoid doses occur by actions at cannabinoid CB1 receptors within the CNS while decreases in urine output produced at higher doses may also involve actions at peripheral cannabinoid CB1 receptors. Finally, we show here that the increased urine output after cannabinoids is weakly naturetic without affecting excretion of Cl− or K+. Further studies addressing the mechanisms of cannabinoid induced diuresis may reveal new insights into the role of cannabinoid receptors in maintaining water homeostasis.
2. Material and methods
2.1 Animals
Male CD-1 mice, weighing 20–25 g at the start of the study (Charles River Laboratories, Wilmington MA), were housed 4/cage in a climate controlled vivarium with food and water available ad libitum. Mice were acclimatized to the animal facility for 7 days, and to study procedures twice, prior to testing. Mice were re-used with a minimum 7 day interval between drug testing. Each group or data point in the paper represents n=6–8 mice. All experiments were performed during the light portion of the light/dark cycle. All studies were approved by the Northeastern University Animal Care and Use Committee, in accordance with guidelines established by the National Research Council.
2.2 Diuresis
Urine output was measured over 6 h during which mice did not have access to food and water. Mice were placed on an elevated grid floor and isolated under a plastic cup (10cm×5cm; d×ht); weigh boats were placed underneath each mouse to collect the voided urine. Voided urine was measured by determining the change in weight of the boats every 2 h to minimize volume loss due to evaporation. Mice were used for 4–8 weeks; doses of drugs and vehicle were always randomized to minimize time dependent bias. Except where noted, injections were delivered s.c. in volumes of 1 ml.100g−1. When drugs were studied in combination, doses were delivered in half volumes, e.g., for antagonism studies 30 min pretreatment with 0.5 ml.100g−1 vehicle or antagonist was followed by 0.5 ml.100g−1 injection of the agonist.
2.3 Measurement of urine pH, Na+, K+ and Cl−
The total urine voided by individual mice over 6 h was collected, weighed, transferred to eppendorf tubes, and stored at – 4°C until analysis. The samples were diluted (1:5 in deionized water) and urine pH and Na+, K+, and Cl− concentrations were measured using ion selective microelectrodes according to manufacturer’s protocol (Lazar Research Laboratory, Inc, Los Angeles, CA, USA). Total amounts of each electrolyte were quantified for each 6 h sample using the formula: 5×diluted sample concentration (μEq/ml)×total volume (ml) of sample.
2.4 Antinociception
Antinociceptive responses were determined using a warm water tail-withdrawal assay. A water bath maintained water temperature at 52.0 ± 0.5°C. Each mouse was gently hand held and the distal 2–3 cm of its tail immersed in the water; latency to tail-withdrawal was measured using a stopwatch and a cut-off time of 8s was established to avoid tissue damage. Baseline latencies were determined twice on each test day with a 10 min interval; only mice with baseline latencies of 1–3s were used in drug studies. Complete dose response curves were generated in each mouse using cumulative dosing procedures similar to those described previously (Paronis and Woods, 1997). Briefly, 30 min (morphine, WIN 55,212-2 and pentobarbital) or 60 min (vehicle, THC, AM7418 and AM4054) after an injection, tail-withdrawal latencies were determined and mice were then injected with the next dose, such that the total cumulative dose was increased by 0.25 or 0.5 log units. This procedure was repeated until the tail-withdrawal latency reached the cut-off or no longer increased with subsequent increase in dose of the test drug.
2.5 Drugs
Δ9-THC and rimonabant were obtained from the National Institute on Drug Abuse [(NIDA), Rockville, MD]; WIN-55-212 [((R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate], U50,488 [trans-(+/-)3,4-dichloro-N-methyl-N-(2-[1-pyrrolidinyl]-cyclohexyl)-benzeneacetamide methane sulfate] and furosemide were purchased from Sigma-Aldrich (St. Louis, MO). AM7418 [Butyl-2-[(6aR, 10aR)-6a, 7, 10, 10a-tetrahydro-1-hydroxy-9-(hydroxymethyl)-6,6-dimethyl-6H-benzo[c]chromen-3-yl]-2-methylpropanoate], AM4054 [9β-(hydroxymethyl)-3-(1-adamantyl)-hexahydrocannabinol], AM6545 [5-(4-(4-cyanobut-1-ynyl)phenyl-1-(2,4-dichlorophenyl)-4-methyl-N-(1,1-ioxothiomorpholino)-1H-pyrazole-3-carboxamide] and AM630 [6-iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-yl](4-methoxyphenyl) methanone] were synthesized at the Center for Drug Discovery, Northeastern University. Morphine and U50,488 were dissolved in saline; furosemide was dissolved in 1% 1N NaOH and sterile water; all other compounds were prepared in 5% ethanol, 5% emulphor-620 (Rhodia, Cranbury, NJ) and 90% saline, and further diluted with saline. Drug doses are expressed in terms of the weight of free base.
2.6 Statistical analysis
Tail withdrawal latencies are expressed as a percentage of maximum possible effect (%MPE), calculated using the formula: %MPE = [(test latency – baseline latency)/ (8 – baseline latency)] × 100. To determine ED50 values for diuresis, 50% of the maximum effect was defined using the formula: [((maximum urine output with the drug – urine output with vehicle)/2) + urine output with vehicle]. ED50 values were calculated using linear regression when more than two data points were available, and otherwise were calculated by interpolation. All drug data were plotted and analyzed using log transformed values of doses. Data were analyzed using one way ANOVA followed by Dunnett’s or Bonferroni’s multiple comparison tests; significance for all tests was set at P ≤ 0.05.
3. Results
3.1 Quantifying diuresis
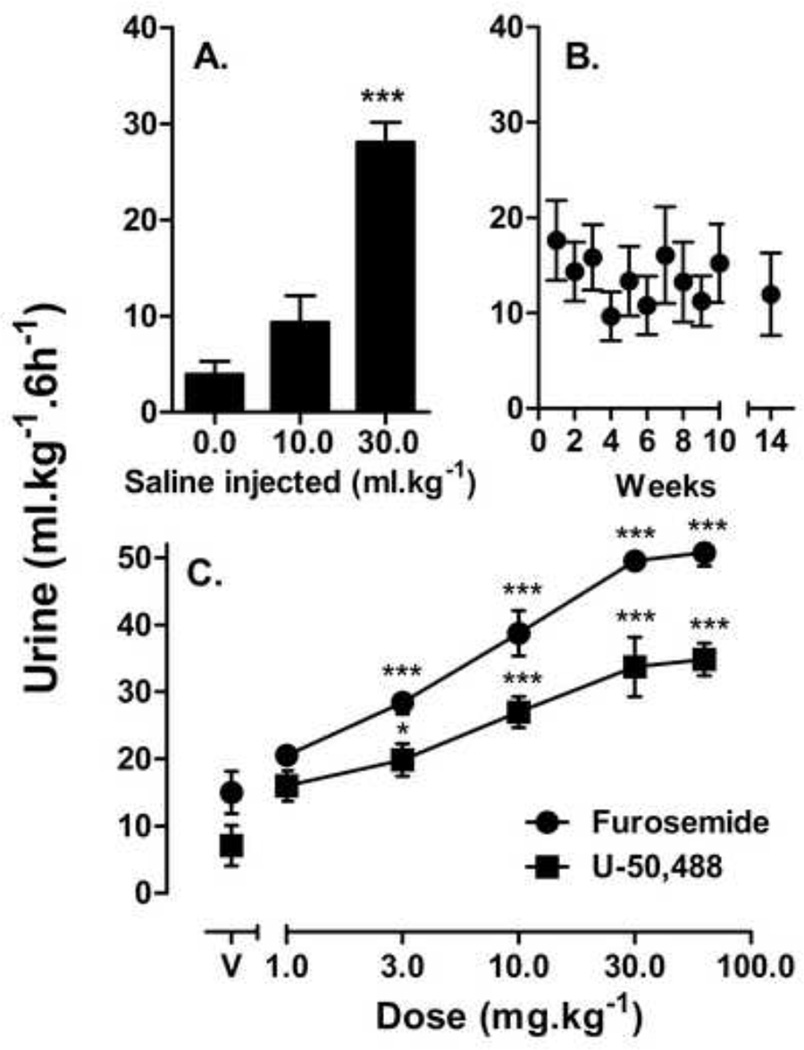
Mice that received sham injections voided, on average, 4 ml•kg−1 urine whereas mice that were injected with 10 or 30 ml•kg−1 saline voided volumes of urine equivalent to amounts of fluid injected (Fig 1A). The effects of 30 ml·kg−1 saline were significantly different from sham injections, whereas the effects of 10 ml•kg−1 saline approached significance (P = 0.06). Mice injected with vehicle or saline, at a total volume of 10 ml·kg−1 once a week for 10–14 weeks, showed no significant changes in urine output after repeated tests as shown in Figure 1B. All subsequent studies maintained the total volumes of pre-session injections at 10 ml•kg−1. Initial studies examined effects of furosemide and U-50,488 on changes in urine output; both furosemide and U-50,488 increased urine output significantly as shown in Figure 1C, with ED50 (95% C.I.) values of 4.8 (3.6,6.3) mg•kg−1 and 3.8 (2.7,4.9) mg•kg−1 respectively. Maximum urine outputs were significantly different for the two drugs (P<0.001), with 30.0–60.0 mg•kg−1 furosemide resulting in ~50 ml•kg−1 urine and 30.0–60.0 mg•kg−1 U-50,488 resulting in ~35 ml•kg−1 urine.
Figure 1.
Diuresis measurements over 6 h. (A) Effects of different volumes of saline (n=8); (B) effects of repeated administration of 10 ml/kg saline (n=8); and (C) effects of furosemide or U50,488 (n=8) (C) on urine output; points above V indicate effects of vehicle injection. Abscissae: volume of saline (A); iteration of saline determination since first determination (B); or drug dose (C). Ordinates: urine output over 6 h in ml per kg body weight. Asterisks indicate doses that had effects significantly different from 10 ml•kg−1 vehicle * P<0.05; *** P<0.001
3.2 Cannabinoid mediated diuresis
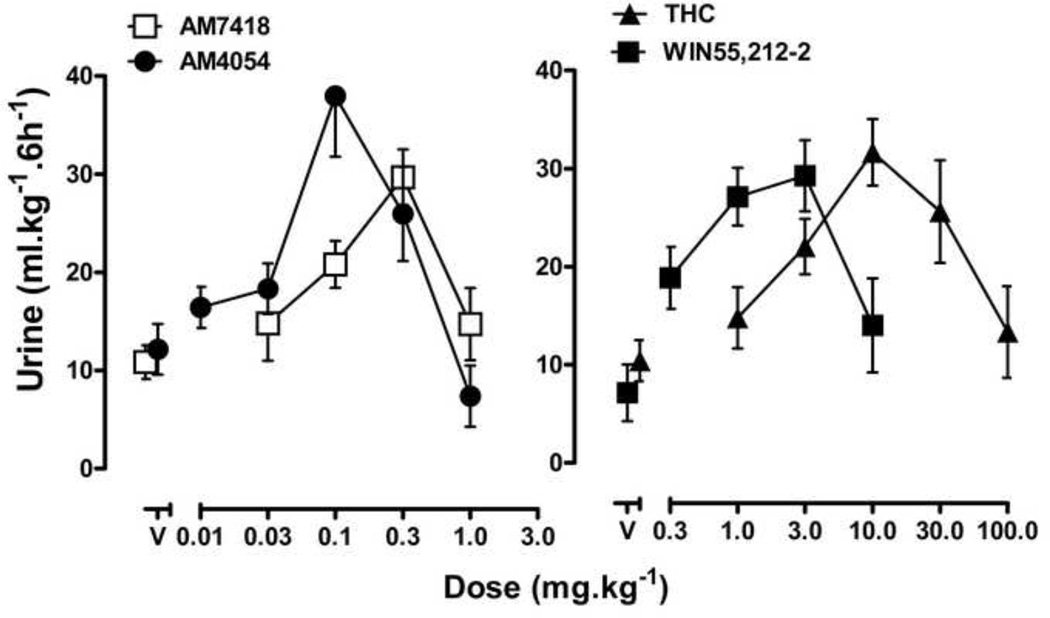
Full dose response curves were generated for four cannabinoid agonists - THC, WIN-55,212-2, AM7418 and AM4054 - and all four drugs increased urine output. THC, at doses of 1.0 −10.0 mg•kg−1, increased urine output in a dose dependent manner with a maximum urine output of 31.6 ± 3.4 ml•kg−1; further increases in THC dose led to decreased urine output, resulting in a biphasic dose response curve. WIN-55212-2, AM7418 and AM4054 had effects similar to THC, with biphasic dose response curves that attained maximum urine outputs of 30 - 36 ml•kg−1, followed by decreases in the magnitude of diuresis (Fig. 2). AM4054 was the most potent drug evaluated; calculated ED50 values for diuresis (with 95% C.I.) were as follows: AM4054 [0.05 (0.01, 0.1) mg•kg−1] ≤ AM7418 [0.09 (0.04, 0.17) mg•kg−1] < WIN 55,212-2 [0.21 (0.0, 0.57) mg•kg−1] < THC [2.2 (1.1, 3.6) mg•kg−1].
Figure 2.
Cannabinoid diuresis. Urine output after AM4054, AM7418, WIN55,212-2 and THC. AM4054 (0.1–0.3 mg•kg−1), AM7418 (0.3 mg•kg−1), WIN55,212-2 (1.0–3.0 mg•kg−1) and THC (10.0–30.0 mg•kg−1) produced statistically significant (P<0.05) increases in urine outputs compared to respective vehicle treatment groups, asterisks omitted for clarity (n=7–8). Other details as in figure 1.
3.3 Cannabinoid mediated antinociception
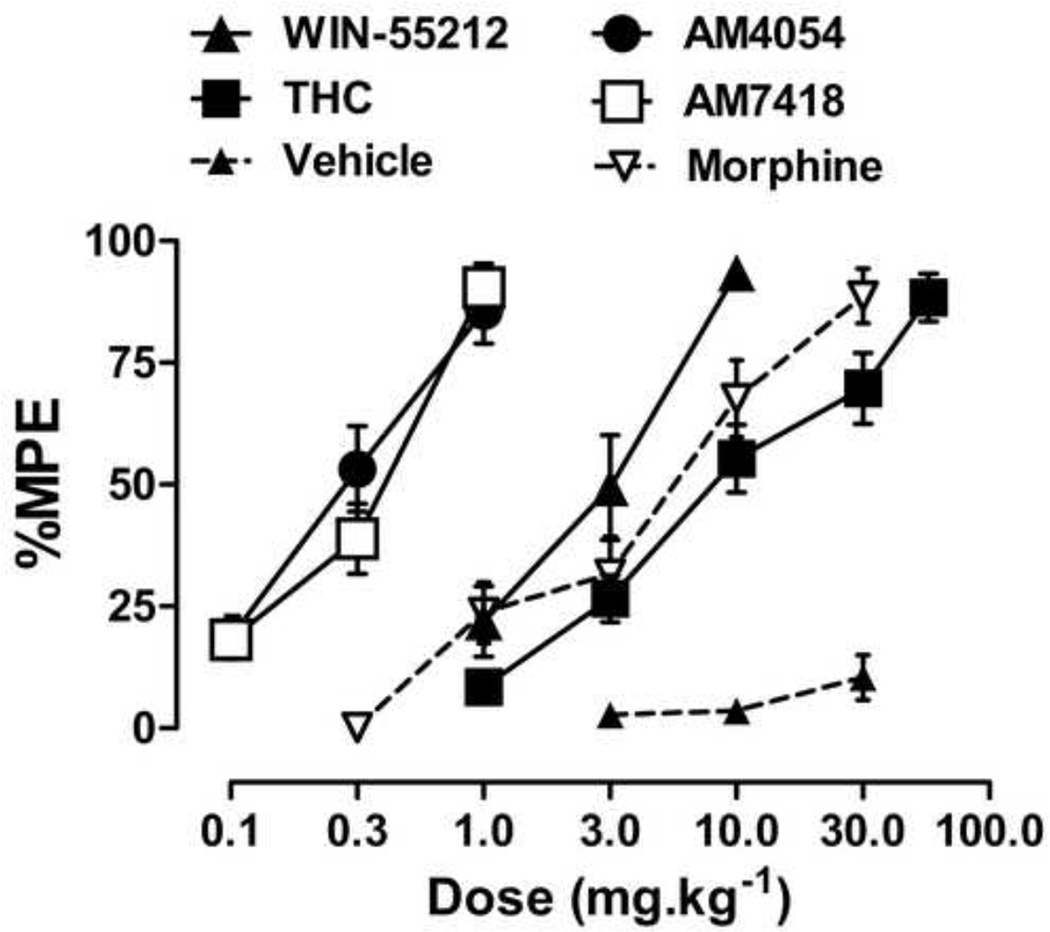
All four cannabinoid agonists dose dependently increased antinociception, with maximum effects similar to those obtained with the μ-opioid agonist morphine (Fig. 3). The ED50 (95% C.I.) values for antinociception for AM4054, AM7418, WIN55,212-2 and THC were 0.3 (0.23,0.37), 0.3 (0.22, 0.39), 2.7 (1.9, 3.7) and 9.2 (7.0, 12.3) mg•kg−1, respectively; thus rank orders of potency of the cannabinoid agonists for increasing tail-withdrawal latency and for diuresis are similar.
Figure 3.
Antinociceptive effects of AM4054, AM7418, THC, WIN55,212-2 and morphine. Vehicle (closed triangles and dotted lines) represent antinociceptive responses following three sequential vehicle injections. Ordinate antinociceptive response, expressed as a percentage of maximum possible effect (%MPE). Abscissa cumulative drug dose in mg•kg−1 body weight.
3.4 Urine electrolyte analysis
The effects of saline, furosemide, THC, and U50,488 were re-determined in separate groups of mice, with results that replicated those shown in Figures 1 and 2. Analysis of urine electrolytes from these studies revealed that furosemide dose-dependently increased the total amounts of Na+ and Cl− excreted in urine over 6 h, whereas the total amounts of K+ excreted over 6 h was decreased after injection of 1.0 mg•kg−1 furosemide, and was increased after 30.0 mg•kg−1 furosemide (Table 1). In contrast, U50,488 did not result in any significant changes in the amounts of Na+, Cl−, or K+ excreted. The effects of THC were intermediate to those of furosemide and U50,488. Doses of THC that significantly increased urine output, 10.0 and 30.0 mg•kg−1 THC, roughly doubled the amount of Na+ excreted over 6 h, without producing any change in the amounts of Cl− or K+ excreted. There were no effects of drug or dose on urine pH values.
Table 1.
Total amount of electrolytes excreted in urine over 6 h (mean ± S.E.M.; n=6–7).
| Na µEq/6h | K µEq/6h | Cl µEq/6h | pH | |
|---|---|---|---|---|
| Saline | 26.5 ± 4.7 | 16.6 ± 3.2 | 130.3 ± 12.8 | 7.5 ± 0.1 |
| Furosemide | ||||
| 1.0 mg·kg−1 | 60.2 ± 11.9 | 5.4 ± 0.9a | 189.2 ± 31.0 | 7.8 ± 1.4 |
| 3.0 mg·kg−1 | 99.1 ± 12.8c | 6.5 ± 1.2 | 249.9 ± 23.5 | 8.1 ± 0.1 |
| 10.0 mg·kg−1 | 149.3 ± 12.9c | 12.6 ± 2.7 | 408.9 ± 32.1c | 8.2 ± 0.3 |
| 30.0 mg·kg−1 | 232.2 ± 17.7c | 30.4 ± 4.5b | 586.3 ± 64.1c | 7.0 ± 0.1 |
| THC | ||||
| 1.0 mg·kg−1 | 17.2 ± 6.8 | 8.4 ± 3.8 | 95.9 ± 30.2 | 7.8 ± 0.3 |
| 3.0 mg·kg−1 | 41.8 ± 15.2 | 14.8 ± 4.4 | 175.1 ± 56.9 | 7.6 ± 0.2 |
| 10.0 mg·kg−1 | 73.2 ± 16.7a | 12.6 ± 3.0 | 202.1 ± 36.6 | 7.6 ± 1.1 |
| 30.0 mg·kg−1 | 71.4 ± 4.6a | 46.4 ± 34.6 | 206.6 ± 6.9 | 7.2 ± 1.0 |
| U-50,488 | ||||
| 1.0 mg·kg−1 | 43.8 ± 22.7 | 6.3 ± 2.4 | 125.5 ± 46.5 | 8.0 ± 0.2 |
| 3.0 mg·kg−1 | 27.9 ± 10.0 | 8.6 ± 2.4 | 117.0 ± 33.0 | 7.5 ± 0.2 |
| 10.0 mg·kg−1 | 27.2 ± 7.2 | 18.8 ± 4.3 | 127.3 ± 27.7 | 7.5 ± 0.2 |
| 30.0 mg·kg−1 | 20.1 ± 3.5 | 26.4 ± 7.8 | 103.8 ± 20.0 | 7.7 ± 0.3 |
P < 0.05, compared to saline
P < 0.01, compared to saline
P < 0.001, compared to saline
3.5 Antagonism studies
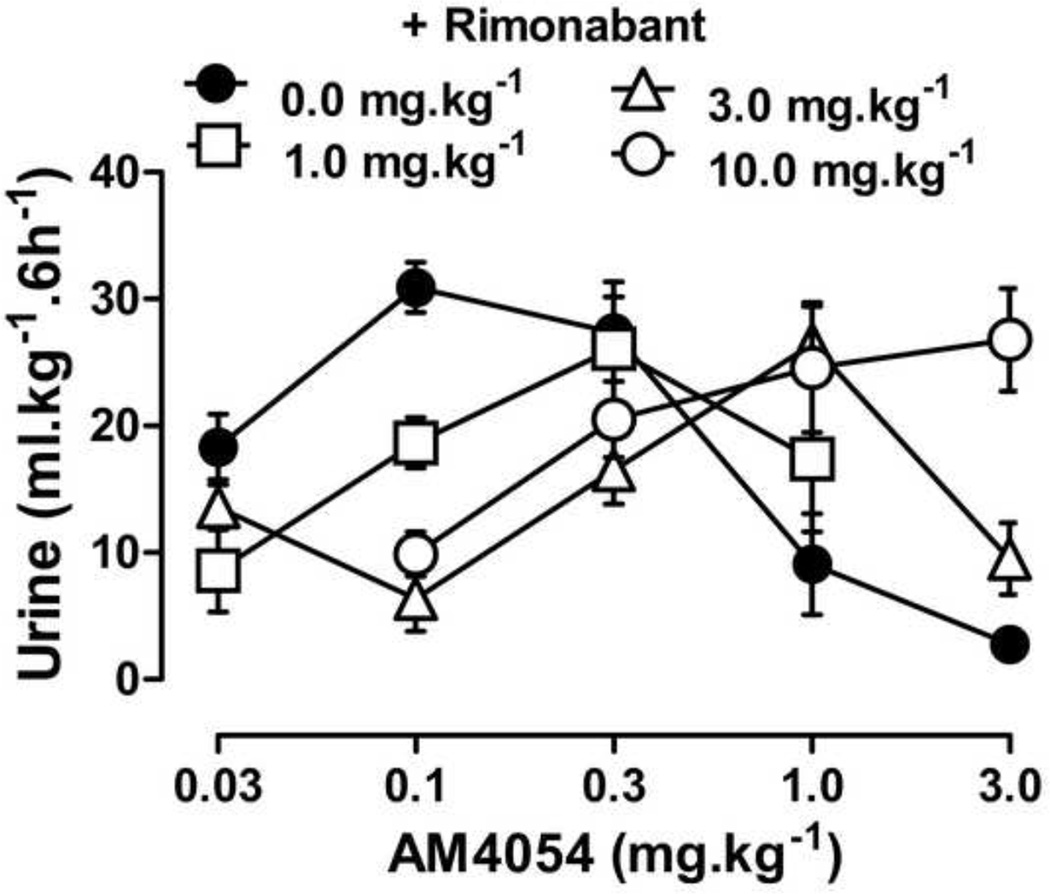
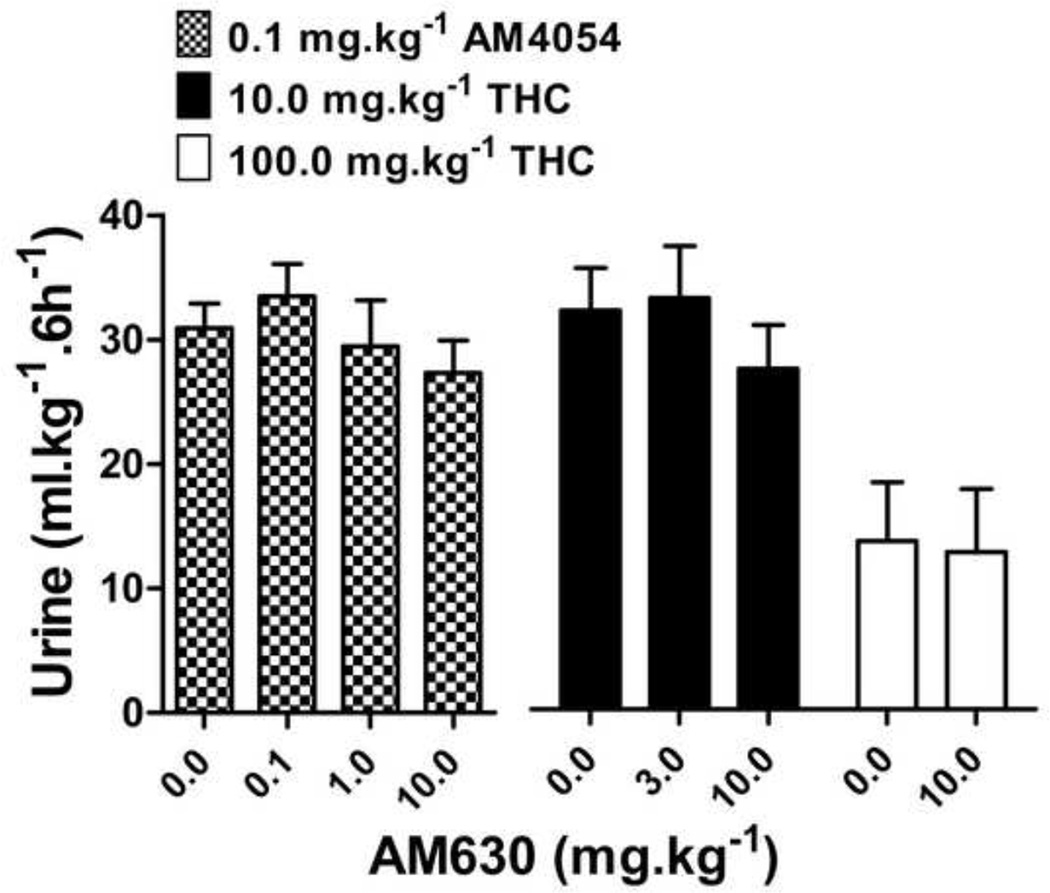
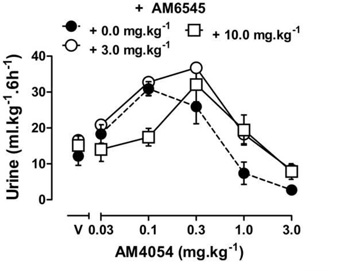
The effects of THC or AM4054 were re-determined after 30 min pretreatment with one of three cannabinoid antagonists: the CB1 selective antagonist rimonabant, the peripherally selective CB1 antagonist AM6545, or the CB2 selective antagonist AM630. Rimonabant alone, 1.0–10.0 mg•kg−1, did not increase or decrease urine output differently from vehicle treatment (data not shown), nor did 10 mg•kg−1 rimonabant affect diuresis produced by either furosemide or U50,488 (data not shown). However, 30 min pretreatment with rimonabant produced dose-dependent rightward shifts of the AM4054 dose response curve, as shown in Figure 4. Rimonabant shifted the ascending limb of the AM4054 dose response curve 4–15 fold to the right, based on the calculated ED50 values listed in Table 2 (the slopes were not significantly different; P=0.15). Rimonabant also shifted the descending limb of the AM4054 dose-response curve to the right (Fig. 4 and Table 2). The CB2 selective antagonist AM630 given alone, at a dose of 10.0 mg•kg−1, had effects similar to saline, resulting in 12.4 ± 2.6 ml•kg−1 urine and pretreatment with AM630 (0.1 - 10.0 mg•kg−1) did not affect the increase in urine output produced by the peak dose of 0.1 mg•kg−1 AM4054 (Fig. 5). Similarly, pretreatment with AM630 did not antagonize the effects of either 10 or 100 mg•kg−1 THC on diuresis (Fig. 5). Pretreatment with the peripherally selective CB1 antagonist AM6545, at a dose of 3.0 mg•kg−1, had no effect on the ED50 for the diuretic effects of AM4054, yet did produce a ~3-fold rightward shift of the descending limb of the AM4054 dose-effect curve (Fig. 6). Increasing the dose of AM6545 to 10 mg.kg−1 failed to produce a greater antagonism of the descending limb of AM4054. However, unlike the lower dose, it did antagonize the ascending limb of the AM4054 dose response curve increasing the ED50 value for diuretic effects from 0.06 mg.kg−1 to 0.18 mg.kg−1 (Fig. 6).
Figure 4.
Cannabinoid diuresis with CB1 antagonist. Dose response curves for AM4054 on diuresis after 30 min pretreatment with rimonabant or vehicle (n=7–8); other details as in figure 1.
Table 2.
ED50 values (with 95% C.I.) and potency ratios calculated from the ascending and descending limb of diuresis dose response curves.
| Ascending limb | Descending limb | |||
|---|---|---|---|---|
| ED50 ( mg·kg−1)a | Potency Ratiob | ED50 ( mg·kg−1)a | Potency Ratiob | |
| AM4054 alone | 0.06 (ND)c | 0.4 (0.04, 1.6) | ||
| + 1.0 mg·kg−1 Rimonabant | 0.26 (ND)c | 4.4 | 0.7 (ND)c | 1.7 |
| + 3.0 mg·kg−1 Rimonabant | 0.85 (0.8, 1.0) | 14.6 | 1.5 (ND)c | 3.6 |
| + 10.0 mg·kg−1 Rimonabant | 0.88 (ND)c | 15.1 | ND | NA |
ED50 values were calculated from grouped data
Potency ratios were calculated by dividing the ED50 value of the agonist alone by the ED50 value obtained after antagonist pretreatment
95% C.I. were not determined because ED50 value was calculated by interpolation of two points
Figure 5.
Cannabinoid diuresis with CB2 antagonist. Effects of combinations of AM630 with doses of AM4054 and THC that produced maximum increases in urine output, and a high dose of THC that produced a maximum decrease in urine output from fig 2 (n=8).
Figure 6.
Cannabinoid diuresis with peripheral CB1 antagonist. Effects of AM4054 following vehicle, 3.0 mg•kg−1 or 10.0 mg.kg−1 AM6545 (n=8); other details as in figure 1.
4. Discussion
The results presented here demonstrate that THC and other synthetic cannabinergic compounds produce diuresis in mice, extending previous reports of the diuretic effects of cannabinoids in rats and humans (Ames, 1958; Paronis et al., 2013; Sofia et al., 1977). The order of potency for the structurally distinct cannabinoid agonists - THC, WIN55,212-2, AM7418 and AM4054 – in producing diuresis was similar to the order of potency for antinociception, although peak diuretic effects occurred at doses lower than peak antinociceptive effects. The cannabinoid agonists increased urine output in a manner qualitatively and quantitatively more similar to that produced by the κ-opioid agonist U50,488 than the loop diuretic, furosemide. Quantitatively, the four cannabinoids produced maximum urine outputs of 30–36 ml•kg−1, equivalent to the outputs achieved with high doses of U50,488, and less than amounts voided after furosemide. Qualitatively, the relatively small Na+ loss following THC indicates weak naturetic effects that are more similar to the free water diuresis produced by U-50,488 than the electrolyte loss that accompanies furosemide diuresis. However, unlike the κ-opioid agonist and the loop diuretic, the cannabinoid agonists had biphasic dose-effect functions as doses above those that yielded 30–36 ml•kg−1 urine led to dose-dependent decreases in urine output. Such biphasic functions were not noted in previous studies in rats and may represent a distinct difference between species.
The involvement of specific cannabinoid receptors in modulating urine output was investigated through pharmacological antagonism studies. To this end, receptor selective antagonists rimonabant or AM630, and the peripherally constrained antagonist AM6545, were used as pretreatment drugs (Rinaldi-Carmona et al., 1995; Ross et al., 1999; Tam et al., 2010). The cannabinoid CB1 antagonist rimonabant had no intrinsic effects on diuresis yet did dose-dependently antagonize both the ascending and descending limbs of the AM4054 dose response curve. In contrast to rimonabant, the CB2 antagonist AM630 did not attenuate the effects of either moderate or high doses of AM4054 or THC. Together, these results suggest that, as in rats, cannabinoid agonists produce their diuretic effects in mice via actions at cannabinoid CB1 receptors with limited, if any, involvement of CB2 receptors. Moreover, since both limbs of the AM4054 dose-response curve were antagonized by rimonabant, our data further indicate that both the increases and subsequent decreases in magnitude of diuresis are CB1-mediated.
Having identified a role for cannabinoid CB1 receptors in modulating diuresis, we were interested in determining whether these effects occur centrally or in the periphery. The quantitative and qualitative similarity between cannabinoid and κ-opioid diuresis suggests central mediation of these effects as U50,488 produces its diuretic effects through central actions (Kapusta and Obih, 1993, 1995). Moreover, cannabinoid CB1 receptors are more commonly associated with the psychoactive and other CNS effects of cannabinoids (McMahon, 2006; Witkin et al., 2005); however, cannabinoid CB1 receptors are found in several peripheral organs as well including, but not limited to, the kidney and bladder (Gatley et al., 1996; Larrinaga et al., 2010; Pertwee, 1997; Pertwee and Fernando, 1996). Together, these encompass multiple possible sites of action through which cannabinergic compounds may modulate urine output. Sofia and colleagues (1971) suggested, based on studies in hypophysectomized and adrenalectomized rats, that THC mediates diuretic effects both centrally and peripherally and a more recent study in intact rats indicated that methanadamide infused directly into the kidney was able to increase urine production (Li and Wang, 2006). On the other hand, results of studies using isolated tissues, have demonstrated that cannabinoid agonists also inhibit contractions in isolated bladder preparations by cannabinoid CB1 receptor mechanisms (Pertwee and Fernando, 1996; Walczak et al., 2009). These contrasting results suggest that cannabinoids may have both CNS-mediated diuretic effects and peripheral anti-micturition effects, which may explain the biphasic dose-response functions obtained in the present studies. To test this hypothesis, the peripherally constrained cannabinoid CB1 antagonist AM6545 (Cluny et al., 2010; Tam et al., 2010) was injected prior to determination of a full AM4054 dose-effect function. A moderate dose of AM6545 did not affect the ascending limb of the AM4054 function, while shifting the descending limb of AM4054 diuresis to the right; a higher dose of AM6545 was able to shift both limbs of the AM4054 dose effect function. Although AM6545 does not readily cross the blood-brain barrier, higher doses will penetrate the CNS and have been associated with blockade of central antinociceptive cannabinoid effects in mice (Chopda et al., 2013). Though limited, these data suggest that diuresis produced by lower doses of agonists are central cannabinoid CB1 receptor effects, however, the decrease in the magnitude of diuresis produced at higher doses of agonists likely involves both central and peripheral cannabinoid CB1 receptors. In addition, indirect effects downstream of actions at cannabinoid CB1 receptors may also have contributed to the descending limb of the cannabinoid dose-effect functions. For example, cannabinoid agonists will produce vasodilation and hypotension via direct actions at cannabinoid CB1 receptors (Wagner et al., 2001); in turn, these actions may stimulate the baroreflex and lead to an increase in vasopressin release which would subsequently decrease urine output.
Clinical studies have reported beneficial effects of smoked or aerosolized cannabis on bladder dysfunction in patients with multiple sclerosis, primarily by decreasing urinary frequency in these subjects following marijuana use (Brady et al., 2004; Consroe et al., 1997). These reports contrast with the earlier clinical reports demonstrating increase in urine output after cannabis administration (Ames, 1958). Our findings in mice demonstrate a dose related increase or decrease in urine output, providing a platform for understanding the mixed effects on urine output observed with marijuana in various clinical studies. As noted earlier in a study with rats (Sofia et al., 1977), the diuresis induced by THC in mice also is weakly naturetic compared to furosemide and further investigations in this area may yield a new, clinically beneficial diuretic. In contrast, our data suggest that development of peripherally selective cannabinoid CB1 agonists may be beneficial for patients suffering from bladder dysfunction.
Acknowledgements
This work was supported by Northeastern University Institutional funds and the National Institutes of Health (DA19205).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of this work were presented previously: Chopda GR, Sharma R, Vemuri K, Thakur GA, Makriyannis A, Paronis CA (2011) Cannabinoid mediated diuresis in mice at the American Society for Pharmacology and Experimental Pharmacology meeting, April 2011 in Washington, D.C, USA.
Statement of conflicts: None
References
- Ames F. A clinical and metabolic study of acute intoxication with Cannabis sativa and its role in the model psychoses. J Ment Sci. 1958;104:972–999. doi: 10.1192/bjp.104.437.972. [DOI] [PubMed] [Google Scholar]
- Barry H, 3rd, Kubena RK, Perhach JL., Jr Pituitary-adrenal activation and related responses to delta1-tetrahydrocannabinol. Prog Brain Res. 1973;39:323–330. doi: 10.1016/S0079-6123(08)64088-1. [DOI] [PubMed] [Google Scholar]
- Brady CM, DasGupta R, Dalton C, Wiseman OJ, Berkley KJ, Fowler CJ. An open-label pilot study of cannabis-based extracts for bladder dysfunction in advanced multiple sclerosis. Mult Scler. 2004;10:425–433. doi: 10.1191/1352458504ms1063oa. [DOI] [PubMed] [Google Scholar]
- Chopda G, Bergman J, Vemuri K, Makriyannis A, Paronis CA. Possible Efficacy Related Differences Among Cannabinoid Agonists. The FASEB journal. 2013;27:2. [Google Scholar]
- Cluny NL, Vemuri VK, Chambers AP, Limebeer CL, Bedard H, Wood JT, Lutz B, Zimmer A, Parker LA, Makriyannis A, Sharkey KA. A novel peripherally restricted cannabinoid receptor antagonist, AM6545, reduces food intake and body weight, but does not cause malaise, in rodents. Br J Pharmacol. 2010;161:629–642. doi: 10.1111/j.1476-5381.2010.00908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consroe P, Musty R, Rein J, Tillery W, Pertwee R. The perceived effects of smoked cannabis on patients with multiple sclerosis. Eur Neurol. 1997;38:44–48. doi: 10.1159/000112901. [DOI] [PubMed] [Google Scholar]
- Dmitrieva N, Berkley KJ. Contrasting effects of WIN 55212-2 on motility of the rat bladder and uterus. J Neurosci. 2002;22:7147–7153. doi: 10.1523/JNEUROSCI.22-16-07147.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatley SJ, Gifford AN, Volkow ND, Lan R, Makriyannis A. 123I-labeled AM251: a radioiodinated ligand which binds in vivo to mouse brain cannabinoid CB1 receptors. Eur J Pharmacol. 1996;307:331–338. doi: 10.1016/0014-2999(96)00279-8. [DOI] [PubMed] [Google Scholar]
- Kapusta DR, Obih JC. Central kappa opioid receptor-evoked changes in renal function in conscious rats: participation of renal nerves. J Pharm Exp Ther. 1993;267:197–204. [PubMed] [Google Scholar]
- Kapusta DR, Obih JC. Central kappa opioids blunt the renal excretory responses to volume expansion by a renal nerve-dependent mechanism. J Pharm Exp Ther. 1995;273:199–205. [PubMed] [Google Scholar]
- Larrinaga G, Varona A, Perez I, Sanz B, Ugalde A, Candenas ML, Pinto FM, Gil J, Lopez JI. Expression of cannabinoid receptors in human kidney. Histol Histopathol. 2010;25:1133–1138. doi: 10.14670/HH-25.1133. [DOI] [PubMed] [Google Scholar]
- Li J, Wang DH. Differential mechanisms mediating depressor and diuretic effects of anandamide. J.Hypertens. 2006;24:2271–2276. doi: 10.1097/01.hjh.0000249706.42230.a8. [DOI] [PubMed] [Google Scholar]
- McMahon LR. Characterization of cannabinoid agonists and apparent pA2 analysis of cannabinoid antagonists in rhesus monkeys discriminating Δ9-tetrahydrocannabinol. J Pharm Exp Ther. 2006;319:1211–1218. doi: 10.1124/jpet.106.107110. [DOI] [PubMed] [Google Scholar]
- Mechoulam R. Marihuana chemistry. Science. 1970;168:1159–1166. doi: 10.1126/science.168.3936.1159. [DOI] [PubMed] [Google Scholar]
- Paronis CA, Thakur GA, Bajaj S, Nikas SP, Vemuri VK, Makriyannis A, Bergman J. Diuretic effects of cannabinoids. J Pharmacol Exp Ther. 2013;344:8–14. doi: 10.1124/jpet.112.199331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paronis CA, Woods JH. Clocinnamox dose-dependently antagonizes morphine-analgesia and [3H]DAMGO binding in rats. Eur J Pharmacol. 1997;337:27–34. doi: 10.1016/s0014-2999(97)01296-x. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Fernando SR. Evidence for the presence of cannabinoid CB1 receptors in mouse urinary bladder. Br J Pharmacol. 1996;118:2053–2058. doi: 10.1111/j.1476-5381.1996.tb15643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor GT, Husain S, Larsen F, McKenzie CE, Carr JD, Braude MC. Interactions between delta9-tetrahydrocannabinol and phencyclidine hydrochloride in rats. Pharmacol Biochem Behav. 1977;6:123–136. doi: 10.1016/0091-3057(77)90168-x. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Héaulme M, Alonso R, Shire D, Congy C, Soubrié P, Brelière JC, Le Fur G. Biochemical and pharmacological characterisation of SR141716A, the first potent and selective brain cannabinoid receptor antagonist. Life Sci. 1995;56:1941–1947. doi: 10.1016/0024-3205(95)00174-5. [DOI] [PubMed] [Google Scholar]
- Ross RA, Brockie HC, Stevenson LA, Murphy VL, Templeton F, Makriyannis A, Pertwee RG. Agonist-inverse agonist characterization at CB1 and CB2 cannabinoid receptors of L759633, L759656 and AM630. Br J Pharmacol. 1999;126:665–672. doi: 10.1038/sj.bjp.0702351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofia RD, Knobloch LC, Harakal JJ, Erikson DJ. Comparative diuretic activity of delta9-tetrahydrocannabinol, cannabidiol, cannabinol and hydrochlorothiazide in the rat. Arch Int Pharmacodyn Ther. 1977;225:77–87. [PubMed] [Google Scholar]
- Tam J, Vemuri KV, Liu J, Bátkai S, Mukhopadhyay B, Godlewski G, Osei-Hyiaman D, Ohnuma S, Ambudkar SV, Pickel J, Makriyannis A, Kunos G. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J. Clin. Invest. 2010;120:2953–2966. doi: 10.1172/JCI42551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JA, Jarai Z, Batkai S, Kunos G. Hemodynamic effects of cannabinoids: coronary and cerebral vasodilation mediated by cannabinoid CB(1) receptors. Eur J Pharmacol. 2001;423:203–210. doi: 10.1016/s0014-2999(01)01112-8. [DOI] [PubMed] [Google Scholar]
- Walczak JS, Price TJ, Cervero F. Cannabinoid CB1 receptors are expressed in the mouse urinary bladder and their activation modulates afferent bladder activity. Neuroscience. 2009;159:1154–1163. doi: 10.1016/j.neuroscience.2009.01.050. [DOI] [PubMed] [Google Scholar]
- Witkin JM, Tzavara ET, Nomikos GG. A role for cannabinoid CB1 receptors in mood and anxiety disorders. Behavioural Pharmacology. 2005;16:315–331. doi: 10.1097/00008877-200509000-00005. [DOI] [PubMed] [Google Scholar]