Abstract
The prefrontal cortex (PFC) is referred to as the visceral motor cortex; however, little is known about whether this region influences respiratory or metabolic outflows. The aim of this study was to describe simultaneous changes in respiratory, metabolic and cardiovascular functions evoked by disinhibition of the medial PFC (mPFC) and adjacent lateral septal nucleus (LSN). In urethane-anaesthetized rats, bicuculline methiodide was microinjected (2 mm; GABA-A receptor antagonist) into 90 sites in the mPFC at 0.72–4.00 mm from bregma. Phrenic nerve amplitude and frequency, arterial pressure, heart rate, splanchnic and lumbar sympathetic nerve activities (SNA), expired CO2, and core and brown adipose tissue temperatures were measured. Novel findings included disturbances to respiratory rhythm evoked from all subregions of the mPFC. Injections into the cingulate cortex evoked reductions in central respiratory function exclusively, whereas in ventral sites, particularly the infralimbic region, increases in respiratory drive and frequency, and metabolic and cardiac outflows were evoked. Disinhibition of sites in surrounding regions revealed that the LSN could evoke cardiovascular changes accompanied by distinct oscillations in SNA, as well as increases in respiratory amplitude. We show that activation of neurons within the mPFC and LSN influence respiratory, metabolic and cardiac outflows in a site-dependent manner. This study has implications with respect to the altered PFC neuronal activity seen in stress-related and mental health disorders, and suggests how basic physiological systems may be affected.
Key points
The medial prefrontal cortex (mPFC) is often referred to as the ‘visceral cortex’, largely based on anatomical connections and cardiovascular influences.
Although an extensive network of inhibitory interneurons regulates PFC function, their roles in modifying central respiratory, metabolic and cardiac functions have not been explored.
This study provides the first integrative investigation describing central respiratory, metabolic and cardiac variables altered following chemical disinhibition of discrete subregions of the mPFC.
Changes were evoked in a site-dependent manner, with central respiratory function modified throughout the mPFC, and exclusively from dorsal regions. By contrast, central respiratory, metabolic and cardiac functions were simultaneously increased in the ventral mPFC, particularly the infralimbic cortex.
These data provide reference material for future investigations into chronic changes in the activity of neurons within the mPFC, as seen in stress and mental health disorders, which are often accompanied by autonomic and respiratory dysfunction.
Introduction
The medial prefrontal cortex (mPFC), defined here as the cingulate (Cg), prelimbic [PrL (dorsal and ventral)], infralimbic (IL) and dorsal peduncular (DP) cortices, plays a major role in cognition, emotion and executive functioning (Miller, 2000; Miller & Cohen, 2001; Kane & Engle, 2002; Etkin et al. 2011). This is achieved primarily by modifying activity in other brain regions. PFC dysfunction is characteristic in mental illness (Gamo & Arnsten, 2011) and the PFC is implicated in the response to stress. For example, the PrL controls stress response inhibition and reward, and the IL regulates the emotional and stress responses to fear (McKlveen et al. 2013). Stress causes remodelling within the PFC (Holmes & Wellman, 2009) and the blockade of GABA-A receptors in the IL cortex has recently been demonstrated to increase anxiety-like behaviours in mice (Bi et al. 2013). As emotion and stress are strongly linked to autonomic function (Hansel & von Kanel, 2008), it is not surprising that the mPFC is also called the ‘visceral motor cortex’ (Neafsey, 1990). Sensitive methods of tract tracing reveal extensive connections between the mPFC and autonomic and respiratory loci (Saper, 1982; Shipley, 1982; Terreberry & Neafsey, 1983; van der Kooy et al. 1984; Hurley-Gius & Neafsey, 1986; Terreberry & Neafsey, 1987; Hurley et al. 1991; Yasui et al. 1991; Barbas et al. 2003), providing multiple sites for potential influence such as the insular cortex, amygdala, lateral and dorsomedial hypothalamus, periaqueductal grey area, parabrachial nucleus, nucleus of the solitary tract, ventral medulla, raphe and spinal cord (Neafsey et al. 1986; Sesack et al. 1989; Hurley et al. 1991; Takagishi & Chiba, 1991; Bacon & Smith, 1993; An et al. 1998; Heidbreder & Groenewegen, 2003; Hoover & Vertes, 2007). Despite this label, only a limited number of autonomic outflows have been monitored following stimulation of this area (for reviews see Verberne & Owens, 1998; Groenewegen & Uylings, 2000; Van Eden & Buijs, 2000; Saper, 2002; Hansel & von Kanel, 2008; Cechetto & Shoemaker, 2009). Moreover, there are grounds for functional differences based on target projections and unique sources of afferent input for each of the prefrontal subregions (Heidbreder & Groenewegen, 2003). Therefore, a comprehensive examination of the influence of subregions of the mPFC on respiratory, cardiovascular and metabolic functions is required.
The effects of electrical stimulation, regional lesioning and a few examples of direct chemical stimulation of the mPFC form the most recent functional data with respect to autonomic effects evoked from this region (Terreberry & Neafsey, 1987; Verberne et al. 1987; Verberne, 1996; Crippa et al. 1999; Owens & Verberne, 2001; Resstel & Correa, 2006). Electrical stimulation of the mPFC in conscious animals generally evokes increases in arterial pressure (AP), transient increases in respiratory rate or depth, measured by pneumotachygraph, and reductions in gastric tone and/or wave amplitude (Anand & Dua, 1956; Buchanan et al. 1984; Burns & Wyss, 1985; Hurley-Gius & Neafsey, 1986; al Maskati & Zbrozyna, 1989; Cechetto & Chen, 1990; Panteleev & Grundy, 2000; Alexandrov et al. 2007). Electrical stimulation of the mPFC in anaesthetized animals evokes decreases in AP predominantly mediated by the sympathoinhibition of abdominal visceral vasculature, as measured by regional haemodynamic changes, splanchnic sympathetic nerve discharge and single unit recordings of medullary sympathoexcitatory neurons (Verberne, 1996; Verberne & Owens, 1998; Owens & Verberne, 2001). Together, these highlight the limited range of autonomic or respiratory variables [mostly heart rate (HR) and AP] that have been measured thus far.
Furthermore, chemical excitation provides a superior alternative to electrical stimulation and ensures the effects are evoked from neurons within the region of interest. In anaesthetized animals, glutamate administration to the mPFC evokes very small depressor responses and mild bradycardia (Owens & Verberne, 2001), whereas, in conscious animals, HR-generated increases in AP are evoked from the IL and PrL subregions and not from the dorsal mPFC (Resstel & Correa, 2005). Acetylcholine injected throughout the mPFC elicits transient hypotension mediated by vasodilatation in the hind limb vascular beds (Crippa et al. 1999, 2000). However, noradrenaline in the Cg1 in conscious animals evokes a pressor response and bradycardia potentially mediated by the release of vasopressin (Fernandes et al. 2003), and HR changes are dependent on the adrenergic receptor type activated (Funk & Stewart, 1996). Thus only very small changes in cardiovascular parameters are evoked by chemical stimulation and these are the only parameters measured so far.
It is clear that the disinhibition or removal of GABAergic input is a potent stimulus in higher brain regions in revealing autonomic responses (DiMicco et al. 1987; Pagani et al. 1987). In the PFC, GABA arising from local cortical interneurons and also from long-range inputs plays a critical role in regulating the excitability of pyramidal projection cells and activity within cortical microcircuits (Somogyi et al. 1998; Gupta et al. 2000; Tamamaki & Tomioka, 2010; Fino & Yuste, 2011). Importantly, the role played by GABA in influencing autonomic outflows from the mPFC has not been examined.
The aims of this study were to comprehensively describe the respiratory, cardiovascular and metabolic outflows altered by disinhibition of subregions of the mPFC, using microinjection of a GABA-A antagonist, bicuculline methiodide (BIC). We extended our study to include adjacent regions of the rostral pole of the lateral septal nucleus (LSN), the medial/ventral orbital cortex and the nucleus of the accumbens shell (NAcbsh) in order to determine how higher-order brain regions differ in their influence on autonomic function. To facilitate the measurement of a comprehensive set of variables, this study was conducted in urethane-anaesthetized rats. The variables measured were phrenic nerve burst amplitude and frequency (respiratory), AP and HR (cardiovascular), splanchnic and lumbar sympathetic nerve activities (SNA) (cardiovascular and metabolic), expired CO2 (respiratory and metabolic), and core temperature and interscapular brown adipose tissue (iBAT) temperature (metabolic).
Methods
Ethical approval
All procedures conformed to the regulations detailed in the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes and were approved by the Macquarie University Animal Care and Ethics Committee.
General procedures and recordings
Ten male Sprague–Dawley rats weighing 350–550 g (Animal Resource Centre, Perth, WA, Australia) were anaesthetized with urethane in saline (10% solution administered at 1.3 mg kg−1i.p.; Sigma-Aldrich Pty Ltd, Sydney, NSW, Australia). The maintenance of anaesthesia was assessed at regular intervals ensuring that noxious pinch did not evoke changes in AP or phrenic nerve discharge rate. Supplemental doses of urethane were given when necessary. The right femoral artery and vein were cannulated for AP measurement and drug administration, respectively. The rats were intubated to permit artificial ventilation with oxygen-enriched air (Ugo Basile SRL, Varese, Italy). End-tidal CO2 was continuously monitored and maintained during control conditions at 3.5–4.5% using a Capstar-100 CO2 analyser (CWE, Inc., Ardmore, PA, USA) by adjusting ventilation volume and rate. Colonic (core) temperature was maintained at 36.5–37.5°C with a thermostatically controlled heating pad (Harvard Apparatus, Holliston, MA, USA). Whole nerve recordings were made from the phrenic nerve (PN) and splanchnic sympathetic nerves (sSN), using bipolar silver hook electrodes bathed in mineral oil. The left phrenic and both vagus nerves were approached dorsally, medial to the scapular, and isolated and cut. The left greater splanchnic preganglionic nerve and lumbar sympathetic chain were isolated using a retro-peritoneal approach as previously described (Burke et al. 2008). In a subset of animals (n= 3), lumbar sympathetic nerve activity (lSNA) and iBAT were measured to assess the possibility of differential sympathetic control, as well as the contribution of thermogenesis in generating core body temperature increases. Brown adipose tissue (BAT) temperature was recorded by inserting thermocouples into the left and right iBAT pads as described previously (Cao et al. 2010; Morrison & Nakamura, 2011).
Neuromuscular blockade was maintained with pancuronium bromide (0.8 mg i.v. induction, 0.4 mg h−1i.v. maintenance; Astra Pharmaceuticals Pty Ltd, Sydney, NSW, Australia) in a constant infusion of physiological saline (1.5 ml h−1) for hydration. Rats were positioned prone in a stereotaxic frame. Using a flat skull orientation, two bore holes were drilled into the skull spanning 0.5–4.0 mm rostral to bregma and ∼3 mm in diameter. The dura was removed for acute microinjection into cortical nuclei.
Nerve recordings were amplified (×10000; CWE Inc.), bandpass filtered (0.1–3 kHz), sampled at 5 kHz [CED The Power 1401-3; Cambridge Electronic Design (CED) Ltd, Cambridge, UK] and recorded on a computer using Spike2 software (CED Ltd). Core and BAT temperatures and end-tidal CO2 were sampled at 2 kHz and also recorded using Spike2. Heart rate was derived from AP. Phrenic nerve frequency (PNf) and amplitude (PNamp) were derived from PN activity (PNA).
Experimental protocol
The region spanning the mPFC and rostral LSN at 0.72–4.00 mm rostrocaudally, 0.70–1.00 mm mediolaterally and 2.00–7.00 mm dorsoventrally from bregma (Paxinos & Watson, 2005) was mapped using microinjections of BIC to locate sites from which autonomic effects were evoked. Injections were made stereotaxically at 1 mm intervals, both rostrocaudally and dorsoventrally. Single glass micropipettes were used for pressure microinjection of BIC (2 mm; Sigma-Aldrich Pty Ltd) dissolved in 10 mm PBS (pH 7.4). This concentration of bicuculline has been used to disinhibit a number of different brain regions in the rat (Cao et al. 2004; Sheriff et al. 2006; McDowall et al. 2007; Nakamura & Morrison, 2007; Iigaya et al. 2012). Injection volumes were 250 nl. In each experiment some sites of injection were marked with rhodamine-labelled microspheres (FluoSpheres; Molecular Probes, Eugene, OR, USA). Using these sites as reference points, the other sites of injection were determined. No more than nine sites were injected in any one animal and sites (rostrocaudal) were injected in a different order in each experiment. A period of > 40 min was allowed to elapse after the injection of each site and baseline levels were attained before the experiment proceeded to the next site. At the end of each experiment, the rat was killed with 3 m KCl i.v. and the cortex removed and placed in fixative overnight (4% formaldehyde in saline). Coronal sections (100 μm) were cut using a vibrating microtome (Leica VT 1000 S; Leica Microsystems Pty Ltd, Sydney, NSW, Australia), mounted on gelatinized glass slides and cover-slipped for microscopic analysis and reconstruction of injection sites (see below).
Data analysis
Nerve recordings were rectified and averaged using a 2 s time constant. Baseline values were obtained by averaging 60 s of data acquired 5 min prior to the microinjection. Peak changes in each variable following BIC injection were determined and responses were graded into four classes. Injection sites and the responses obtained for each variable [splanchnic SNA (sSNA), mean AP (MAP), HR, phrenic nerve burst amplitude (PNamp) and phrenic nerve burst frequency (PNf), expired CO2, core temperature] were initially reconstructed onto 20 anatomically appropriate sections ranging from 0.72 mm to 4.20 mm rostral to bregma using a brain atlas (Paxinos & Watson, 2005). These response maps were then merged into four maps (presented here) representing responses obtained from 0.72–0.84 mm, 2.04–2.28 mm, 2.52–3.00 mm and 3.72–4.00 mm rostral to bregma. For the levels 0.72–0.84 mm and 2.52–3.00 mm rostral to bregma, peak responses were also calculated along the ventral axis at 1 mm intervals approximately aligning with specific subregions of the PFC and LSN. The resulting column graphs express the mean ± standard error of the mean (s.e.m.) of responses from all sites tested within the region. One-way ANOVA and Bonferroni multiple comparison were used to determine differences across subregions. Differences were considered significant if they achieved a P-value of < 0.05.
Results
Unilateral injections of BIC were made on both sides of the mPFC (e.g. Fig. 1Aa). No differences between the hemispheres were seen in the responses obtained for any of the outputs measured; consequently, all the responses presented refer to one side of the brain. Injections of vehicle solution (PBS) in all subregions of the mPFC and LSN failed to evoke a response in any variable measured (Fig. 1Ab).
Figure 1. Respiratory, cardiovascular and metabolic outputs altered by bicuculline methiodide (BIC) in the rostral medial prefrontal cortex (mPFC).
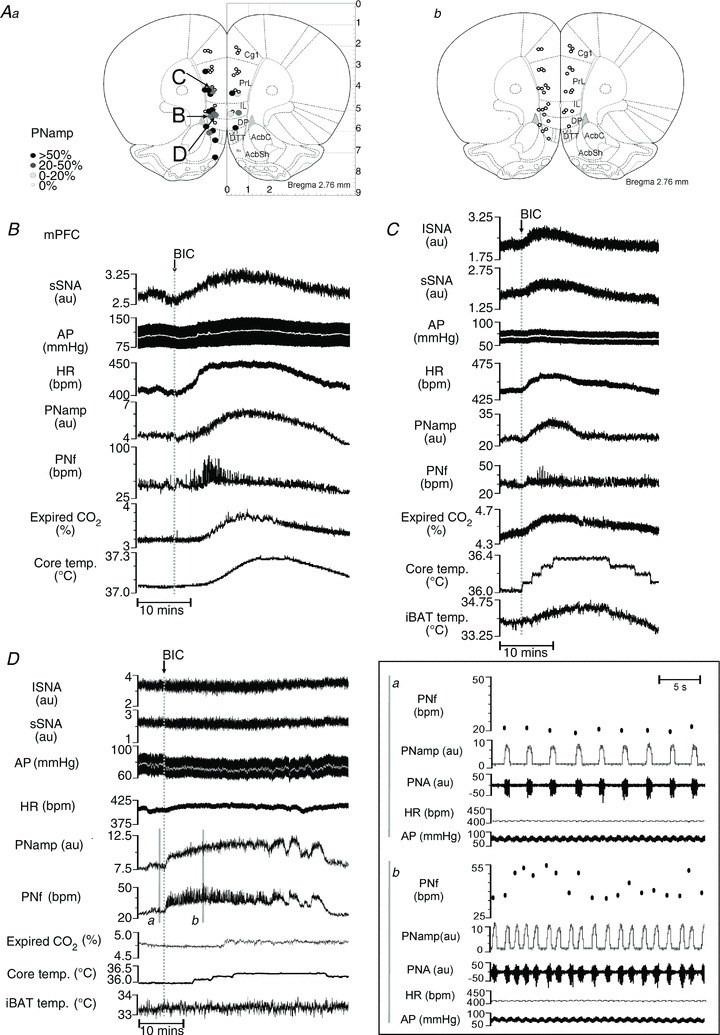
Aa, map of phrenic nerve amplitude (PNamp) responses evoked from left and right hemisphere sites of the midline cortex from 2.52–3.00 mm rostral to bregma. No hemispheric lateralization is evident. Ab, map of PNamp responses evoked by vehicle injections. B, effects on splanchnic sympathetic nerve activity (sSNA), arterial pressure (AP), heart rate (HR), PNamp, phrenic nerve frequency (PNf), expired CO2 and core temperature following BIC microinjection into the infralimbic cortex (IL). C, effects on the same variables as in B with the addition of lumbar sympathetic nerve activity (lSNA) and interscapular brown adipose tissue (iBAT) temperature following BIC into a different site within the IL. D, changes in PNf and PNamp, but not in lSNA, sSNA, AP, HR, PNamp, PNf and expired CO2, after BIC microinjection into the ventral prelimbic cortex: a, excerpt from control period showing PNf, integrated PNamp, raw PNA, HR and AP; b, excerpt after BIC microinjection showing the variable frequency and increased bursting rate and depth of PNA with no change in HR or AP.
Microinjections of BIC were made into 90 sites focused on the mPFC and the rostral LSN.
Medial prefrontal cortex
Representative examples of responses evoked from the mPFC are shown in Fig. 1. Figure 1B shows the response evoked by BIC injection in the IL region of the mPFC (3 mm rostral, 0.7 mm lateral, 5 mm ventral from bregma). Increases in sSNA (30%), MAP (9 mmHg), HR (40 bpm), PNamp (33%), PNf (21 bpm), expired CO2 (0.23%) and core temperature (0.22°C) were seen. Figure 1C shows another similar response also elicited from the IL region to show increases in iBAT. This response pattern was commonly associated with ventral parts of the mPFC. At some sites in the dorsal mPFC, changes in only respiratory variables (PNf and PNamp) were evoked by BIC (Fig. 1D) (PrL: rostral 2.6 mm, lateral 0.6 mm, ventral 4.0 mm from bregma). Increases in PNamp (43%) and PNf (17 bpm), with no inherent rhythmicity [Fig. 1Da (before BIC) and 1Db (after BIC)] were evoked without eliciting changes in lumbar or splanchnic SNA, MAP (< 5 mmHg), HR, expired CO2 or iBAT temperature.
Grouped data obtained from subregions of the mPFC [cingulate 1 (Cg1), PrL, IL, DP and dorsal tenia tecta (DTT)] and surrounding regions (ventral and medial orbital cortex and olfactory bulb) are shown as response maps and graphically (Figs 2 and 3) and in summary (Table 1).
Figure 2. Response maps showing respiratory, metabolic and cardiovascular changes evoked by bicuculline methiodide (BIC) microinjection in the medial prefrontal cortex (mPFC) and surrounding area (3.72–4.00 mm rostral to bregma).
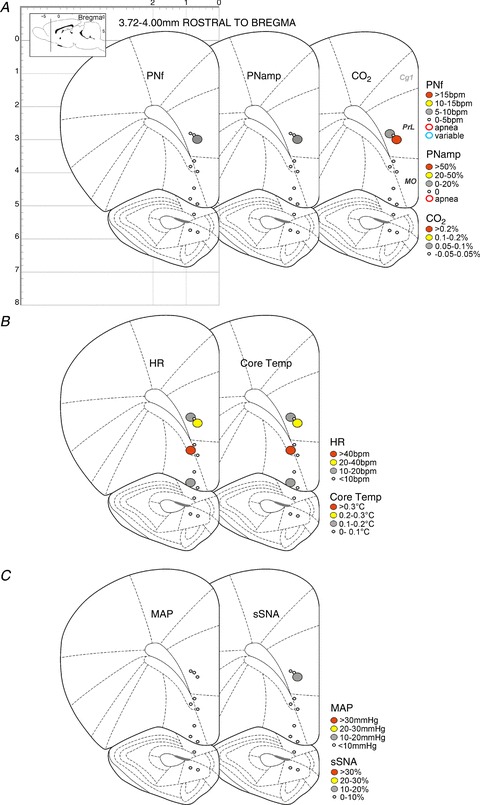
A, maps showing changes in phrenic nerve frequency (PNf), phrenic nerve amplitude (PNamp) and expired CO2 (respiratory and metabolic). B, maps showing changes in heart rate (HR) and core temperature (cardiovascular and metabolic). C, maps showing changes in mean arterial pressure (MAP) and splanchnic sympathetic nerve activity (sSNA) (cardiovascular and metabolic).
Figure 3. Response maps showing respiratory, metabolic and cardiovascular changes evoked by bicuculline methiodide (BIC) microinjection in the medial prefrontal cortex (mPFC) and surrounding area (2.52–3.00 mm rostral to bregma).
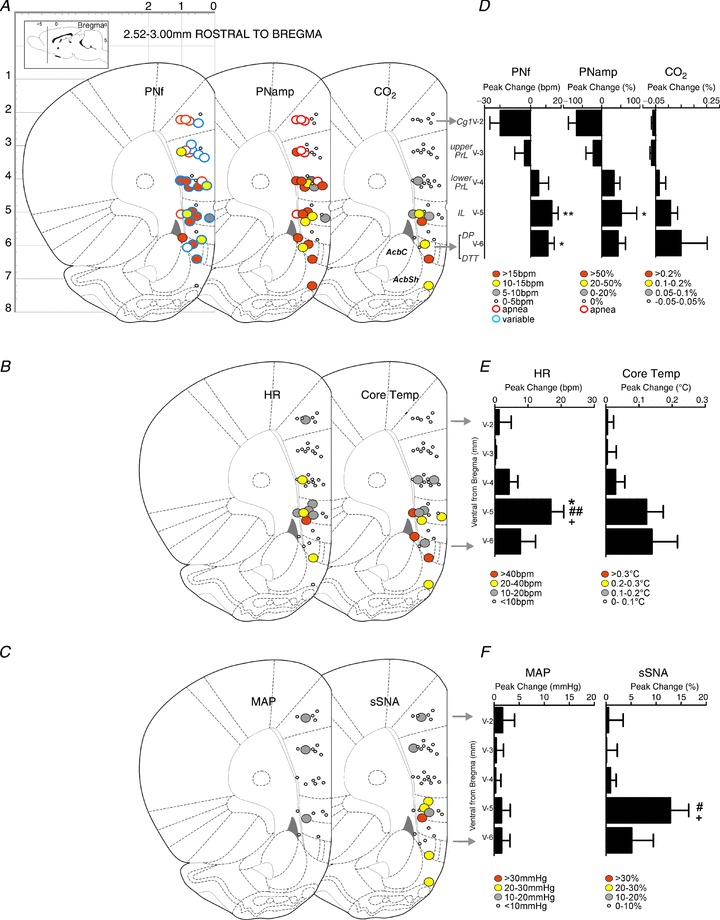
A, maps showing changes in phrenic nerve frequency (PNf), phrenic nerve amplitude (PNamp) and expired CO2 (respiratory and metabolic). B, maps showing changes in heart rate (HR) and core temperature (cardiovascular and metabolic). C, maps showing changes in mean arterial pressure (MAP) and splanchnic sympathetic nerve activity (sSNA) (cardiovascular and metabolic). D–F, column graphs of responses shown in A–C evoked by BIC microinjection at 1 mm dorsoventral intervals. Bars represent the peak changes ± standard error of the mean obtained from all sites located in the cluster. *P < 0.05 compared to ventral level v-2 (Cg1); **P < 0.01 compared to v-2 (Cg1); #P < 0.05 compared to v-3 (upper PrL); ##P < 0.01 compared to v-3 (upper PrL); +P < 0.05 compared to v-4 (lower PrL).
Table 1.
Cardiovascular, respiratory and metabolic responses to bicuculline methiodide in the medial prefrontal cortex and lateral septal nucleus
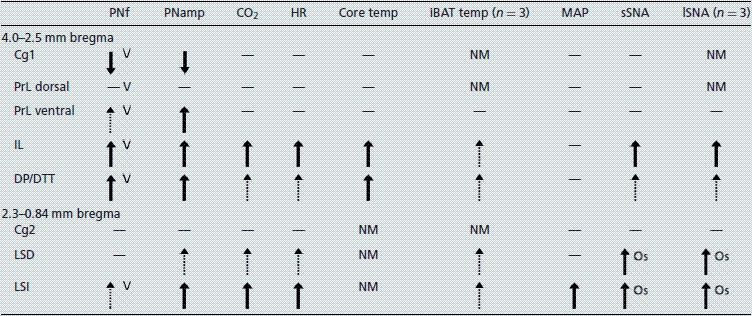 |
PNf, phrenic nerve frequency; PNamp, phrenic nerve amplitude; HR, heart rate; iBAT, interscapular brown adipose tissue; MAP, mean arterial pressure; sSNA, splanchnic sympathetic nerve activity; lSNA, lumbar sympathetic nerve activity; Cg, cingulate cortex; PrL, prelimbic cortex; IL, infralimbic cortex; DP, dorsal peduncle; DTT, dorsal tenia tecta; LSD, dorsal lateral septal nucleus; LSI, intermediate lateral septal nucleus; NM, not measured; V, variable frequency response; Os, oscillating sympathetic response; dash, no response; dashed arrow, small response; thick arrow, large response.
Figure 2 shows that BIC injected into a few sites in the PrL in the most rostral mPFC evoked modest increases in PNamp, PNf, expired CO2, HR, core temperature and sSNA. These are likely to represent the rostral extension of the same regions more caudally depicted in Fig. 3. Little response was elicited from the medial or ventral orbital cortex at more ventral locations (Fig. 2).
Responses obtained from more caudal parts of the mPFC are described by system (Fig. 3 and Table 1).
Respiration
Figure 3A shows that changes in PNf and PNamp were commonly evoked by BIC in all subregions of the mPFC. Dorsoventral analysis shows that reductions in PNf and PNamp (including apnoea) were commonly evoked in Cg1 and dorsal PrL; conversely, increases in PNf and PNamp were regularly elicited in ventral Prl, IL and DP/DTT (Fig. 3B). One-way ANOVA comparison of grouped responses across the dorsoventral regions of the mPFC showed significant differences in PNf (F(4,30)= 4.43, P= 0.0062) and PNamp (F(4,30)= 3.632, P= 0.0158) (Fig. 3B). Bonferroni multiple comparison between subregions of the mPFC revealed significant differences between the Cg1 and ventral regions of the mPFC in PNf (Cg1 vs. IL: P < 0.01; Cg1 vs. DP/DTT: P < 0.05) and in PNamp (Cg1 vs. IL: P < 0.05). Figure 1B shows a response obtained from a site in IL with a steady rise in PNamp that appears to be coordinated with sSNA, HR and expired CO2, whereas PNf reaches its peak and returns to baseline within half the duration of the PNamp response.
Apnoea was evoked from seven of 36 sites, (red-outlined circles, Fig. 3A), restricted mainly dorsally to the Cg1 and PrL (five of seven sites). Increases in PNf and PNamp were evoked from 15 of 36 sites and 18 of 40 sites, respectively, in the ventral PrL, IL and DP/DTT region.
A disturbed irregular rhythm in PNf was evoked in 21 of 36 sites throughout all subregions of the mPFC (blue-outlined circles, Fig. 3A). A high degree of variability in phrenic burst frequency resulted from changes in the expiratory period, as shown in Fig. 1D in an example in the PrL. In dorsal regions the variability in rhythm evoked was independent of PNamp changes and mean frequency tended not to change.
Expired CO2
Figure 3A shows that changes in expired CO2 were evoked only at sites at which PNf or PNamp were altered by BIC. Increases in expired CO2 were elicited in eight of 40 sites, mainly in the IL, DP and DTT (Fig. 3B); these sites were confined to the outermost cortical laminae (Fig. 3A).
Heart rate
Bradycardia was never evoked from the mPFC, whereas modest tachycardia was evoked from nine of 40 sites (Fig. 3C). Tachycardia was elicited mainly from the IL region. One-way ANOVA of grouped responses across the dorsoventral regions of the mPFC showed significant differences in HR (F(4,34)= 4.832, P= 0.0034). A mean increase in HR of 17 ± 4 bpm was evoked from eight sites in the IL region (Fig. 3C, D). Bonferroni multiple comparison revealed significant differences between the Cg1 (P < 0.05), and upper (P < 0.01) and lower (P < 0.05) PrL with the IL. Typical HR responses elicited by BIC exhibited a steady rise to plateau that was sustained before returning to baseline in a more protracted manner (Fig. 1B, C). It should be noted that experiments were conducted in vagotomized rats and therefore the observed HR effects were most likely mediated by the sympathetic nervous system.
Thermoregulation
Microinjection of BIC in 11 of 40 sites caused modest increases in core temperature in the IL, DP and DTT from the outer lamina (Fig. 3C, D). Figure 1B shows a distinct increase in core temperature of 0.22°C from a site in the IL. These increases in core temperature consistently occurred at sites from which increases in expired CO2 were obtained. A comparison of grouped responses across the dorsoventral regions of the mPFC showed a trend to an increased core temperature (F(4,34)= 2.323, P= 0.077).
Additional experiments were conducted to assess the contribution of thermogenesis in generating the increase in core body temperature. Figure 1D shows an example of one such site in the IL, demonstrating an increase in iBAT temperature of 0.66°C. Injection of BIC evoked increases in iBAT temperature at 15 of 18 sites in the lower IL and DP/DTT and none of 12 sites in the Cg1 and PrL. This was consistently associated with increases in core temperature and expired CO2. Not surprisingly, on all such occasions respiratory and cardiovascular parameters reached peak values more rapidly than temperature responses (Fig. 1B, C).
Mean arterial pressure
Changes in MAP were rarely evoked from the mPFC (three of 24 sites; Fig. 3E, F) and never exceeded 15 mmHg.
Sympathetic nerve activity
Increases in sSNA were evoked from eight of 40 sites, found mainly in the IL, and were rarely > 30% from baseline (Fig. 3E, F). Increases in sSNA were not always associated with vasomotor activity as only three of eight sites had corresponding increases in MAP (Fig. 3E). One-way ANOVA of grouped responses across the dorsoventral regions of the mPFC showed significant differences in sSNA (F(4,34)= 4.001, P= 0.009). A significant increase was seen when comparing the Cg1 and IL (P < 0.05), and upper PrL and IL regions (P < 0.05) using Bonferroni multiple comparison.
In the subset of animals in which iBAT was measured, SNA was also measured from the lumbar nerve to assess differences in sympathetic outflows to different targets. In the IL, similar small increases were seen together with sSNA and responses of < 30% were generated (e.g. Fig. 1C). No evidence for the differential control of lSNA and sSNA was seen within the IL.
Table 1 shows a summary of the site-specific effects of disinhibition of the mPFC. Distinct changes to respiratory, cardiovascular (HR but rarely AP) and metabolic functions were generated from the mPFC in the IL and DP/DTT. Furthermore, changes in only respiratory function were generated from the Cg1 and dorsal and ventral PrL regions. Disturbances in PNf, observed as a highly variable frequency rate, were generated throughout the dorsoventral extent of the mPFC.
Lateral septal nucleus and adjacent regions
In order to determine whether the changes evoked by BIC were restricted to the mPFC, injections were made into more caudal regions focusing particularly on the LSN (Figs 4–6). Figure 4A, B shows that responses obtained at the rostral pole of the LSN were not surprisingly similar to responses obtained from the adjacent more rostral mPFC. Increases in MAP (5 mmHg), HR (19 bpm), sSNA (22%), PNamp (34%), PNf (7 bpm), expired CO2 (0.19%), core temperature (0.4°C) and iBAT temperature (0.66°C) were elicited from the rostral LSN (cf. Figs 4B and 1B).
Figure 4. Respiratory, cardiovascular and metabolic outputs altered by bicuculline methiodide (BIC) in the lateral septal nucleus (LSN).
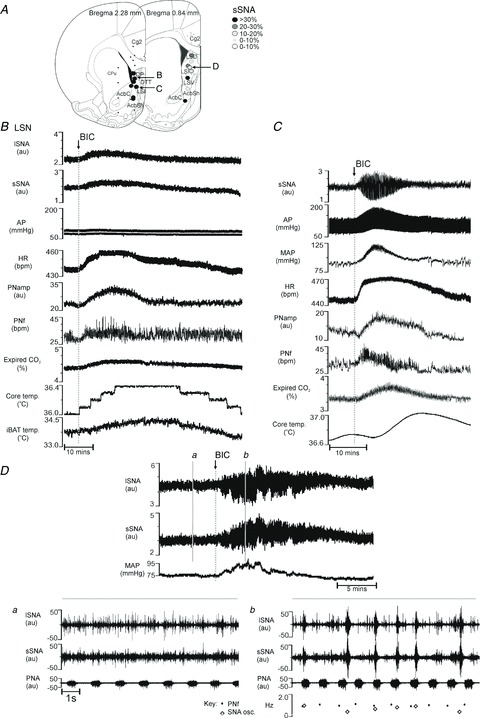
A, locations of responses shown in B–D. B, effects on lumbar sympathetic nerve activity (lSNA), splanchnic sympathetic nerve activity (sSNA), arterial pressure (AP), heart rate (HR), phrenic nerve amplitude (PNamp), phrenic nerve frequency (PNf), expired CO2, core temperature and interscapular brown adipose tissue (iBAT) temperature following BIC microinjection in the intermediate LSN (LSI), a response similar to that evoked in the infralimbic cortex. C, effects on sSNA, AP, mean AP (MAP), HR, PNamp, PNf, expired CO2 and core temperature following BIC microinjection into LSI. Note the oscillation evoked in sSNA associated with the increase in MAP. D, lumbar and splanchnic SNA and MAP before and after BIC microinjection into another site in LSI: a, control period, and b, BIC-evoked large bursts in both lSNA and sSNA unrelated to PNf, at variable frequencies of 0.3–0.8 Hz.
Figure 6. Response maps showing respiratory, metabolic and cardiovascular changes evoked by bicuculline methiodide (BIC) microinjection in the midline cortex and surrounding area (0.72–0.84 mm rostral to bregma).
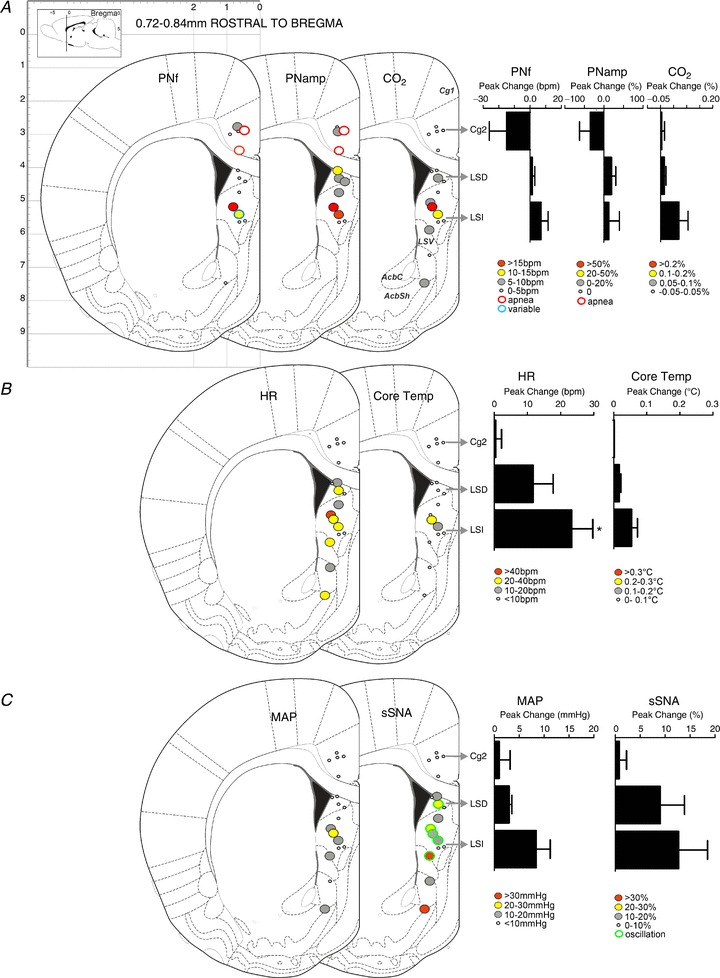
A, maps showing changes in phrenic nerve frequency (PNf), phrenic nerve amplitude (PNamp) and expired CO2 (respiratory and metabolic). B, maps showing changes in heart rate (HR) and core temperature (cardiovascular and metabolic). C, maps showing changes in mean arterial pressure (MAP) and splanchnic sympathetic nerve activity (sSNA) (cardiovascular and metabolic). D–F, column graphs of responses shown in A–C evoked by BIC microinjection at 1 mm dorsoventral intervals. Bars represent the peak changes ± standard error of the mean obtained from all sites located in the ventral cluster. *P < 0.05 compared to Cg2.
However, a different response evoked only from the LSN and distinguished by large bursts in the sSNA was also identified (Fig. 4C, D). Figure 4C shows that the distinct pattern of bursting in sSNA (termed ‘sympathetic oscillation’) was often coupled with pronounced increases in MAP (30 mmHg) and HR (26 bpm) and accompanied by increases in PNamp (34%), PNf (8 bpm), expired CO2 (0.26%) and core temperature (0.32°C). In order to determine whether the sympathetic oscillation was generated only in the splanchnic nerve, a subset of experiments (n= 3) also measured lSNA. The sympathetic oscillation was consistently generated in both splanchnic and lumbar sympathetic nerves (Fig. 4Da, Db). The very large bursts generated were additional to normal nerve discharge and each large burst was followed by low levels of activity in the nerve. The large bursts were generated at a frequency of 0.3–0.8 Hz and were not correlated with PNf (Fig. 4Db).
Response maps centred at 2.28 mm and 0.84 mm, respectively, rostral to bregma are shown in Figs 5 and 6. Grouped data from injection sites shown in Fig. 6 are also graphically represented; these responses are summarized in Table 1. Responses in any parameters were rarely evoked from sites within the Cg2, the caudate putamen and, not surprisingly, the corpus callosum. By contrast, changes in all variables were evoked commonly from sites within the LSN [intermediate LSN (LSI) and dorsal LSN (LSD)], as well as in the NAcbSh. Only the rostral part of the LSN was explored.
Figure 5. Response maps showing respiratory, metabolic and cardiovascular changes evoked by bicuculline methiodide (BIC) microinjection in the midline cortex and surrounding area (2.04–2.28 mm rostral to bregma).
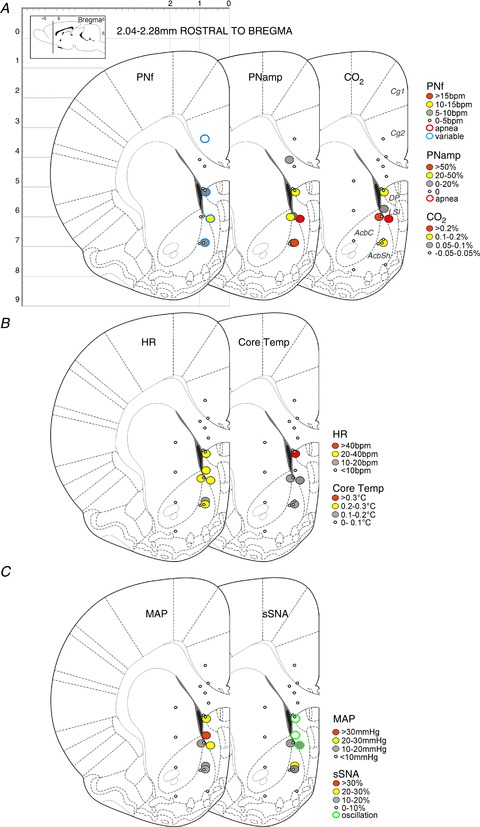
A, maps showing changes in phrenic nerve frequency (PNf), phrenic nerve amplitude (PNamp) and expired CO2 (respiratory and metabolic). B, maps showing changes in heart rate (HR) and core temperature (cardiovascular and metabolic). C, maps showing changes in mean arterial pressure (MAP) and splanchnic sympathetic nerve activity (sSNA) (cardiovascular and metabolic).
Respiration
Microinjections of BIC evoked apnoea in two of six sites located in the Cg2 (Figs 5A and 6A). BIC injection into the LSN evoked increases in PNf in four of 10 sites and in the NAcbSh in one of six sites (Figs 5A and 6A). Irregular variations in frequency similar to those evoked in the mPFC were seen in three of 10 sites located rostrally. Increases in PNamp were evoked in eight of 10 sites in the LSN and in four of five sites in the NAcbSh (Figs 5A and 6A).
Expired CO2
No changes in CO2 were generated from Cg2. Increases in expired CO2 often accompanied increases in PNamp in both the LSN (eight of 14 sites) and NAcbSh (three of eight sites) (Figs 5A and 6A).
Heart rate
No effects on HR were evoked from Cg2. Marked tachycardia was elicited from 10 of 13 sites in the LSN with increases in HR of > 20 bpm evoked from eight of these 10 sites (Figs 5B and 6B). Within the NAcbSh, tachycardia was evoked from five of nine sites. One-way ANOVA of grouped responses across the dorsoventral regions of the LSN showed significant differences in HR (F(2,13)= 4.589, P= 0.0311). Bonferroni multiple comparison revealed a significant difference between the Cg2 and IL regions (P < 0.05).
Thermoregulation
Little to no change was seen in core temperature when BIC was injected into Cg2 (none of five sites), but small increases in core temperature were evoked from four of 13 sites within the LSN (Fig. 6B); a similar pattern was seen in the NAcbSh (two of nine sites). The small core temperature changes were evoked with concurrent increases in expired CO2 and PNamp. The rare and small temperature responses generated in LSN contrasted with the more commonly evoked effect in the mPFC (see Table 1).
Mean arterial pressure
Little change in MAP was evoked in Cg2. Increases in MAP were evoked from seven of 13 sites within the LSN (Figs 5C and 6C). Small increases in MAP were evoked from the NAcbSh in four of nine sites. Larger increases in MAP were evoked from the LSN than from the mPFC (Table 1).
Sympathetic nerve activity
Little change in sSNA was evoked from Cg2. Increases in sSNA (eight of 13 sites) were elicited from the LSN (Figs 5C and 6C) with distinct sympathetic oscillations seen at eight of 13 sites; no increase in mean SNA occurred at two of these eight oscillation sites. Although differences were insignificant as a result of the variability of responses generated, sympathoexcitation tended to be elicited from the LSD and LSI. In the NAcbSh, increases in SNA were seen in five of nine sites. Sites at which the sympathetic oscillation was generated were located consistently within the LSN (Figs 5 and 6).
Table 1 summarizes the responses obtained from the subregions LSN and Cg2. Only respiratory responses were evoked from Cg2. By contrast, disinhibition in the LSN resulted in distinct changes in HR, MAP and SNA, with small effects on PNamp and CO2, which were largest in the LSI. Table 1 also compares responses obtained in the LSN and mPFC. Responses in the LSN were distinguished from those in the IL by the generation of a low-frequency oscillation in both lumbar and splanchnic SNA, larger pressor responses and fewer PNf effects.
Discussion
This study provides a comprehensive assessment of simultaneously and directly measured cardiovascular, respiratory and metabolic responses evoked by the blockade of GABA-A receptors disinhibiting subregions of the mPFC and the rostral part of the LSN. The major findings of this study are:
Medial PFC: apnoea was commonly evoked from Cg1. Increases in PNamp and PNf, sympathetically dependent HR, core and iBAT temperatures, splanchnic and lumbar SNA with few changes in AP were commonly evoked from the ventral PrL, IL and DP/DTT and consistently elicited from the IL. Disturbances in the rhythm of phrenic burst discharge were generated from all subregions of the mPFC.
Rostral LSN: apnoea was commonly evoked from Cg2. Increases in PNamp, sympathetically dependent HR, splanchnic and lumbar SNA and AP were commonly evoked from LSN and most consistently from LSI. Core temperature and PNf changes were not commonly elicited. The irregular low-frequency oscillation generated in both lumbar and splanchnic SNA and evident in AP was evoked only from LSN and was unrelated to PNf.
Some responses were evoked in the NAcbSh. However, BIC rarely elicited responses from the medial or ventral orbital cortex, anterior olfactory nucleus ventroposterior part, dorsal transition zone navicular nucleus of the basal forebrain or corpus callosum.
These data indicate that neurons within the ventral mPFC (IL, DP/DTT), LSN and NAcbsh regulate cardiac and respiratory function and, at least in the IL, also metabolic function. Respiratory activity alone was modulated by neuronal activation in the dorsal PFC (Cg1, Cg2, PrL). These findings significantly extend those of previous reports in which only small cardiovascular changes were reported following chemical modulation in only some of these subregions. It is well established that the mPFC plays a critical role in responses to stress and emotion and that its function is modified in mental illness (Bennett, 2008; Arnsten, 2009, 2011; Gilabert-Juan et al. 2012). That multiple physiological systems are modulated by activation of the IL is not surprising as these systems permit the body to adapt to behavioural changes evoked by environmental cues or stressors. Thus, when neuronal function is changed in the PFC, physiological systems are also affected. It is possible therefore, that this may contribute to the higher risk for premature death in patients with mental health disorders that involve a dysfunctional PFC, than in the general population (Bradley & Dinan, 2010; Vogelzangs et al. 2010).
Considerations
The present data show that directly modulating the activity of neurons in the injected brain sites alters respiratory, cardiac and/or metabolic function. BIC did not evoke responses at all sites injected. This was evident in sites located laterally, dorsally or ventrally to the mPFC and LSN and indicates that the effects evoked were site-specific. Similarly, distinctly different patterns of response were evoked when injections were separated by 1 mm in any direction, a finding which, again, represents an indication of site-specific responses. The volume of injection (250 nl) was selected based on those used to modify behavioural responses in mPFC and the likely area of diffusion. Behavioural studies consistently use volumes of 250–1000 nl to evoke responses (LaLumiere et al. 2012; Kim et al. 2013; Pardey et al. 2013). Diffusion parameters indicate that the diameter of spread of injection is approximately 1 mm (Nicholson, 1985).
Perhaps surprisingly, the present study did not show functional bias between the hemispheres although behavioural and cognitive lateralization and hemisphere-specific remodelling changes associated with stress have been described previously in the rat (Carlson et al. 1993; Sullivan & Szechtman, 1994; Sullivan & Gratton, 1999, 2002; Czeh et al. 2008). Further studies correlating behavioural and autonomic functions may be required to determine if lateralization can be detected.
Urethane-induced anaesthesia was used to facilitate the assessment of the wide range of variables measured in the present study. Previous studies show that anaesthesia may invert centrally mediated AP responses and it is possible that the direction of response described here may differ; nevertheless, this study identifies the parameters that will be affected when a specific site in the PFC is activated (Bergmann & Gutman, 1966; Burns & Wyss, 1985; Fisk & Wyss, 1997; Verberne & Owens, 1998; Fernandes et al. 2003; Tavares et al. 2004).
In the present study, central mechanisms were responsible for the changes evoked in phrenic and sympathetic nerves, whereas changes in HR, AP, expired CO2 and iBAT temperature could be altered by central mechanisms or indirectly by humeral factors. Tachycardia reported here was likely to be mediated via increased cardiac sympathetic activity as the animals were vagotomized and the responses were rapid. In support of this, Tavares et al. (2004) reported that changes in HR elicited by electrical stimulation in the PFC were blocked by ganglionic blockade but not by adrenal demedullation. The PFC can modulate glucocorticoid (Feldman & Conforti, 1985; Diorio et al. 1993; Herman & Cullinan, 1997) and vasopressin (Peres-Polon & Correa, 1994; Fernandes et al. 2003; Pelosi et al. 2006) release and these can influence AP. However, electrical stimulation of the mPFC in conscious rats has previously evoked pressor responses independent of vasopressin, angiotensin II or the pituitary gland, which were abolished by ganglionic blockade (Tavares et al. 2004). In the present report, we demonstrate that disinhibition of the PFC evoked little change in MAP and that pressor responses generated from the LSN were associated with concurrent increases in SNA and thus are unlikely to be mediated humerally. Increases in expired CO2 were also evoked in the present study and it is arguable whether these generated the increases seen in PNf and PNamp. However, it was clear that changes in CO2 were not always correlated with changes in PNf or PNamp, particularly in the dorsal PFC.
Few studies have investigated the influence of the NAcbsh on autonomic function. Previous studies showed that stimulation of this area had little effect on cardiovascular function but did inhibit cardiovascular components of the defence reaction (al Maskati & Zbrozyna, 1989). These novel data show that chemical disinhibition of the few sites tested in the NAcbsh can increase activity in respiratory and cardiovascular outflows and thus this issue deserves further investigation.
Functional effects following disinhibition of the LSN
Disinhibition of the LSN evoked cardiovascular and respiratory effects. Pressor responses accompanied by tachycardia and sympathoexcitation were evoked particularly from the LSI. By contrast, small depressor responses were previously reported following small injections of excitatory dl-homocysteate in the LSN (Gelsema & Calaresu, 1987). Our findings, however, are in keeping with previous reports that electrical stimulation of the LSN can alter AP and HR (Calaresu & Mogenson, 1972) and that muscimol (GABA-A agonist) injection into the PFC abolished pressor responses induced by immobilization stress (Kubo et al. 2002). Furthermore, acetylcholine (Peres-Polon & Correa, 1994) and noradrenaline (Fernandes et al. 2005) injection also evoked pressor effects in the rostral LSN in conscious rats, although these responses appeared to have been mediated by vasopressin and not sympathetic activation, as shown here.
Of particular note, the present study revealed the consistent eliciting of low-frequency oscillatory SNA bursts from the LSN. Although Calaresu & Mogenson (1972) noted a ‘rhythmic oscillation’ in AP after electrical stimulation of the LSN, they did not measure any sympathetic outputs. In agreement with our findings, these authors also reported no correlation with indirect measures of respiratory frequency and stated that this oscillation occurred at 0.4 Hz (Calaresu & Mogenson, 1972), which is similar to the 0.3–8.0 Hz range seen in our data.
In addition to this sympathetic oscillation, the present study shows that changes in PNamp, expired CO2 and, on occasion, core temperature can be evoked from the LSN, and demonstrates that cardiovascular and respiratory systems, at least, can be altered by disinhibition of neurons within the LSN, particularly in the LSI (Table 1).
Functional effects following disinhibition of the mPFC
All subregions of the mPFC
The data presented here represent the first measures of respiratory variables directly assessed from phrenic nerve discharge following disinhibition of the mPFC. A distinct variability in phrenic burst frequency was generated by disinhibition throughout the dorsoventral extent of the mPFC. Changes were seen mainly in the expiratory rather than the inspiratory period, which may assist future researchers in defining the mechanisms responsible for generating this response.
Dorsal mPFC
In the present study, only respiratory responses, and mainly apnoea, were consistently evoked by disinhibition of Cg1 and dorsal PrL, indicating that these regions turn off respiratory control systems. The negligible responses in MAP and HR evoked here are in agreement with the findings of previous studies in which dl-homocysteate was injected in the Cg1 and dorsal PrL (Fisk & Wyss, 1997; Verberne & Owens, 1998) or following electrical stimulation of the PrL (Owens et al. 1999), and showed little change in vascular conductance in multiple beds (Owens & Verberne, 2001). Together, these studies indicate that the dorsal PFC has little influence on cardiovascular function. However, acetylcholine in the Cg1 can elicit a dose-dependent decrease in AP in conscious animals (Crippa et al. 1999), which indicates that some neurotransmitter-specific effects may be generated here.
Ventral mPFC
Cardiovascular (except MAP), respiratory and metabolic responses were all evoked by disinhibition of the PrL, IL and DP/DTT and most potently from the IL.
Arterial pressure changes of > 5 mmHg were rarely evoked from the mPFC in the present study; however, increases in sSNA, lSNA and HR (most probably mediated by cardiac sympathetic activation) were elicited. These were similar in magnitude but not in direction with those identified in previous studies, in which electrical or glutamate stimulation of the IL elicited small decreases in gastric motility (Panteleev & Grundy, 2000), and small increases in vascular conductance in the renal, mesenteric and iliac vascular beds were found to accompany depressor responses (Owens et al. 1999; Owens & Verberne, 2001). The direction of response may be anaesthetic- or stimulus-dependent. Pressor responses have been described in conscious animals inhibited by ganglion blockers (Tavares et al. 2004) and increases in HR and AP elicited from the IL were inhibited not only by ganglionic blockade but also by atenolol (β-adrenergic receptor antagonist) (Resstel & Correa, 2005), which suggests a cardiac sympathetic effect. These latter data, together with the present results, suggest that activation of the IL evokes increases in HR and in the activity of sympathetic beds innervated by splanchnic and lumbar sympathetic nerves.
Increases in PNf and PNamp, indicating increases in both frequency and the drive to breathe, were evoked by disinhibition of the ventral mPFC. In support of these data, an increase in respiratory depth and rate assessed by pneumotachography was previously reported after electrical stimulation of the IL in the rat (Alexandrov et al. 2007), cat and monkey (Anand & Dua, 1956) or suggested by association as an NMDA-induced lesion of the IL altered the respiratory rate evoked during a footshock-conditioned stimulus (Frysztak & Neafsey, 1991).
The present study shows that iBAT and core temperature, as well as expired CO2 levels, increased following disinhibition of the ventral mPFC, predominantly the IL. It has been shown that increases in the local temperature of iBAT correlate with metabolically driven non-shivering thermogenic activation in iBAT (Morrison & Nakamura, 2011). The tachycardic response seen following disinhibition of the ventral mPFC also supports this notion and represents an attempt to maintain cardiac output and distribute heat efficiently (Morrison & Nakamura, 2011). The increase in core temperature and the inability to efficiently expire (under mechanical ventilation) the increasing levels of CO2 produced as a result of increased metabolism also indicate thermogenesis. Although the circuitry underlying central thermoregulation is now well understood (Morrison & Nakamura, 2011), these data suggest that inputs arising from the IL influence this pathway (see below). Increases in respiratory parameters evoked in the IL may be associated with thermogenic and/or metabolic effects.
The clear functional dorsoventral mPFC organization shown in Table 1 is supported by extensive anatomical data showing differences in projection targets between the dorsal and ventral mPFC. Polysynaptic pathways, at least to the heart and adrenal gland, originate strictly from the IL cortex (Westerhaus & Loewy, 2001). Furthermore, the IL sends dense projections to targets, including the bed nucleus of the stria terminalis, the medial and lateral preoptic nuclei, the dorsomedial and lateral hypothalamus, the medial and central amygdala, the parabrachial nucleus and the nucleus of the solitary tract (Sesack et al. 1989; Hurley et al. 1991; Takagishi & Chiba, 1991; Vertes, 2004), all of which are strongly implicated in cardiovascular, respiratory and metabolic control, which may mediate the responses described here. Furthermore, Table 1 demonstrates differences in responses evoked between subregions of the mPFC, together with subtle but distinct rostrocaudal differences between mPFC and LSN subregions.
Conclusions
Our extensive functional data demonstrate that central respiratory, cardiac and metabolic function can be directly altered by disinhibition of discrete regions of the ventral mPFC, particularly the IL. A distinct sympathetic oscillation accompanied by cardiovascular and respiratory effects can be generated from the LSN. Only respiratory responses can be evoked from the dorsal mPFC (Cg1 and PrL). These data, together with previous substantial anatomical evidence, suggest that the mPFC and LSN recruit downstream autonomic and respiratory loci in order to regulate cardiac, respiratory and/or metabolic systems. The mPFC is important in responses to stress, reward and emotion. Our data demonstrate that the changes in neural activity in these regions evoked by such psychological stimuli may provide parallel cardiovascular, respiratory and metabolic outcomes. The functional integration of these systems is highlighted by the recent finding that disinhibition of the IL evokes anxiety-like behaviour in mice (Bi et al. 2013); the current study describes a correlational change in respiratory, cardiac and sympathetic activity. It is perhaps not surprising that chronic changes in the activity of these regions, which occur following stress and in mental health disorders, alter not only behavioural and cognitive function, but may also contribute to the autonomic and respiratory dysfunction seen in these conditions.
Acknowledgments
The authors would like to thank Peter G. R. Burke for his helpful comments.
Glossary
Abbreviations
- AP
arterial pressure
- BIC
bicuculline methiodide
- Cg
cingulate cortex
- LSD
dorsal lateral septal nucleus
- DP
dorsal peduncle
- DTT
dorsal tenia tecta
- HR
heart rate
- iBAT
interscapular brown adipose tissue
- IL
infralimbic cortex
- LSI
intermediate lateral septal nucleus
- LSN
lateral septal nucleus
- lSNA
lumbar sympathetic nerve activity
- MAP
mean arterial pressure
- mPFC
medial prefrontal cortex
- NAcbsh
nucleus accumbens shell
- PN
phrenic nerve
- PNA
phrenic nerve activity
- PNamp
phrenic nerve amplitude
- PNf
phrenic nerve frequency
- PrL
prelimbic cortex
- SNA
sympathetic nerve activity
- sSN
splanchnic sympathetic nerve
- sSNA
splanchnic sympathetic nerve activity
Additional information
Competing interests
None declared.
Author contributions
A.K.G and S.F.H. contributed to the conception and design of the study. S.F.H. contributed to the collection of data. S.F.H. and A.K.G contributed to the analysis and interpretation of data. All authors contributed to the drafting and revision of the article for important intellectual content and approved the final version for publication.
Funding
This work was supported by the National Health and Medical Research Council (Australia) (APP1028183), the National Heart Foundation (Australia) (G09S4340), Macquarie University Safety Net and the Hillcrest Foundation (Perpetual Sydney, NSW, Australia).
References
- al Maskati HA, Zbrozyna AW. Stimulation in prefrontal cortex area inhibits cardiovascular and motor components of the defence reaction in rats. J Auton Nerv Syst. 1989;28:117–125. doi: 10.1016/0165-1838(89)90084-2. [DOI] [PubMed] [Google Scholar]
- Alexandrov VG, Ivanova TG, Alexandrova NP. Prefrontal control of respiration. J Physiol Pharmacol. 2007;58(Suppl 5):17–23. [PubMed] [Google Scholar]
- An X, Bandler R, Ongur D, Price JL. Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeys. J Comp Neurol. 1998;401:455–479. [PubMed] [Google Scholar]
- Anand BK, Dua S. Circulatory and respiratory changes induced by electrical stimulation of limbic system (visceral brain) J Neurophysiol. 1956;19:393–400. doi: 10.1152/jn.1956.19.5.393. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Prefrontal cortical network connections: key site of vulnerability in stress and schizophrenia. Int J Dev Neurosci. 2011;29:215–223. doi: 10.1016/j.ijdevneu.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon SJ, Smith AD. A monosynaptic pathway from an identified vasomotor centre in the medial prefrontal cortex to an autonomic area in the thoracic spinal cord. Neuroscience. 1993;54:719–728. doi: 10.1016/0306-4522(93)90242-8. [DOI] [PubMed] [Google Scholar]
- Barbas H, Saha S, Rempel-Clower N, Ghashghaei T. Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC Neurosci. 2003;4:25. doi: 10.1186/1471-2202-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MR. Stress and anxiety in schizophrenia and depression: glucocorticoids, corticotropin-releasing hormone and synapse regression. Aust N Z J Psychiatry. 2008;42:995–1002. doi: 10.1080/00048670802512073. [DOI] [PubMed] [Google Scholar]
- Bergmann F, Gutman Y. Central regulation of blood pressure. Acta Physiol Lat Am. 1966;16:49–58. [PubMed] [Google Scholar]
- Bi LL, Wang J, Luo ZY, Chen SP, Geng F, Chen YH, Li SJ, Yuan CH, Lin S, Gao TM. Enhanced excitability in the infralimbic cortex produces anxiety-like behaviors. Neuropharmacology. 2013;72C:148–156. doi: 10.1016/j.neuropharm.2013.04.048. [DOI] [PubMed] [Google Scholar]
- Bradley AJ, Dinan TG. A systematic review of hypothalamic-pituitary-adrenal axis function in schizophrenia: implications for mortality. J Psychopharmacol. 2010;24:91–118. doi: 10.1177/1359786810385491. [DOI] [PubMed] [Google Scholar]
- Buchanan SL, Powell DA, Buggy J. 3H-2-deoxyglucose uptake after electrical stimulation of cardioactive sites in anterior medial cortex in rabbits. Brain Res Bull. 1984;13:371–382. doi: 10.1016/0361-9230(84)90087-x. [DOI] [PubMed] [Google Scholar]
- Burke PG, Li Q, Costin ML, McMullan S, Pilowsky PM, Goodchild AK. Somatostatin 2A receptor-expressing presympathetic neurons in the rostral ventrolateral medulla maintain blood pressure. Hypertension. 2008;52:1127–1133. doi: 10.1161/HYPERTENSIONAHA.108.118224. [DOI] [PubMed] [Google Scholar]
- Burns SM, Wyss JM. The involvement of the anterior cingulate cortex in blood pressure control. Brain Res. 1985;340:71–77. doi: 10.1016/0006-8993(85)90774-7. [DOI] [PubMed] [Google Scholar]
- Calaresu FR, Mogenson GJ. Cardiovascular responses to electrical stimulation of the septum in the rat. Am J Physiol. 1972;223:777–782. doi: 10.1152/ajplegacy.1972.223.4.777. [DOI] [PubMed] [Google Scholar]
- Cao WH, Fan W, Morrison SF. Medullary pathways mediating specific sympathetic responses to activation of dorsomedial hypothalamus. Neuroscience. 2004;126:229–240. doi: 10.1016/j.neuroscience.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Cao WH, Madden CJ, Morrison SF. Inhibition of brown adipose tissue thermogenesis by neurons in the ventrolateral medulla and in the nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol. 2010;299:R277–R290. doi: 10.1152/ajpregu.00039.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JN, Fitzgerald LW, Keller RW, Jr, Glick SD. Lateralized changes in prefrontal cortical dopamine activity induced by controllable and uncontrollable stress in the rat. Brain Res. 1993;630:178–187. doi: 10.1016/0006-8993(93)90655-7. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Chen SJ. Subcortical sites mediating sympathetic responses from insular cortex in rats. Am J Physiol Regul Integr Comp Physiol. 1990;258:R245–R255. doi: 10.1152/ajpregu.1990.258.1.R245. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Shoemaker JK. Functional neuroanatomy of autonomic regulation. Neuroimage. 2009;47:795–803. doi: 10.1016/j.neuroimage.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Crippa GE, Lewis SJ, Johnson AK, Correa FM. Medial prefrontal cortex acetylcholine injection-induced hypotension: the role of hindlimb vasodilation. J Auton Nerv Syst. 2000;79:1–7. doi: 10.1016/s0165-1838(99)00091-0. [DOI] [PubMed] [Google Scholar]
- Crippa GE, Peres-Polon VL, Kuboyama RH, Correa FM. Cardiovascular response to the injection of acetylcholine into the anterior cingulate region of the medial prefrontal cortex of unanesthetized rats. Cereb Cortex. 1999;9:362–365. doi: 10.1093/cercor/9.4.362. [DOI] [PubMed] [Google Scholar]
- Czeh B, Perez-Cruz C, Fuchs E, Flugge G. Chronic stress-induced cellular changes in the medial prefrontal cortex and their potential clinical implications: does hemisphere location matter. Behav Brain Res. 2008;190:1–13. doi: 10.1016/j.bbr.2008.02.031. [DOI] [PubMed] [Google Scholar]
- DiMicco JA, Abshire VM, Shekhar A, Wilbe JH., Jr Role of GABAergic mechanisms in the central regulation of arterial pressure. Eur Heart J. 1987;8(Suppl B):133–138. doi: 10.1093/eurheartj/8.suppl_b.133. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman S, Conforti N. Modifications of adrenocortical responses following frontal cortex simulation in rats with hypothalamic deafferentations and medial forebrain bundle lesions. Neuroscience. 1985;15:1045–1047. doi: 10.1016/0306-4522(85)90253-2. [DOI] [PubMed] [Google Scholar]
- Fernandes KB, Crippa GE, Tavares RF, Antunes-Rodrigues J, Correa FM. Mechanisms involved in the pressor response to noradrenaline injection into the cingulate cortex of unanesthetized rats. Neuropharmacology. 2003;44:757–763. doi: 10.1016/s0028-3908(03)00067-4. [DOI] [PubMed] [Google Scholar]
- Fernandes KB, Tavares RF, Correa FM. The lateral septal area is involved in the pressor pathway activated by microinjection of norepinephrine into the rat brain cingulate cortex. Neuropharmacology. 2005;49:564–571. doi: 10.1016/j.neuropharm.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Fino E, Yuste R. Dense inhibitory connectivity in neocortex. Neuron. 2011;69:1188–1203. doi: 10.1016/j.neuron.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisk GD, Wyss JM. Pressor and depressor sites are intermingled in the cingulate cortex of the rat. Brain Res. 1997;754:204–212. doi: 10.1016/s0006-8993(97)00076-0. [DOI] [PubMed] [Google Scholar]
- Frysztak RJ, Neafsey EJ. The effect of medial frontal cortex lesions on respiration, ‘freezing’, and ultrasonic vocalizations during conditioned emotional responses in rats. Cereb Cortex. 1991;1:418–425. doi: 10.1093/cercor/1.5.418. [DOI] [PubMed] [Google Scholar]
- Funk D, Stewart J. Role of catecholamines in the frontal cortex in the modulation of basal and stress-induced autonomic output in rats. Brain Res. 1996;741:220–229. doi: 10.1016/s0006-8993(96)00931-6. [DOI] [PubMed] [Google Scholar]
- Gamo NJ, Arnsten AF. Molecular modulation of prefrontal cortex: rational development of treatments for psychiatric disorders. Behav Neurosci. 2011;125:282–296. doi: 10.1037/a0023165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelsema AJ, Calaresu FR. Chemical microstimulation of the septal area lowers arterial pressure in the rat. Am J Physiol Regul Integr Comp Physiol. 1987;252:R760–R767. doi: 10.1152/ajpregu.1987.252.4.R760. [DOI] [PubMed] [Google Scholar]
- Gilabert-Juan J, Castillo-Gomez E, Guirado R, Molto MD, Nacher J. Chronic stress alters inhibitory networks in the medial prefrontal cortex of adult mice. Brain Struct Funct. 2012;1448:129–136. doi: 10.1007/s00429-012-0479-1. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Uylings HB. The prefrontal cortex and the integration of sensory, limbic and autonomic information. Prog Brain Res. 2000;126:3–28. doi: 10.1016/S0079-6123(00)26003-2. [DOI] [PubMed] [Google Scholar]
- Gupta A, Wang Y, Markram H. Organizing principles for a diversity of GABAergic interneurons and synapses in the neocortex. Science. 2000;287:273–278. doi: 10.1126/science.287.5451.273. [DOI] [PubMed] [Google Scholar]
- Hansel A, von Kanel R. The ventro-medial prefrontal cortex: a major link between the autonomic nervous system, regulation of emotion, and stress reactivity. Biopsychosoc Med. 2008;2:21. doi: 10.1186/1751-0759-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobehav Rev. 2009;33:773–783. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Hurley-Gius KM, Neafsey EJ. The medial frontal cortex and gastric motility: microstimulation results and their possible significance for the overall pattern of organization of rat frontal and parietal cortex. Brain Res. 1986;365:241–248. doi: 10.1016/0006-8993(86)91635-5. [DOI] [PubMed] [Google Scholar]
- Hurley KM, Herbert H, Moga MM, Saper CB. Efferent projections of the infralimbic cortex of the rat. J Comp Neurol. 1991;308:249–276. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- Iigaya K, Muller-Ribeiro FC, Horiuchi J, McDowall LM, Nalivaiko E, Fontes MA, Dampney RA. Synchronized activation of sympathetic vasomotor, cardiac, and respiratory outputs by neurons in the midbrain colliculi. Am J Physiol Regul Integr Comp Physiol. 2012;303:R599–R610. doi: 10.1152/ajpregu.00205.2012. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: an individual-differences perspective. Psychon Bull Rev. 2002;9:637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- Kim WY, Jang JK, Lee JW, Jang H, Kim JH. Decrease of GSK3beta phosphorylation in the rat nucleus accumbens core enhances cocaine-induced hyper-locomotor activity. J Neurochem. 2013;125:642–648. doi: 10.1111/jnc.12222. [DOI] [PubMed] [Google Scholar]
- Kubo T, Kanaya T, Numakura H, Okajima H, Hagiwara Y, Fukumori R. The lateral septal area is involved in mediation of immobilization stress-induced blood pressure increase in rats. Neurosci Lett. 2002;318:25–28. doi: 10.1016/s0304-3940(01)02463-6. [DOI] [PubMed] [Google Scholar]
- LaLumiere RT, Smith KC, Kalivas PW. Neural circuit competition in cocaine-seeking: roles of the infralimbic cortex and nucleus accumbens shell. Eur J Neurosci. 2012;35:614–622. doi: 10.1111/j.1460-9568.2012.07991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowall LM, Horiuchi J, Dampney RA. Effects of disinhibition of neurons in the dorsomedial hypothalamus on central respiratory drive. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1728–R1735. doi: 10.1152/ajpregu.00503.2007. [DOI] [PubMed] [Google Scholar]
- McKlveen JM, Myers B, Flak JN, Bundzikova J, Solomon MB, Seroogy KB, Herman JP. Role of prefrontal cortex glucocorticoid receptors in stress and emotion. Biol Psychiatry. 2013;74:672–679. doi: 10.1016/j.biopsych.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front Biosci. 2011;16:74–104. doi: 10.2741/3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2007;292:R127–R136. doi: 10.1152/ajpregu.00427.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neafsey EJ. Prefrontal cortical control of the autonomic nervous system: anatomical and physiological observations. Prog Brain Res. 1990;85:147–165. doi: 10.1016/s0079-6123(08)62679-5. discussion 165–166. [DOI] [PubMed] [Google Scholar]
- Neafsey EJ, Hurley-Gius KM, Arvanitis D. The topographical organization of neurons in the rat medial frontal, insular and olfactory cortex projecting to the solitary nucleus, olfactory bulb, periaqueductal gray and superior colliculus. Brain Res. 1986;377:561–570. doi: 10.1016/0006-8993(86)90867-x. [DOI] [PubMed] [Google Scholar]
- Nicholson C. Diffusion from an injected volume of a substance in brain tissue with arbitrary volume fraction and tortuosity. Brain Res. 1985;333:325–329. doi: 10.1016/0006-8993(85)91586-0. [DOI] [PubMed] [Google Scholar]
- Owens NC, Sartor DM, Verberne AJ. Medial prefrontal cortex depressor response: role of the solitary tract nucleus in the rat. Neuroscience. 1999;89:1331–1346. doi: 10.1016/s0306-4522(98)00389-3. [DOI] [PubMed] [Google Scholar]
- Owens NC, Verberne AJ. Regional haemodynamic responses to activation of the medial prefrontal cortex depressor region. Brain Res. 2001;919:221–231. doi: 10.1016/s0006-8993(01)03017-7. [DOI] [PubMed] [Google Scholar]
- Pagani FD, DiMicco JA, Hamilton BL, Souza JD, Schmidt B, Gillis RA. Stress-induced changes in the function of the parasympathetic nervous system are mimicked by blocking GABA in the CNS of the cat. Neuropharmacology. 1987;26:155–160. doi: 10.1016/0028-3908(87)90203-6. [DOI] [PubMed] [Google Scholar]
- Panteleev S, Grundy D. Descending influences from the infralimbic cortex on vago-vagal reflex control of gastric motor activity in the rat. Auton Neurosci. 2000;86:78–83. doi: 10.1016/S1566-0702(00)00249-6. [DOI] [PubMed] [Google Scholar]
- Pardey MC, Kumar NN, Goodchild AK, Cornish JL. Catecholamine receptors differentially mediate impulsive choice in the medial prefrontal and orbitofrontal cortex. J Psychopharmacol. 2013;27:203–212. doi: 10.1177/0269881112465497. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Amsterdam, Boston, MA: Elsevier Academic Press; 2005. [Google Scholar]
- Pelosi GG, Peres-Polon VL, Correa FM. Pressor effects of the injection of noradrenaline into different cerebroventricular spaces in unanesthetized rats. Neurosci Lett. 2006;397:165–169. doi: 10.1016/j.neulet.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Peres-Polon VL, Correa FM. Pressor effects of acetylcholine injected into the lateral septal area of conscious rats. Neuropharmacology. 1994;33:1537–1544. doi: 10.1016/0028-3908(94)90127-9. [DOI] [PubMed] [Google Scholar]
- Resstel LB, Correa FM. Pressor and tachycardic responses evoked by microinjections of l-glutamate into the medial prefrontal cortex of unanaesthetized rats. Eur J Neurosci. 2005;21:2513–2520. doi: 10.1111/j.1460-9568.2005.04078.x. [DOI] [PubMed] [Google Scholar]
- Resstel LB, Correa FM. Involvement of the medial prefrontal cortex in central cardiovascular modulation in the rat. Auton Neurosci. 2006;126–127:130–138. doi: 10.1016/j.autneu.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Saper CB. Convergence of autonomic and limbic connections in the insular cortex of the rat. J Comp Neurol. 1982;210:163–173. doi: 10.1002/cne.902100207. [DOI] [PubMed] [Google Scholar]
- Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci. 2002;25:433–469. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Sheriff MJ, Fontes MA, Killinger S, Horiuchi J, Dampney RA. Blockade of AT1 receptors in the rostral ventrolateral medulla increases sympathetic activity under hypoxic conditions. Am J Physiol Regul Integr Comp Physiol. 2006;290:R733–R740. doi: 10.1152/ajpregu.00410.2005. [DOI] [PubMed] [Google Scholar]
- Shipley MT. Insular cortex projection to the nucleus of the solitary tract and brainstem visceromotor regions in the mouse. Brain Res Bull. 1982;8:139–148. doi: 10.1016/0361-9230(82)90040-5. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Tamas G, Lujan R, Buhl EH. Salient features of synaptic organization in the cerebral cortex. Brain Res Brain Res Rev. 1998;26:113–135. doi: 10.1016/s0165-0173(97)00061-1. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A. Lateralized effects of medial prefrontal cortex lesions on neuroendocrine and autonomic stress responses in rats. J Neurosci. 1999;19:2834–2840. doi: 10.1523/JNEUROSCI.19-07-02834.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A. Prefrontal cortical regulation of hypothalamic-pituitary-adrenal function in the rat and implications for psychopathology: side matters. Psychoneuroendocrinology. 2002;27:99–114. doi: 10.1016/s0306-4530(01)00038-5. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Szechtman H. Left/right nigrostriatal asymmetry in susceptibility to neurotoxic dopamine depletion with 6-hydroxydopamine in rats. Neurosci Lett. 1994;170:83–86. doi: 10.1016/0304-3940(94)90244-5. [DOI] [PubMed] [Google Scholar]
- Takagishi M, Chiba T. Efferent projections of the infralimbic (area 25) region of the medial prefrontal cortex in the rat: an anterograde tracer PHA-L study. Brain Res. 1991;566:26–39. doi: 10.1016/0006-8993(91)91677-s. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Tomioka R. Long-range GABAergic connections distributed throughout the neocortex and their possible function. Front Neurosci. 2010;4:202. doi: 10.3389/fnins.2010.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares RF, Antunes-Rodrigues J, de Aguiar Correa FM. Pressor effects of electrical stimulation of medial prefrontal cortex in unanesthetized rats. J Neurosci Res. 2004;77:613–620. doi: 10.1002/jnr.20195. [DOI] [PubMed] [Google Scholar]
- Terreberry RR, Neafsey EJ. Rat medial frontal cortex: a visceral motor region with a direct projection to the solitary nucleus. Brain Res. 1983;278:245–249. doi: 10.1016/0006-8993(83)90246-9. [DOI] [PubMed] [Google Scholar]
- Terreberry RR, Neafsey EJ. The rat medial frontal cortex projects directly to autonomic regions of the brainstem. Brain Res Bull. 1987;19:639–649. doi: 10.1016/0361-9230(87)90050-5. [DOI] [PubMed] [Google Scholar]
- van der Kooy D, Koda LY, McGinty JF, Gerfen CR, Bloom FE. The organization of projections from the cortex, amygdala, and hypothalamus to the nucleus of the solitary tract in rat. J Comp Neurol. 1984;224:1–24. doi: 10.1002/cne.902240102. [DOI] [PubMed] [Google Scholar]
- Van Eden CG, Buijs RM. Functional neuroanatomy of the prefrontal cortex: autonomic interactions. Prog Brain Res. 2000;126:49–62. doi: 10.1016/S0079-6123(00)26006-8. [DOI] [PubMed] [Google Scholar]
- Verberne AJ. Medullary sympathoexcitatory neurons are inhibited by activation of the medial prefrontal cortex in the rat. Am J Physiol Regul Integr Comp Physiol. 1996;270:R713–R719. doi: 10.1152/ajpregu.1996.270.4.R713. [DOI] [PubMed] [Google Scholar]
- Verberne AJ, Lewis SJ, Worland PJ, Beart PM, Jarrott B, Christie MJ, Louis WJ. Medial prefrontal cortical lesions modulate baroreflex sensitivity in the rat. Brain Res. 1987;426:243–249. doi: 10.1016/0006-8993(87)90878-x. [DOI] [PubMed] [Google Scholar]
- Verberne AJ, Owens NC. Cortical modulation of the cardiovascular system. Prog Neurobiol. 1998;54:149–168. doi: 10.1016/s0301-0082(97)00056-7. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vogelzangs N, Seldenrijk A, Beekman AT, van Hout HP, de Jonge P, Penninx BW. Cardiovascular disease in persons with depressive and anxiety disorders. J Affect Disord. 2010;125:241–248. doi: 10.1016/j.jad.2010.02.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerhaus MJ, Loewy AD. Central representation of the sympathetic nervous system in the cerebral cortex. Brain Res. 2001;903:117–127. doi: 10.1016/s0006-8993(01)02453-2. [DOI] [PubMed] [Google Scholar]
- Yasui Y, Breder CD, Saper CB, Cechetto DF. Autonomic responses and efferent pathways from the insular cortex in the rat. J Comp Neurol. 1991;303:355–374. doi: 10.1002/cne.903030303. [DOI] [PubMed] [Google Scholar]


