Abstract
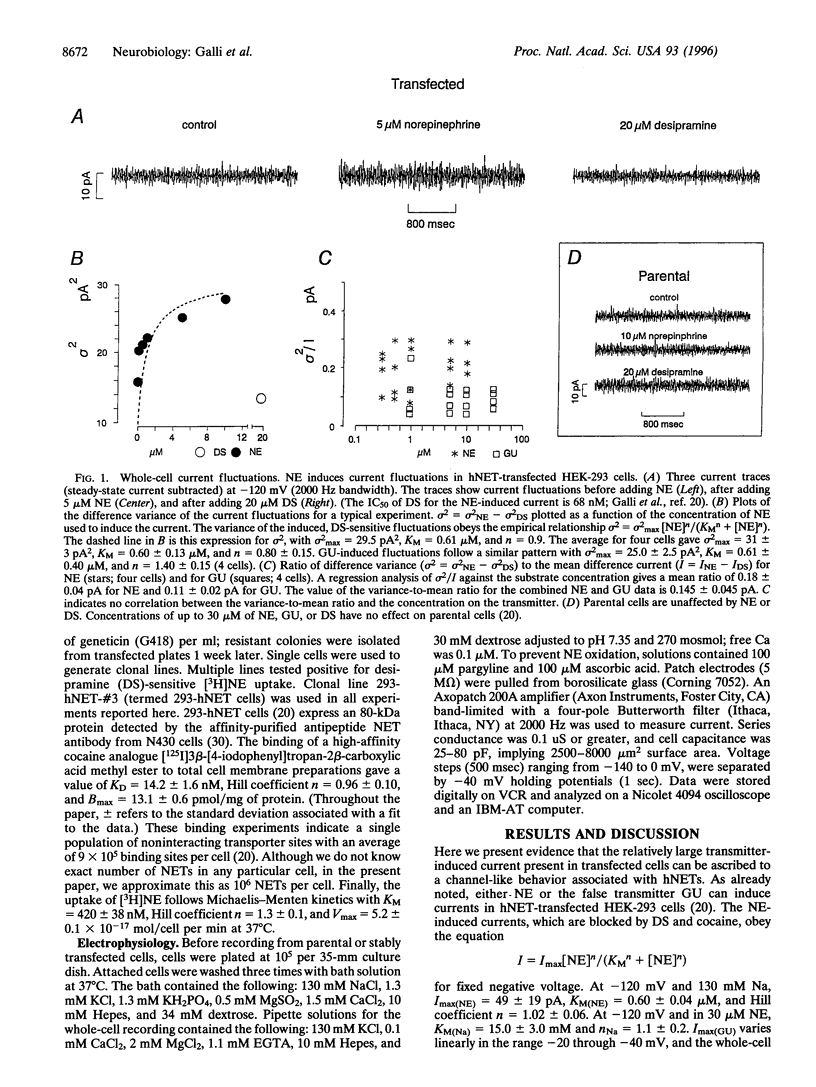
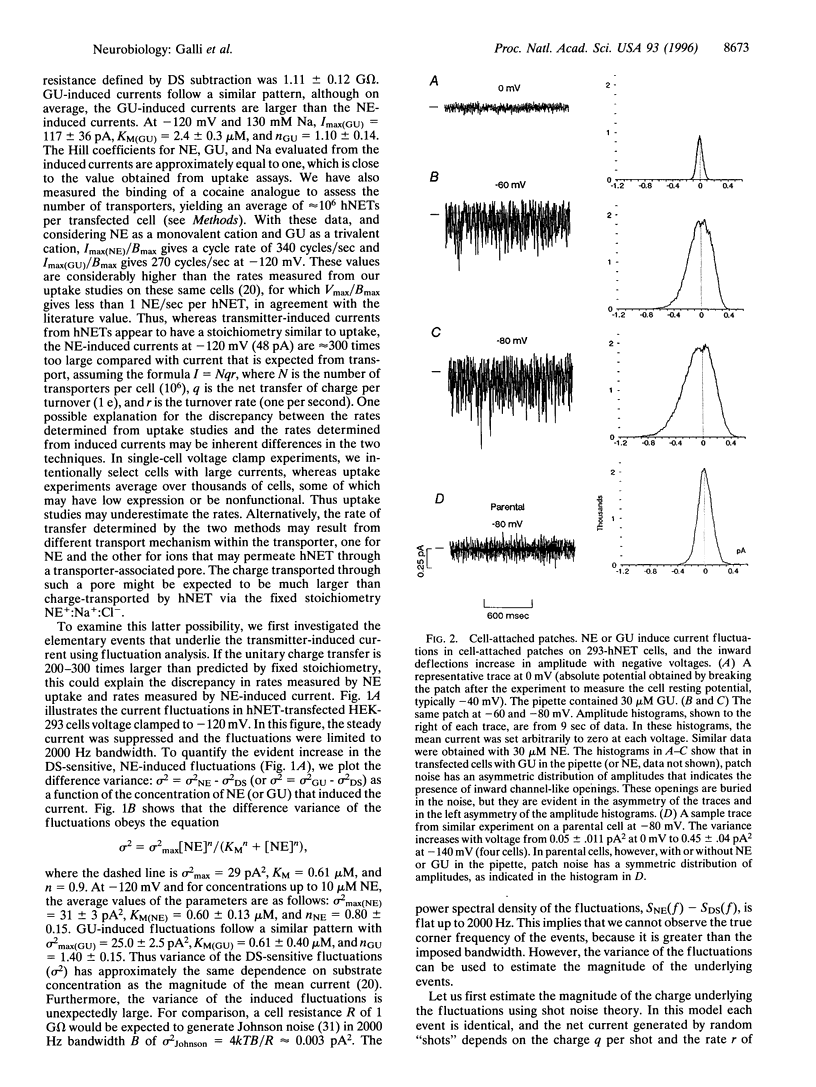
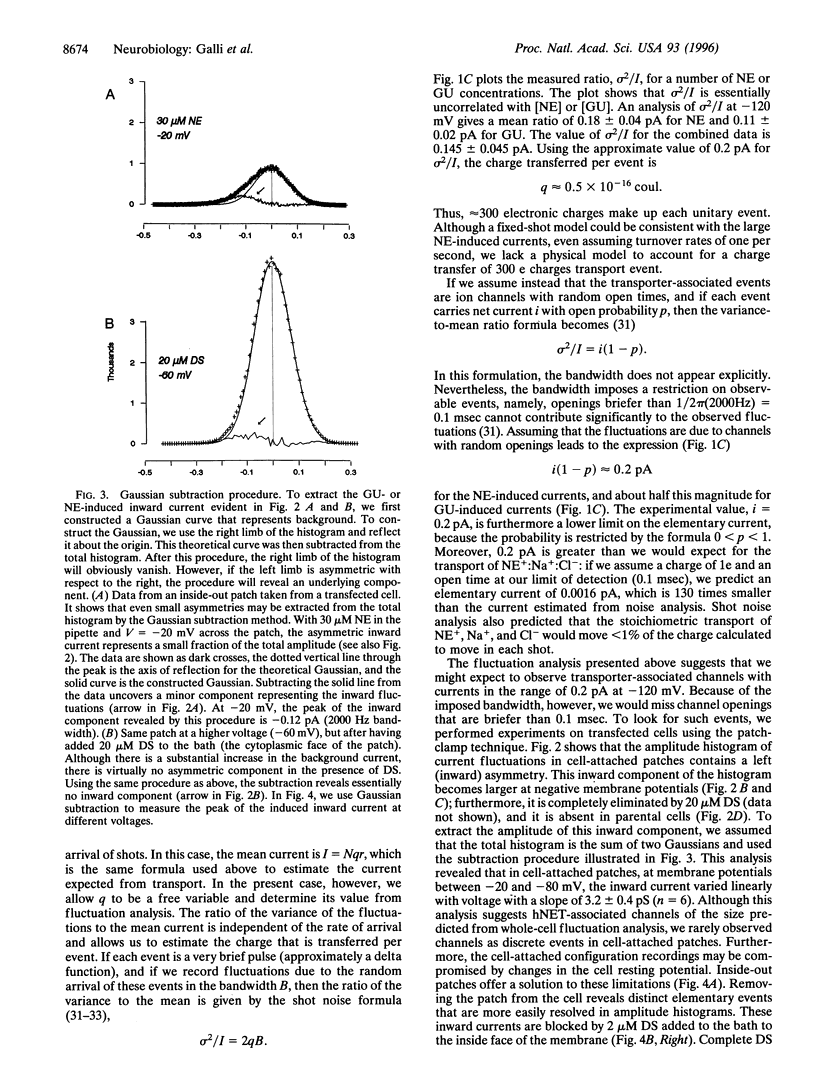
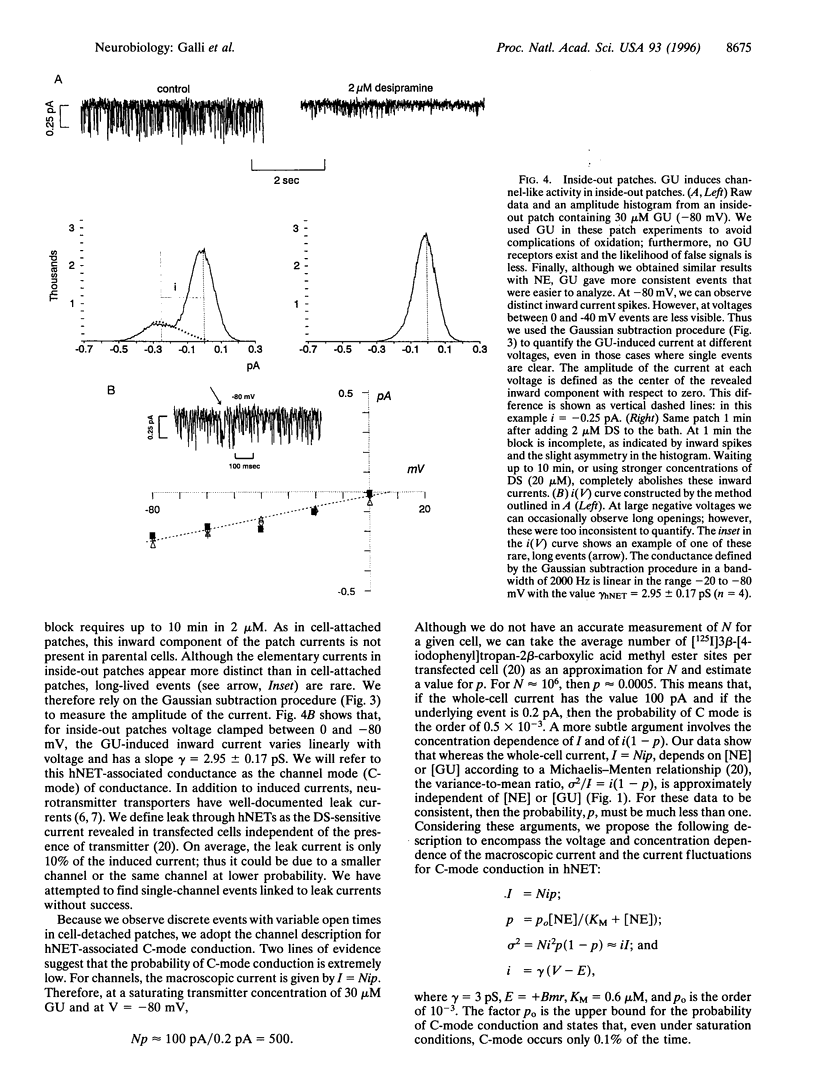
Neurotransmitter transporters couple to existing ion gradients to achieve reuptake of transmitter into presynaptic terminals. For coupled cotransport, substrates and ions cross the membrane in fixed stoichiometry. This is in contrast to ion channels, which carry an arbitrary number of ions depending on the channel open time. Members of the gamma-aminobutyric acid transporter gene family presumably function with fixed stoichiometry in which a set number of ions cotransport with one transmitter molecule. Here we report channel-like events from a presumably fixed stoichiometry [norepinephrine (NE)+, Na+, and Cl-], human NE (hNET) in the gamma-aminobutyric acid transporter gene family. These events are stimulated by NE and by guanethidine, an hNET substrate, and they are blocked by cocaine and the antidepressant desipramine. Voltage-clamp data combined with NE uptake data from these same cells indicate that hNETs have two functional modes of conduction: a classical transporter mode (T-mode) and a novel channel mode (C-mode). Both T-mode and C-mode are gated by the same substrates and antagonized by the same blockers. T-mode is putatively electrogenic because the transmitter and cotransported ions sum to one net charge. However, C-mode carries virtually all of the transmitter-induced current, even though it occurs with low probability. This is because each C-mode opening transports hundreds of charges per event. The existence of a channel mode of conduction in a previously established fixed-stoichiometry transporter suggests the appearance of an aqueous pore through the transporter protein during the transport cycle and may have significance for transporter regulation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELROD J., WHITBY L. G., HERTTING G. Effect of psychotropic drugs on the uptake of H3-norepinephrine by tissues. Science. 1961 Feb 10;133(3450):383–384. doi: 10.1126/science.133.3450.383. [DOI] [PubMed] [Google Scholar]
- Amara S. G., Kuhar M. J. Neurotransmitter transporters: recent progress. Annu Rev Neurosci. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- Axelrod J. Noradrenaline: fate and control of its biosynthesis. Science. 1971 Aug 13;173(3997):598–606. doi: 10.1126/science.173.3997.598. [DOI] [PubMed] [Google Scholar]
- Blakely R. D., De Felice L. J., Hartzell H. C. Molecular physiology of norepinephrine and serotonin transporters. J Exp Biol. 1994 Nov;196:263–281. doi: 10.1242/jeb.196.1.263. [DOI] [PubMed] [Google Scholar]
- Bruns D., Engert F., Lux H. D. A fast activating presynaptic reuptake current during serotonergic transmission in identified neurons of Hirudo. Neuron. 1993 Apr;10(4):559–572. doi: 10.1016/0896-6273(93)90159-o. [DOI] [PubMed] [Google Scholar]
- Bönisch H., Harder R. Binding of 3H-desipramine to the neuronal noradrenaline carrier of rat phaeochromocytoma cells (PC-12 cells). Naunyn Schmiedebergs Arch Pharmacol. 1986 Dec;334(4):403–411. doi: 10.1007/BF00569378. [DOI] [PubMed] [Google Scholar]
- Cammack J. N., Rakhilin S. V., Schwartz E. A. A GABA transporter operates asymmetrically and with variable stoichiometry. Neuron. 1994 Oct;13(4):949–960. doi: 10.1016/0896-6273(94)90260-7. [DOI] [PubMed] [Google Scholar]
- Cammack J. N., Schwartz E. A. Channel behavior in a gamma-aminobutyrate transporter. Proc Natl Acad Sci U S A. 1996 Jan 23;93(2):723–727. doi: 10.1073/pnas.93.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson C. W., Chang C., Stolfi A., George W. J., Yamasaki S., Pickoff A. S. Electrophysiological effects of high cocaine concentrations on intact canine heart. Evidence for modulation by both heart rate and autonomic nervous system. Circulation. 1993 Mar;87(3):950–962. doi: 10.1161/01.cir.87.3.950. [DOI] [PubMed] [Google Scholar]
- Crouzy S. C., Sigworth F. J. Fluctuations in ion channel gating currents. Analysis of nonstationary shot noise. Biophys J. 1993 Jan;64(1):68–76. doi: 10.1016/S0006-3495(93)81341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairman W. A., Vandenberg R. J., Arriza J. L., Kavanaugh M. P., Amara S. G. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature. 1995 Jun 15;375(6532):599–603. doi: 10.1038/375599a0. [DOI] [PubMed] [Google Scholar]
- Friedrich U., Bönisch H. The neuronal noradrenaline transport system of PC-12 cells: kinetic analysis of the interaction between noradrenaline, Na+ and Cl- in transport. Naunyn Schmiedebergs Arch Pharmacol. 1986 Jul;333(3):246–252. doi: 10.1007/BF00512937. [DOI] [PubMed] [Google Scholar]
- Galli A., DeFelice L. J., Duke B. J., Moore K. R., Blakely R. D. Sodium-dependent norepinephrine-induced currents in norepinephrine-transporter-transfected HEK-293 cells blocked by cocaine and antidepressants. J Exp Biol. 1995 Oct;198(Pt 10):2197–2212. doi: 10.1242/jeb.198.10.2197. [DOI] [PubMed] [Google Scholar]
- Gu H., Wall S. C., Rudnick G. Stable expression of biogenic amine transporters reveals differences in inhibitor sensitivity, kinetics, and ion dependence. J Biol Chem. 1994 Mar 11;269(10):7124–7130. [PubMed] [Google Scholar]
- Harder R., Bönisch H. Effects of monovalent ions on the transport of noradrenaline across the plasma membrane of neuronal cells (PC-12 cells). J Neurochem. 1985 Oct;45(4):1154–1162. doi: 10.1111/j.1471-4159.1985.tb05536.x. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Kravitz E. A. Sodium dependence of transmitter uptake at adrenergic nerve terminals. Mol Pharmacol. 1966 Jul;2(4):360–362. [PubMed] [Google Scholar]
- Larsson H. P., Picaud S. A., Werblin F. S., Lecar H. Noise analysis of the glutamate-activated current in photoreceptors. Biophys J. 1996 Feb;70(2):733–742. doi: 10.1016/S0006-3495(96)79613-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester H. A., Mager S., Quick M. W., Corey J. L. Permeation properties of neurotransmitter transporters. Annu Rev Pharmacol Toxicol. 1994;34:219–249. doi: 10.1146/annurev.pa.34.040194.001251. [DOI] [PubMed] [Google Scholar]
- Läuger P. Current noise generated by electrogenic ion pumps. Eur Biophys J. 1984;11(2):117–128. doi: 10.1007/BF00276627. [DOI] [PubMed] [Google Scholar]
- Mager S., Naeve J., Quick M., Labarca C., Davidson N., Lester H. A. Steady states, charge movements, and rates for a cloned GABA transporter expressed in Xenopus oocytes. Neuron. 1993 Feb;10(2):177–188. doi: 10.1016/0896-6273(93)90309-f. [DOI] [PubMed] [Google Scholar]
- Malchow R. P., Ripps H. Effects of gamma-aminobutyric acid on skate retinal horizontal cells: evidence for an electrogenic uptake mechanism. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8945–8949. doi: 10.1073/pnas.87.22.8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melikian H. E., McDonald J. K., Gu H., Rudnick G., Moore K. R., Blakely R. D. Human norepinephrine transporter. Biosynthetic studies using a site-directed polyclonal antibody. J Biol Chem. 1994 Apr 22;269(16):12290–12297. [PubMed] [Google Scholar]
- Nirenberg M. J., Vaughan R. A., Uhl G. R., Kuhar M. J., Pickel V. M. The dopamine transporter is localized to dendritic and axonal plasma membranes of nigrostriatal dopaminergic neurons. J Neurosci. 1996 Jan 15;16(2):436–447. doi: 10.1523/JNEUROSCI.16-02-00436.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacholczyk T., Blakely R. D., Amara S. G. Expression cloning of a cocaine- and antidepressant-sensitive human noradrenaline transporter. Nature. 1991 Mar 28;350(6316):350–354. doi: 10.1038/350350a0. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S., Prasad P. D., Kulanthaivel P., Leibach F. H., Blakely R. D., Ganapathy V. Expression of a cocaine-sensitive norepinephrine transporter in the human placental syncytiotrophoblast. Biochemistry. 1993 Feb 9;32(5):1346–1353. doi: 10.1021/bi00056a021. [DOI] [PubMed] [Google Scholar]
- Risso S., DeFelice L. J., Blakely R. D. Sodium-dependent GABA-induced currents in GAT1-transfected HeLa cells. J Physiol. 1996 Feb 1;490(Pt 3):691–702. doi: 10.1113/jphysiol.1996.sp021178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnick G., Clark J. From synapse to vesicle: the reuptake and storage of biogenic amine neurotransmitters. Biochim Biophys Acta. 1993 Oct 4;1144(3):249–263. doi: 10.1016/0005-2728(93)90109-s. [DOI] [PubMed] [Google Scholar]
- Sammet S., Graefe K. H. Kinetic analysis of the interaction between noradrenaline and Na+ in neuronal uptake: kinetic evidence for CO-transport. Naunyn Schmiedebergs Arch Pharmacol. 1979 Nov;309(2):99–107. doi: 10.1007/BF00501216. [DOI] [PubMed] [Google Scholar]
- Sánchez-Armáss S., Orrego F. A major role for chloride in (3H)- noradrenaline transport by rat heart adrenergic nerves. Life Sci. 1977 Jun 1;20(11):1829–1838. doi: 10.1016/0024-3205(77)90218-1. [DOI] [PubMed] [Google Scholar]
- WHITBY L. G., AXELROD J., WEIL-MALHERBE H. The fate of H3-norepinephrine in animals. J Pharmacol Exp Ther. 1961 May;132:193–201. [PubMed] [Google Scholar]
- Wadiche J. I., Amara S. G., Kavanaugh M. P. Ion fluxes associated with excitatory amino acid transport. Neuron. 1995 Sep;15(3):721–728. doi: 10.1016/0896-6273(95)90159-0. [DOI] [PubMed] [Google Scholar]



