Abstract
We have recently engineered an in vivo endothelial cell-specific retroviral gene transfer system and found that a single Kaposi's sarcoma (KS)-associated herpesvirus/human herpesvirus 8 gene encoding a G protein-coupled receptor (vGPCR), is sufficient to induce KS-like tumors in mice. By using this system, we show here that the Akt signaling pathway plays a central role in vGPCR oncogenesis. Indeed, a constitutively active Akt was sufficient to induce benign hemangiomas in mice, whereas heterozyogosity for PTEN (the phosphatase and tension homologue deleted on chromosome 10), modestly enhancing basal Akt activity, dramatically enhanced vGPCR sarcomagenesis. Examination of KS biopsies from AIDS patients revealed active Akt as a prominent feature, supportive of a role for Akt in human Kaposi's sarcomagenesis. By using a vGPCR agonist-dependent mutant, we further establish constitutive activity as a requirement for vGPCR sarcomagenesis, validating targeted inhibition of key vGPCR signaling pathways as an approach for preventing its oncogenic potential. These observations prompted us to explore the efficacy of inhibiting Akt activation as a molecular approach to KS treatment. Pharmacological inhibition of the Akt pathway with the chemotherapeutic agent 7-hydroxystaurosporine prevented proliferation of vGPCR-expressing endothelial cells in vitro and inhibited their tumorigenic potential in vivo. Both were associated with a decrease in Akt activity. These results identify Akt as an essential player in vGPCR sarcomagenesis and demonstrate the therapeutic potential of drugs targeting this pathway in the treatment of KS.
Keywords: human herpesvirus 8, Kaposi's sarcoma-associated herpesvirus, 7-hydroxystaurosporine
Kaposi's sarcoma (KS)-associated herpesvirus (KSHV)/human herpesvirus 8 has been identified as the etiologic agent for KS, the most common tumor arising in HIV-infected patients (1). The KSHV genome encodes several putative transforming proteins that bear potential for KS pathogenesis. To explore the relative contribution of candidate KSHV oncogenes to Kaposi's sarcomagenesis, we have engineered a transgenic mouse line expressing the avian leukosis virus (ALV) receptor TVA (2, 3) under the control of the vascular endothelial cell-specific TIE2 promoter (4). Because only the mammalian cells engineered to express the tva transgene can be transduced by infection with ALV (5–7), the use of ALV-derived recombinant viruses and TIE2-tva mice provides an endothelial cell-specific retroviral gene transfer system in which the infectious process by which KSHV targets endothelial cells in vivo (8) can be recapitulated (9). By using this recently developed animal model, we have observed that retroviral delivery of a KSHV-encoded G protein-coupled receptor (vGPCR) can promote the initiation of KS-like tumors in mice (9). Furthermore, by the use of immortalized murine endothelial cells expressing key latent KSHV genes, we obtained evidence that cells expressing vGPCR cooperate with cells expressing latent genes to synergistically promote tumor progression in a KS allograft model (9). These results suggest that latent genes might require the paracrine secretions from vGPCR-expressing cells to exhibit their transforming potential, further implicating vGPCR in the progression of KS.
Here we took advantage of the flexibility afforded by the high throughput TIE2-tva system to identify critical molecules involved in vGPCR oncogenesis that may therefore represent molecular targets to inhibit Kaposi's sarcomagenesis. We demonstrate that Akt is a central player in the initiation of vGPCR oncogenesis, and represents a suitable and effective therapeutic target for the development of rationally designed, molecularly targeted therapies for KS.
Materials and Methods
Expression Plasmids and Reagents. The expression plasmids for vGPCR and enhanced GFP have been described in ref. 10. The vGPCR mutants R143Q and R143A were obtained with a site-directed mutagenesis kit (Stratagene). To prepare the viral constructs, cDNAs were transferred into the avian retroviral vector RCAS (replication-competent ALV long-terminal repeat with splice acceptor) (11). RCAS-myr-Akt, encoding a myristoylated (myr)Akt, was prepared as described in ref. 12.
Recombinant human IL-8 was purchased from PeproTech (Rocky Hill, NJ). The phosphoinositide-3-OH kinase (PI3K) inhibitor wortmannin (Promega) was dissolved in DMSO as 1000 fold concentrated stock solutions, and used at the indicated concentrations. Cells were treated with a single dose for up to 12 h. In each case, the final concentration of DMSO was ≤0.1%. The Development Therapeutics Program of the National Cancer Institute received 7-hydroxystaurosporine (UCN-01) from Kyowa Hakko Kogyo (Tokyo). For in vitro studies, the UCN-01 compound was reconstituted in DMSO as a 10 mM stock solution, which was further diluted to the working concentration (0–1,000 nM) in culture media. The final concentration of DMSO in the culture medium was ≤0.1%. The diluent for the in vivo studies was 2% sodium citrate, pH 3.5.
Cell Lines and Transfections. Simian virus 40, large T-antigen-immortalized, murine endothelial cells (SVECs), 293T cells, and NIH 3T3 cells were grown in DMEM supplemented with 10% FBS (SVECs and 293T) or 10% calf serum (NIH 3T3), and 1% penicillin/streptomycin. DF-1 chicken fibroblasts were maintained in DMEM with high glucose supplemented with 10% FBS and 1% penicillin/streptomycin. Transfection was performed with FuGENE reagent (Roche Applied Science) (SVEC), Lipofectamine Plus reagent (Invitrogen) (293T and NIH 3T3), or Superfect reagent (Qiagen, Valencia, CA) (DF-1) according to the manufacturers' protocols. NIH and SVEC stable cell lines were obtained by stable transfection of the corresponding pCEFL-derived plasmids, as has been described (13).
Western Blots and Akt Kinase Assay. Western blots were performed as described in ref. 14. Mouse monoclonal anti-hemagglutinin was purchased from Babco (Richmond, CA). Levels of endogenous Akt and phosphorylated Akt were assessed by using antibody against Akt-1 (C-20, Santa Cruz Biotechnology) or phospho-Akt (pSer472/473/474, Pharmingen), respectively. Akt kinase assays were performed as described in ref. 14.
ELISA. Conditioned media from NIH-vGPCR, NIH-R143Q, NIH-R143A, or NIH parental cell lines or from transiently transfected 293T cells were prepared by incubating subconfluent cultured cells for 24 h in DMEM without supplements. Harvested conditioned media were then filtered through a 0.22-μm low protein-binding polyethylsulfonate membrane filter. Vascular endothelial growth factor (VEGF) secretion was detected in the media with a VEGF immunoassay kit (R & D Systems) as indicated in the standard protocol provided by the manufacturer.
Assessment of Thymidine Incorporation. Assessment of cell proliferation by uptake of [3H]thymidine (ICN) was essentially as described (14).
TIE2-tva System. Generation and characterization of the TIE2-tva transgenic mouse line has been described (9). Transgenic mice were generated in FVB/N mice with standard techniques. PTEN+/– (PTEN, the phosphatase and tension homologue deleted on chromosome 10) mice were described in ref. 15; the mice were bred for eight generations into an FVB/N background before breeding with TIE2-tva transgenic mice. Genotypes were determined by Southern blotting and by PCR with tail DNA. For infections, DF-1 cells were transfected with RCAS vectors encoding indicated genes to produce recombinant viruses. Viral stocks were isolated, tittered, and then injected i.p. into 5-day-old littermates as described in ref. 9.
Establishment and Treatment of Tumor Allografts in Athymic nu/nu Mice. All animal studies were carried out by following the appropriate National Institutes of Health animal care and user protocol. The 8-week-old, 22- to 24-g athymic (nu/nu) nude mice (Harlan Sprague–Dawley, Frederick, MD) used in the study were housed in appropriate sterile filter-capped cages and fed and watered ad libitum. All handling and transplantation procedures were conducted in a laminar-flow biosafety hood. SVECs stably expressing vGPCR (EC-vGPCR) were used to induce allografts as described in ref. 9. Briefly, early passage, exponentially growing EC-vGPCRs were harvested after stable selection with G418, washed with PBS, and resuspended in DMEM. Viable cells (5 × 105) were then transplanted s.c. in the right flank of the mouse. The animals were monitored three times weekly for tumor formation, and drug treatment was commenced when tumor volume reached ≈0.1 g. For drug treatment, tumor-bearing animals were randomly grouped (control, n = 10; test, n = 10) and treated with UCN-01 (7.5 mg/kg per day) or an equal volume of diluent (2% sodium citrate, pH 3.5). Treatment schedule was a single injection per animal, given i.p. for 5 consecutive days, as described in ref. 16. On completion of the 5-day treatment, animals were monitored (three times weekly) for tumor growth and body weight. For analysis, tumor weight was determined as described in ref. 16, whereby tumor volume (LW2/2, where L and W represent longest length and shortest width of the tumor) was converted to weight. Results of animal experiments were expressed as mean ± SEM. At the end of the study period, animals were killed for tissue retrieval.
Immunofluorescence and Immunohistochemistry. Immunofluorescence was performed as described in ref. 9. Human tissues were fixed in 10% neutrally buffered formalin and routinely processed to paraffin blocks. Murine tissues were fixed in 4% paraformaldehyde/1× PBS for 36 h, transferred to 70% ethanol/PBS, and embedded in paraffin. Immunohistochemical analysis of tissues has been described (9). For BrdUrd studies, mice were first given an i.p. injection of BrdUrd (100 mg/kg) 2 h before being killed. Rabbit anti-phospho-Akt (Ser-473) polyclonal antibody was purchased from Cell Signaling Technology (Beverly, MA).
Results
vGPCR Activates Akt Through Both Direct and Indirect (Paracrine) Mechanisms. By using the TIE2-tva endothelial-specific retroviral gene transfer system (Fig. 1A) (9), we have previously shown that endothelial infection with a modified ALV-derived vector, RCAS, encoding the KSHV vGPCR (RCAS-vGPCR) causes KS-like tumors in mice (ref. 9 and Fig. 1 B and C). vGPCR is a member of the CXC chemokine G protein-coupled receptor family, that has significant homology to the IL-8 receptors, CXCR1 and CXCR2 (17, 18), but unlike its cellular homologues, vGPCR exhibits ligand-independent activity (18) that is further augmented in the presence of certain chemokines, including IL-8 and growth-regulated oncogene α, that act as vGPCR agonists (14). Because the Akt signaling pathway has been previously implicated in the promotion of endothelial cell survival by vGPCR in vitro (14), we set out to examine the contribution of this signaling route to vGPCR sarcomagenesis in vivo. Surprisingly, despite evidence that vGPCR is only expressed in rare tumor cells in both human KS lesions (19) and vGPCR experimental tumors (refs. 9, 20, and 21 and results not shown), immunohistochemical analysis of vGPCR-induced tumors demonstrated that almost all tumor cells expressed elevated levels of activated (phosphorylated) Akt (Fig. 1D) when compared with adjacent normal tissue (Fig. 1E). These results suggested that Akt is activated in vGPCR-induced tumor cells, even if they do not express vGPCR.
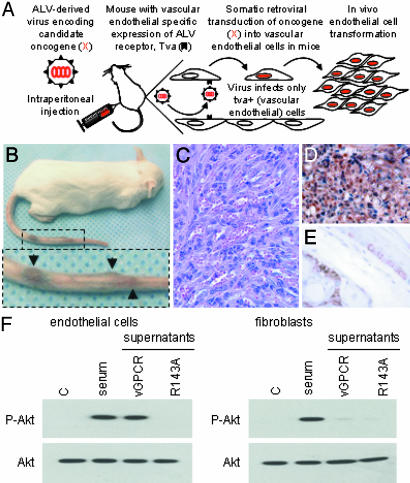
Fig. 1.
vGPCR paracrine activation of Akt in vitro and in vivo. (A) Schematic demonstrating use of the TIE2-tva mouse model for the somatic retroviral transduction of candidate oncogenes into vascular endothelial cells in vivo. (B) Kaposi-like vascular tumors (arrows) found in the tail of mice injected with RCAS-vGPCR (105 units). Similar lesions were found systemically. (C) Representative hematoxylin/eosin-stained section of lesions found in the tail of TIE2-tva mice. (D and E) Anti-phospho-Akt staining of vGPCR-induced tumors demonstrates activated Akt in a majority of tumor cells (D) but only in a few basal and hair follicle cells (E) in adjacent normal skin. (F) Activation of Akt in murine endothelial cells (SVECs) or fibroblasts (NIH 3T3) after a 15-min treatment with conditioned media (supernatant) from vGPCR- or vGPCR(R143A)-expressing NIH 3T3 cells. Ten percent FBS and conditioned media from parental NIH 3T3 cells were used as controls (C).
Although we have previously shown that vGPCR can stimulate the direct activation of Akt in vGPCR-expressing endothelial cells in vitro (14), we next sought an explanation for the activation of Akt in bystander endothelial cells in vGPCR-induced tumors. In this regard, it has recently been reported that cells expressing vGPCR potently induce the persistent secretion of angiogenic growth factors and chemokines in vitro (10, 13, 22–24) and in vivo (9, 20, 21). To begin addressing whether these paracrine secretions could activate Akt in bystander endothelial cells, we treated cells with conditioned media from cell lines ectopically expressing vGPCR or its inactive mutant, R143A (see below). Only vGPCR-conditioned media induced Akt activation in treated endothelial cells (SVECs), and this enhanced Akt activity was similar to that provoked by serum, that potently stimulates Akt in a variety of cell types (25) and thus served as a control (Fig. 1F). However, fibroblasts treated with vGPCR-conditioned media showed little induction of Akt, suggesting that Akt activation by vGPCR-conditioned media may be a consequence of signaling cascades activated by endothelial-specific receptors. Indeed, treatment of endothelial cells with recombinant growth factors (e.g., VEGF) or chemokines (e.g., IL-8 and growth-regulated oncogene α) secreted by vGPCR-expressing cells all potently activated Akt (results not shown). Thus, vGPCR can activate Akt through both direct and indirect (paracrine) mechanisms.
Akt Is a Key Component by Which vGPCR Induces Endothelial Cell Transformation in Vivo. These results suggested that Akt may represent a key point of convergence for vGPCR direct and paracrine neoplasia. Because overactivation of this pathway is essential in many human cancers (26), we set out to investigate whether Akt activation may contribute to the initiation of endothelial cell transformation in vGPCR sarcomagenesis. To this end, we generated virus expressing a constitutively active myr-Akt (12) (Fig. 2A) and infected TIE2-tva mice with high titer (107 units) virus. Animals infected with RCAS-myr-Akt virus developed systemic vascular lesions within 6 months of infection, ranging from vascular angiectasias to benign endothelial tumors (Fig. 2B), suggesting that constitutive activation of Akt is sufficient to initiate endothelial cell transformation in vivo. These results suggest that Akt, as a point of convergence for vGPCR direct and paracrine neoplasia, may contribute to the initiation of endothelial cell transformation by vGPCR.
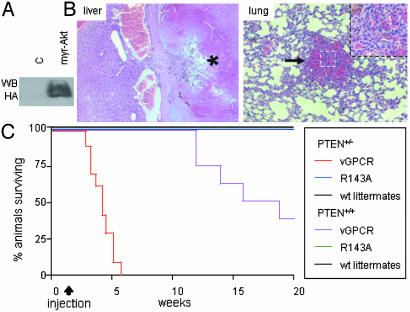
Fig. 2.
Akt is a key component by which vGPCR induces endothelial cell transformation in vivo. (A) Western blot (WB) of DF-1 cells infected with virus encoding a hemagglutinin-tagged myr-Akt (RCAS-myr-Akt). (B) Representative hematoxylin/eosin-stained sections of thrombosed (*) angiectasia found in the liver and a benign endothelial tumor (arrow and Inset) found in the lung in TIE2-tva mice infected with RCAS-myr-Akt. Most vascular tissues examined (e.g., spleen, liver, lungs, mesentery, etc.) in myr-Akt-infected TIE2-tva animals (n = 23) showed similar lesions (data not shown). Sections initially photographed at 20× magnification. (C) Surviving mice (%) after i.p. infection with RCAS-vGPCR (n = 22 total; PTEN+/–, n = 14; PTEN+/+, n = 8) or RCAS-vGPCR(R143A) (n = 13 total) virus. Five-day-old mice born from TIE2-tva × PTEN+/– breeding pairs were injected with 106 units of respective virus. Data are from a single representative experiment from two independent trials. PTEN status (+/+ or +/–) are indicated.
To investigate more directly the importance of the Akt signaling pathway to the initiation of vGPCR sarcomagenesis, we next bred the TIE2-tva mouse line into the PTEN heterozygous background. Pten is a tumor suppressor gene that modulates cell survival by reducing levels of phosphatidylinositol 4,5-triphosphate, thereby diminishing the activity of Akt (27–30). Thus, reduction of PTEN manifests as prolonged activation of Akt (31). In the context of the TIE2-tva PTEN+/– background, viral transduction of vGPCR resulted in the rapid death of infected mice in only a few weeks (Fig. 2C). The median survival of TIE2-tva PTEN+/+ animals infected with RCAS-vGPCR virus (106 units) was 19 weeks, whereas the median survival of their TIE2-tva PTEN+/– littermates was dramatically reduced to only 4 weeks. Histological examination revealed numerous KS-like tumors compromising the function of multiple organs in both wt and PTEN+/– animals (results not shown). In contrast, animals infected with the inactive receptor mutant RCAS-vGPCR R143A, showed no gross or histopathology, even when killed up to 6 months after injection (Fig. 2C and results not shown). Thus, haploinsufficiency for PTEN, which modestly elevates the basal Akt activity, has a profound effect on the timing of vGPCR sarcomagenesis, which is consistent with a role for Akt in the initiation of vGPCR sarcomagenesis.
Activation of Akt Is a Molecular Hallmark of Human KS. The important contribution of Akt to experimental vGPCR sarcomagenesis prompted us to examine the levels of activated Akt in human KS. To this end, we examined biopsies of cutaneous and visceral KS lesions from patients with HIV-associated KS. The human KS lesions demonstrated characteristic whorls of spindle-shaped tumor cells and erythrocyte-replete vascular slits (Fig. 3) similar to vGPCR-induced tumors in TIE2-tva mice. Immunohistochemical analysis further revealed high levels of phosphorylated Akt in human KS tumor cells in all samples tested (eight of eight) (Fig. 3), strongly supporting a role for this pathway in human Kaposi's sarcomagenesis.
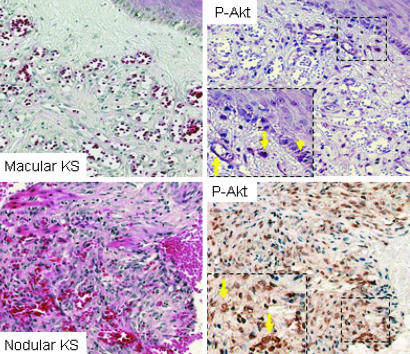
Fig. 3.
Akt activation in human Kaposi's sarcomagenesis. (Left) Representative hematoxylin/eosin-stained sections from early (macular) and late (nodular) stage human KS lesions. (Right) Relative levels of phospho-Akt (arrows) in macular and nodular KS. Rare basal-layer proliferating keratinocytes stain positive for phospho-Akt (arrowhead) in normal adjacent skin. Sections initially photographed at ×20 magnification.
Constitutive Activity Is Required for vGPCR Sarcomagenesis. Unlike its cellular homologues, vGPCR exhibits ligand-independent activities because of the presence of an Asp142Val mutation in a highly conserved Asp-Arg-Lys (DRY) sequence (32), enabling constitutive (ligand-independent) activity. To determine whether constitutive signaling is required for vGPCR sarcomagenesis, we generated point mutations in the viral receptor's DRY sequence that were either agonist-dependent (vGPCR R143Q) or remained inactive despite the presence of agonist (vGPCR R143A) (Fig. 4 A and B). We then generated virus expressing the two mutants (RCAS-vGPCR R143Q or RCAS-vGPCR R143A) and confirmed their expression in vitro (Fig. 4C) before infecting TIE2-tva mice. Although all animals infected with high titer (107 units) RCAS-vGPCR died 6–9 weeks after injection, animals infected with either the RCAS-vGPCR R143Q or the RCAS-vGPCR R143A virus were not affected and did not present any gross or histopathology when killed 6 months after injection (Fig. 4D and results not shown). These results suggested that constitutive activity is required for vGPCR sarcomagenesis.
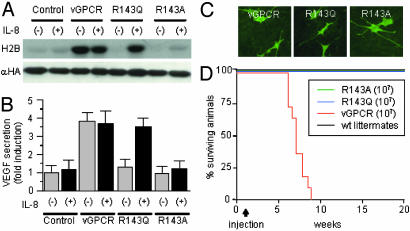
Fig. 4.
Constitutive activity required for vGPCR sarcomagenesis. (A and B) Activation of Akt (A) or induction VEGF secretion (B) by vGPCR or the agonist-dependent [vGPCR(R143Q)] and inactive [vGPCR(R143A)] receptor mutants in the presence (+) or absence (–) of IL-8 [50 nM for 15 min (A) or 50 nM × 2 for 4 h and 8 h(B)] in NIH 3T3 cells. For Akt assays, histone 2B (H2B) was used as a substrate in an in vitro Akt kinase assay. VEGF secretion is reported as a fold induction compared with control (NIH 3T3-GFP) cells. (C) AU5-tagged receptors were detected by immunofluorescence in endothelial cells stably expressing TVA after infection with RCAS-vGPCR, RCAS-vGPCR(R143Q), or RCAS-vGPCR(R143A) virus. (D) Surviving mice (%) after i.p. infection with RCAS-vGPCR (n = 8) and its mutants (R143Q, n = 10; R143A, n = 9). Five-day-old mice born from TIE2-tva × FVB/N breeding pairs were injected with 107 units of virus. Data are from a single representative experiment from three independent trials. wt, wild type. αHA, antibody against hemagglutinin tag.
Antitumor Effects with Systemic Administration of Chemotherapeutic Agent UCN-01 Correlate with Inhibition of Akt. The requirement for constitutive activity for vGPCR sarcomagenesis indicates that transient stimulation of vGPCR activated pathways may not be sufficient to initiate sarcomagenesis. This finding further suggests that inhibition of critical vGPCR transforming pathways may be an effective approach for preventing vGPCR sarcomagenesis. In this regard, as a point of convergence for vGPCR direct and paracrine neoplasia, Akt may represent an ideal target to inhibit vGPCR oncogenesis. Indeed, the PI3K inhibitor wortmannin (33), which potently inhibits activation of Akt by vGPCR (Fig. 5A), prevented vGPCR-stimulated endothelial cell proliferation (Fig. 6, which is published as supporting information on the PNAS web site) and induced apoptosis in vitro (results not shown). Similar results were obtained by using the PI3K inhibitor LY 294002 (results not shown), thus confirming that the PI3K/Akt pathway may play an essential role in the enhanced proliferative capacity of vGPCR-transformed endothelial cells, in addition to its previously described role in their survival (14).
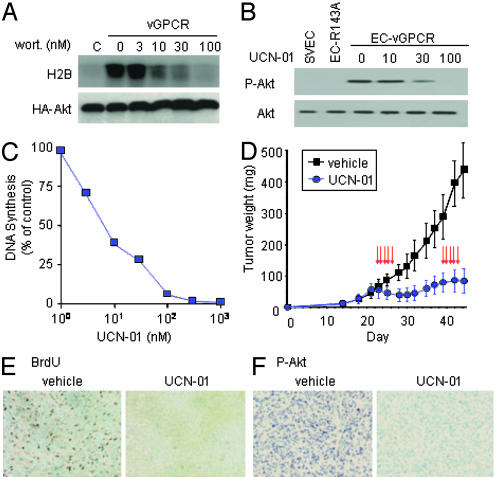
Fig. 5.
Pharmacologic inhibition of Akt blocks proliferation of vGPCR-expressing endothelial cells in vitro and their ability to form tumors in vivo. (A) In vitro kinase assay in NIH 3T3 cells ectopically expressing vGPCR, demonstrating inhibition of Akt activation by wortmannin (wort.). H2B, histone 2B; HA-Akt, hemagglutinin-tagged WT Akt. (B) Treatment of EC-vGPCR cells with UCN-01 (1 h) reduces active (phosphorylated) Akt (P-Akt) to basal levels. SVEC and endothelial cells stably expressing vGPCR R143A inactive mutant were used as controls. (C) EC-vGPCR cells were grown for 24 h in the presence of UCN-01 (1–1,000 nM) or DMSO (0.1% final concentration). The antiproliferative effect of UCN-01 in EC-vGPCR cells is illustrated as percent of [3H]thymidine incorporation relative to control. The results are the mean of triplicate samples from a single representative experiment that was repeated three times with similar results. (D) Athymic nu/nu mice with EC-vGPCR allografts were treated with two cycles (arrows) of either UCN-01 (7.5 mg/kg; n = 10) or equivalent volume of 2% sodium citrate, pH 3.5, as described in Materials and Methods. The results are expressed as mean tumor weight (mg) ± SEM. Data are from a representative independent experiment that was repeated two times with similar results. (E and F) BrdUrd incorporation (E) and relative levels of phosphorylated (active) Akt (F) in representative sections of vehicle- and UCN-01-treated EC-vGPCR tumor tissue. Both cell proliferation (BrdUrd incorporation) and Akt activation (phosphorylation) were dramatically reduced in UCN-01 treated versus control tissues. Sections initially photographed at ×20 magnification.
These observations, combined with the lack of effective treatment strategies for KS, prompted us to examine pharmacological agents that may target the Akt pathway, with the aim of assessing the potential clinical benefit of such drugs for the treatment of KS patients. Unfortunately, wortmannin and LY 294002 are toxic (34) and would not be appropriate drugs for anti-KS therapies. We therefore sought a candidate chemotherapeutic agent that could both target this signaling pathway and be tolerated in a clinical setting. To this end, we investigated the effects of UCN-01, a recently described chemotherapeutic agent that inhibits the Akt upstream modulator 3′-phosphoinositide-dependent protein kinase 1 (35), on EC-vGPCR cells. Exposure to UCN-01 for as little as 1 h was sufficient to cause a potent dose-dependent reduction of Akt activity, which reached near basal levels at 100 nM (Fig. 5B), validating its use as an inhibitor of the Akt pathway in our system.
EC-vGPCR cells exposed to UCN-01 (0–1,000 nM) for 24 h resulted in a dose-dependent inhibition of [3H]thymidine uptake (Fig. 5C). Time-course analysis of EC-vGPCR cells exposed to UCN-01 at therapeutic levels (36) demonstrated a decrease in proliferation in as early as 3 h of treatment (Fig. 7, which is published as supporting information on the PNAS web site). A corresponding increase in endothelial cell apoptosis was observed as early as 6 h following treatment (results not shown). Thus, inhibition of Akt activity in UCN-01 treated EC-vGPCR cells precedes the inhibition of cell proliferation and induction of apoptosis observed in these cells.
Because endothelial cells expressing vGPCR were remarkably sensitive to the antiproliferative effects of UCN-01 in vitro, we next sought to determine whether UCN-01 possessed similar activities in vivo. To address this issue, we established allografts with EC-vGPCR cells in athymic nu/nu mice and then treated animals with either a proven tolerable dose of UCN-01 (7.5 mg/kg per day) (16) or vehicle (2% sodium citrate, pH 3.5) i.p. for 5 consecutive days. Drug toxicity, as assessed by weight loss, was minimal in the UCN-01-treated group (reduction <5%) during the treatment period (results not shown). Tumor growth was monitored for 3 weeks after initiation of treatment. Tumor regression in UCN-01-treated animals was observed 2 d after initiation of treatment, and growth inhibition was sustained for >10 d (Fig. 5D). To determine the feasibility of repeated chemotherapeutic cycles of UCN-01 in the treatment of KS, EC-vGPCR allografts were treated with a second cycle of either UCN-01 or vehicle. Tumor regrowth was again inhibited after the second cycle of UCN-01 treatment (Fig. 5D). At the end of the study (day 44), although vehicle-treated tumors weighed 440 mg, an almost 10-fold increase in 21 d, the UCN-01-treated group demonstrated minimal growth over the same period, with an average tumor weight of 88.2 mg on day 44 (Fig. 8, which is published as supporting information on the PNAS web site), representing only a 1.6-fold increase in tumor mass in 21 d after the initiation of the treatment.
Tissue sections excised from animals 5 d after completion of the second cycle of UCN-01 treatment demonstrated a significant decrease in cell proliferation (as determined by BrdUrd incorporation), compared with corresponding vehicle control tissues (Fig. 5E). This inhibitory effect correlated with a significant decrease in activated Akt (Fig. 5F). Taken together, these data demonstrate that the administration of UCN-01 at tolerable doses causes a significant antitumor effect that is sustained with repeated therapeutic cycles of this drug, suggesting that inhibitors of the Akt pathway, including UCN-01, may be appropriate candidate therapeutics for KS patients in clinical trials.
Discussion
The growing field of rational drug design, in combination with advanced molecular understanding of cell signaling, will make it possible to develop a new generation of gene-product target-specific and molecular-based drugs. The recent success of this approach for chronic myelogenous leukemia illustrates the potential of rationally designed molecularly targeted therapies for cancers that have previously failed conventional chemotherapeutic approaches (37). In this regard, mechanism-based therapies directed against specific KSHV oncogenes may prove to be an effective approach to treating KS. Identification of the gene(s) necessary for KSHV oncogenesis and the nature of the molecular events mediating their oncogenic potential is an essential first step for the successful development of such therapies.
Because KSHV latent genes are expressed in almost all spindle cells in late KS lesions, they were predicted to play a critical role in the development of KS (38). Indeed, several recent studies have demonstrated that a number of the KSHV latent genes, including LANA-1 (39–41), LANA-2 (42), vCyclin (43, 44), vFlip (45), and Kaposin (46), harbor transforming potential in vitro. However, these genes were unable to initiate endothelial cell transformation in vivo in the TIE2-tva KS model (9). Although this inability does not rule out a role for these genes in the progression of KS or in the pathogenesis of KSHV-associated B cell-derived tumors (multicentric Castleman's disease and primary effusion lymphoma), it would suggest that endothelial expression of KSHV latent genes may not be sufficient to initiate Kaposi's sarcomagenesis. Conversely, expression of the KSHV-encoded lytic gene, vGPCR, consistently induced KS-like tumors in several animal models (9, 20, 21), identifying this viral protein as a promising target for the generation of rationally designed therapies for KS. However the molecular mechanisms by which vGPCR promotes its remarkable biological activity are still poorly understood.
Genetic alterations leading to overactivation of the Akt pathway are a frequent theme in a wide variety of human cancers (26). vGPCR activation of this pathway may play an analogous role in initiating Kaposi's sarcomagenesis. However, tumor cells in lesions induced by myr-Akt remained well differentiated, failing to exhibit all histological features of human KS lesions, which are recapitulated in vGPCR-induced experimental tumors. Thus, although activation of Akt can initiate endothelial cell transformation, our results suggest that the complex inter-play between direct and paracrine Akt activation, together with yet-to-be-identified additional molecular mechanisms, may explain the potent oncogenic potential of vGPCR. Analysis of biopsy samples from patients with AIDS-associated KS demonstrated that active Akt is a molecular hallmark of human KS, supportive of a role for this pathway in human Kaposi's sarcomagenesis.
These observations, combined with the lack of effective treatment strategies for KS, prompted us to explore drugs that target Akt, with the aim of assessing the potential clinical benefit of such drugs for the treatment of KS patients. We demonstrate that endothelial cells transformed by vGPCR were exquisitely sensitive to the antiproliferative properties of UCN-01, a chemotherapeutic drug recently documented to potently inhibit the Akt modulator, 3′-phosphoinositide-dependent protein kinase 1 (35, 47, 48). The antiproliferative effects of UCN-01 had an average IC50 concentration of ≈9 nM, which is significantly lower than that estimated for the NCI 60 cancer cell line panel (≈37 nM) (49), and well within the therapeutic levels of unbound UCN-01 easily achieved in humans (≈110 nM) (36). We further observed a significant antitumor effect after only one treatment cycle of UCN-01, which was sustained for 10 d following initiation of treatment. Of note, s.c. injection of UCN-01 appears to be an equally effective route for administration of this drug (50), given that injection of UCN-01 directly into EC-vGPCR allografts demonstrated a similar inhibitory effect on tumor growth (results not shown), suggesting that intralesional UCN-01 may be an effective treatment for cutaneous KS.
Because UCN-01 is known to inhibit other proliferative pathways, there may be additional mechanisms through which this drug achieves its antitumor effects (51). Indeed, UCN-01 was originally developed as a PKC inhibitor (52) and was later shown to target cell cycle regulators (53). However, although inhibition of PI3K with wortmannin and LY 294002, and thus of Akt, prevented vGPCR-stimulated endothelial cell proliferation, the treatment of EC-vGPCR with a PKC-specific inhibitor (GFX) at effective concentrations (1–10 μM) failed to reproduce the profound inhibitory effect on cell proliferation demonstrated by therapeutic concentrations of UCN-01 (results not shown). Moreover, the antitumor effects of UCN-01 in our KS allograft model correlated well with its inhibition of Akt, thus suggesting that the ability to inhibit 3′-phosphoinositide-dependent protein kinase 1 and the Akt pathway is likely to mediate the remarkable activities of UCN-01. These effects were achievable at doses that were well tolerated by experimental animals and at levels similar to those achieved in humans in a recently completed Phase I clinical trial (36). Taken together, our data provide the basis for the early assessment of molecules inhibiting the Akt pathway, including UCN-01, in those patients with cutaneous and/or systemic KS.
Supplementary Material
Acknowledgments
We thank M. Kriete and the Veterinary Resources Core Facility for assistance with animal care, SAIC-Frederick (a subsidiary of Science Applications International Corporation) for tissue preparation and immunohistochemical staining, and K. Rogers for technical assistance. This work has been supported by National Institutes of Health Grant R0-1 AI46145-01A2 and Department of Defense Grant BC972195 (to E.T.S.).
Abbreviations: KS, Kaposi's sarcoma; PI3K, phosphoinositide-3-OH kinase; KSHV, KS herpesvirus; vGPCR, KSHV-encoded G protein-coupled receptor; SVEC, simian virus 40, large T antigen-immortalized, murine endothelial cell; EC-vGPCR, SVECs stably expressing vGPCR; UCN-01, 7-hydroxystaurosporine; ALV, avian leukosis virus; RCAS, replication-competent ALV long-terminal repeat with splice acceptor; VEGF, vascular endothelial growth factor; myr, myristoylated.
References
- 1.Chang, Y., Cesarman, E., Pessin, M. S., Lee, F., Culpepper, J., Knowles, D. M. & Moore, P. S. (1994) Science 266, 1865–1869. [DOI] [PubMed] [Google Scholar]
- 2.Bates, P., Young, J. A. & Varmus, H. E. (1993) Cell 74, 1043–1051. [DOI] [PubMed] [Google Scholar]
- 3.Young, J. A., Bates, P. & Varmus, H. E. (1993) J. Virol. 67, 1811–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlaeger, T. M., Bartunkova, S., Lawitts, J. A., Teichmann, G., Risau, W., Deutsch, U. & Sato, T. N. (1997) Proc. Natl. Acad. Sci. USA 94, 3058–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Federspiel, M. J., Bates, P., Young, J. A., Varmus, H. E. & Hughes, S. H. (1994) Proc. Natl. Acad. Sci. USA 91, 11241–11245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher, G. H., Orsulic, S., Holland, E., Hively, W. P., Li, Y., Lewis, B. C., Williams, B. O. & Varmus, H. E. (1999) Oncogene 18, 5253–5260. [DOI] [PubMed] [Google Scholar]
- 7.Orsulic, S. (2002) Mamm. Genome 13, 543–547. [DOI] [PubMed] [Google Scholar]
- 8.Flore, O., Rafii, S., Ely, S., O'Leary, J. J., Hyjek, E. M. & Cesarman, E. (1998) Nature 394, 588–592. [DOI] [PubMed] [Google Scholar]
- 9.Montaner, S., Sodhi, A., Molinolo, A., Bugge, T. H., Sawai, E. T., He, Y., Li, Y., Ray, P. E. & Gutkind, J. S. (2003) Cancer Cell 3, 23–36. [DOI] [PubMed] [Google Scholar]
- 10.Sodhi, A., Montaner, S., Patel, V., Zohar, M., Bais, C., Mesri, E. A. & Gutkind, J. S. (2000) Cancer Res. 60, 4873–4880. [PubMed] [Google Scholar]
- 11.Bos, T. J., Monteclaro, F. S., Mitsunobu, F., Ball, A. R., Jr., Chang, C. H., Nishimura, T. & Vogt, P. K. (1990) Genes Dev. 4, 1677–1687. [DOI] [PubMed] [Google Scholar]
- 12.Orsulic, S., Li, Y., Soslow, R. A., Vitale-Cross, L. A., Gutkind, J. S. & Varmus, H. E. (2002) Cancer Cell 1, 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bais, C., Santomasso, B., Coso, O., Arvanitakis, L., Raaka, E. G., Gutkind, J. S., Asch, A. S., Cesarman, E., Gershengorn, M. C., Mesri, E. A. & Gerhengorn, M. C. (1998) Nature 391, 86–89. [DOI] [PubMed] [Google Scholar]
- 14.Montaner, S., Sodhi, A., Pece, S., Mesri, E. A. & Gutkind, J. S. (2001) Cancer Res. 61, 2641–2648. [PubMed] [Google Scholar]
- 15.Podsypanina, K., Ellenson, L. H., Nemes, A., Gu, J., Tamura, M., Yamada, K. M., Cordon-Cardo, C., Catoretti, G., Fisher, P. E. & Parsons, R. (1999) Proc. Natl. Acad. Sci. USA 96, 1563–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel, V., Lahusen, T., Leethanakul, C., Igishi, T., Kremer, M., Quintanilla-Martinez, L., Ensley, J. F., Sausville, E. A., Gutkind, J. S. & Senderowicz, A. M. (2002) Clin. Cancer Res. 8, 3549–3560. [PubMed] [Google Scholar]
- 17.Cesarman, E., Nador, R. G., Bai, F., Bohenzky, R. A., Russo, J. J., Moore, P. S., Chang, Y. & Knowles, D. M. (1996) J. Virol. 70, 8218–8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arvanitakis, L., Geras-Raaka, E., Varma, A., Gershengorn, M. C. & Cesarman, E. (1997) Nature 385, 347–350. [DOI] [PubMed] [Google Scholar]
- 19.Chiou, C. J., Poole, L. J., Kim, P. S., Ciufo, D. M., Cannon, J. S., ap Rhys, C. M., Alcendor, D. J., Zong, J. C., Ambinder, R. F. & Hayward, G. S. (2002) J. Virol. 76, 3421–3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang, T. Y., Chen, S. C., Leach, M. W., Manfra, D., Homey, B., Wiekowski, M., Sullivan, L., Jenh, C. H., Narula, S. K., Chensue, S. W. & Lira, S. A. (2000) J. Exp. Med. 191, 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo, H. G., Sadowska, M., Reid, W., Tschachler, E., Hayward, G. & Reitz, M. (2003) J. Virol. 77, 2631–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwarz, M. & Murphy, P. M. (2001) J. Immunol. 167, 505–513. [DOI] [PubMed] [Google Scholar]
- 23.Shepard, L. W., Yang, M., Xie, P., Browning, D. D., Voyno-Yasenetskaya, T., Kozasa, T. & Ye, R. D. (2001) J. Biol. Chem. 276, 45979–45987. [DOI] [PubMed] [Google Scholar]
- 24.Polson, A. G., Wang, D., DeRisi, J. & Ganem, D. (2002) Cancer Res. 62, 4525–4530. [PubMed] [Google Scholar]
- 25.Andjelkovic, M., Jakubowicz, T., Cron, P., Ming, X. F., Han, J. W. & Hemmings, B. A. (1996) Proc. Natl. Acad. Sci. USA 93, 5699–5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vivanco, I. & Sawyers, C. L. (2002) Nat. Rev. Cancer 2, 489–501. [DOI] [PubMed] [Google Scholar]
- 27.Cantley, L. C. & Neel, B. G. (1999) Proc. Natl. Acad. Sci. USA 96, 4240–4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simpson, L. & Parsons, R. (2001) Exp. Cell Res. 264, 29–41. [DOI] [PubMed] [Google Scholar]
- 29.Myers, M. P., Pass, I., Batty, I. H., Van der Kaay, J., Stolarov, J. P., Hemmings, B. A., Wigler, M. H., Downes, C. P. & Tonks, N. K. (1998) Proc. Natl. Acad. Sci. USA 95, 13513–13518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li, J., Yen, C., Liaw, D., Podsypanina, K., Bose, S., Wang, S. I., Puc, J., Miliaresis, C., Rodgers, L., McCombie, R., et al. (1997) Science 275, 1943–1947. [DOI] [PubMed] [Google Scholar]
- 31.Sun, R., Lin, S. F., Staskus, K., Gradoville, L., Grogan, E., Haase, A. & Miller, G. (1999) J. Virol. 73, 2232–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ho, H. H., Ganeshalingam, N., Rosenhouse-Dantsker, A., Osman, R. & Gershengorn, M. C. (2001) J. Biol. Chem. 276, 1376–1382. [DOI] [PubMed] [Google Scholar]
- 33.Carpenter, C. L. & Cantley, L. C. (1996) Curr. Opin. Cell Biol. 8, 153–158. [DOI] [PubMed] [Google Scholar]
- 34.Berrie, C. P. (2001) Expert Opin. Invest. Drugs 10, 1085–1098. [DOI] [PubMed] [Google Scholar]
- 35.Sato, S., Fujita, N. & Tsuruo, T. (2002) Oncogene 21, 1727–1738. [DOI] [PubMed] [Google Scholar]
- 36.Sausville, E. A., Arbuck, S. G., Messmann, R., Headlee, D., Bauer, K. S., Lush, R. M., Murgo, A., Figg, W. D., Lahusen, T., Jaken, S., et al. (2001) J. Clin. Oncol. 19, 2319–2333. [DOI] [PubMed] [Google Scholar]
- 37.Druker, B. J. (2002) Trends Mol. Med. 8, S14–S18. [DOI] [PubMed] [Google Scholar]
- 38.Jenner, R. G. & Boshoff, C. (2002) Biochim. Biophys. Acta 1602, 1–22. [DOI] [PubMed] [Google Scholar]
- 39.Friborg, J., Jr., Kong, W., Hottiger, M. O. & Nabel, G. J. (1999) Nature 402, 889–894. [DOI] [PubMed] [Google Scholar]
- 40.Radkov, S. A., Kellam, P. & Boshoff, C. (2000) Nat. Med. 6, 1121–1127. [DOI] [PubMed] [Google Scholar]
- 41.Fujimuro, M., Wu, F. Y., ApRhys, C., Kajumbula, H., Young, D. B., Hayward, G. S. & Hayward, S. D. (2003) Nat. Med. 9, 300–306. [DOI] [PubMed] [Google Scholar]
- 42.Rivas, C., Thlick, A. E., Parravicini, C., Moore, P. S. & Chang, Y. (2001) J. Virol. 75, 429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li, M., Lee, H., Yoon, D. W., Albrecht, J. C., Fleckenstein, B., Neipel, F. & Jung, J. U. (1997) J. Virol. 71, 1984–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Godden-Kent, D., Talbot, S. J., Boshoff, C., Chang, Y., Moore, P., Weiss, R. A. & Mittnacht, S. (1997) J. Virol. 71, 4193–4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Belanger, C., Gravel, A., Tomoiu, A., Janelle, M. E., Gosselin, J., Tremblay, M. J. & Flamand, L. (2001) J. Hum. Virol. 4, 62–73. [PubMed] [Google Scholar]
- 46.Kliche, S., Nagel, W., Kremmer, E., Atzler, C., Ege, A., Knorr, T., Koszinowski, U., Kolanus, W. & Haas, J. (2001) Mol. Cell 7, 833–843. [DOI] [PubMed] [Google Scholar]
- 47.Davies, S. P., Reddy, H., Caivano, M. & Cohen, P. (2000) Biochem. J. 351, 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wan, X., Yokoyama, Y., Shinohara, A., Takahashi, Y. & Tamaya, T. (2002) Cell Death Differ. 9, 414–420. [DOI] [PubMed] [Google Scholar]
- 49.Monga, M. & Sausville, E. A. (2002) Leukemia 16, 520–526. [DOI] [PubMed] [Google Scholar]
- 50.Hill, D. L., Tillery, K. F., Rose, L. M. & Posey, C. F. (1994) Cancer Chemother. Pharmacol. 35, 89–92. [DOI] [PubMed] [Google Scholar]
- 51.Senderowicz, A. M. & Sausville, E. A. (2000) J. Natl. Cancer Inst. 92, 376–387. [DOI] [PubMed] [Google Scholar]
- 52.Mizuno, K., Noda, K., Ueda, Y., Hanaki, H., Saido, T. C., Ikuta, T., Kuroki, T., Tamaoki, T., Hirai, S., Osada, S., et al. (1995) FEBS Lett. 359, 259–261. [DOI] [PubMed] [Google Scholar]
- 53.Seynaeve, C. M., Stetler-Stevenson, M., Sebers, S., Kaur, G., Sausville, E. A. & Worland, P. J. (1993) Cancer Res. 53, 2081–2086. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.