Background: The human enzyme β-carotene 15,15′-oxygenase (BCO1) produces vitamin A from carotenoids in food.
Results: BCO1 catalyzes the oxidative cleavage of the 15–15′ double bond of major dietary provitamin A carotenoids, β-apocarotenals, and lycopene.
Conclusion: BCO1 reacts only with carotenoids and apocarotenoids that yield retinal or acycloretinal.
Significance: Elucidating the substrate specificity of BCO1 is crucial for understanding how humans metabolize carotenoids.
Keywords: Carotenoid, Enzymes, Lipid Metabolism, Metabolism, Retinoid, Vitamin A, Acycloretinal, Apocarotenal, Lycopene, Retinal
Abstract
Humans cannot synthesize vitamin A and thus must obtain it from their diet. β-Carotene 15,15′-oxygenase (BCO1) catalyzes the oxidative cleavage of provitamin A carotenoids at the central 15–15′ double bond to yield retinal (vitamin A). In this work, we quantitatively describe the substrate specificity of purified recombinant human BCO1 in terms of catalytic efficiency values (kcat/Km). The full-length open reading frame of human BCO1 was cloned into the pET-28b expression vector with a C-terminal polyhistidine tag, and the protein was expressed in the Escherichia coli strain BL21-Gold(DE3). The enzyme was purified using cobalt ion affinity chromatography. The purified enzyme preparation catalyzed the oxidative cleavage of β-carotene with a Vmax = 197.2 nmol retinal/mg BCO1 × h, Km = 17.2 μm and catalytic efficiency kcat/Km = 6098 m−1 min−1. The enzyme also catalyzed the oxidative cleavage of α-carotene, β-cryptoxanthin, and β-apo-8′-carotenal to yield retinal. The catalytic efficiency values of these substrates are lower than that of β-carotene. Surprisingly, BCO1 catalyzed the oxidative cleavage of lycopene to yield acycloretinal with a catalytic efficiency similar to that of β-carotene. The shorter β-apocarotenals (β-apo-10′-carotenal, β-apo-12′-carotenal, β-apo-14′-carotenal) do not show Michaelis-Menten behavior under the conditions tested. We did not detect any activity with lutein, zeaxanthin, and 9-cis-β-carotene. Our results show that BCO1 favors full-length provitamin A carotenoids as substrates, with the notable exception of lycopene. Lycopene has previously been reported to be unreactive with BCO1, and our findings warrant a fresh look at acycloretinal and its alcohol and acid forms as metabolites of lycopene in future studies.
Introduction
Carotenoids are yellow and orange pigments found in fruits and vegetables. They are a major dietary source of vitamin A. In humans and other animals, provitamin A carotenoids are converted to retinal (vitamin A) via the oxidative cleavage of the central double bond by the enzyme β, β-carotene 15,15′-oxygenase (BCO1)2 (1–7). Although it is clear that BCO1 cleaves primarily at the central double bond, the possibility that it can also perform eccentric cleavage has not been well studied.
BCO1 is expressed in several tissues, and high enzymatic activity has been shown for homogenates of liver and intestine (8–13). Early studies of substrate specificity of BCO1 used liver and intestine homogenates to show the enzymatic production of retinal from provitamin A carotenoids (β-carotene, α-carotene, β-cryptoxanthin) and β-apocarotenals (β-apo-8′-, β-apo-10′-, and β-apo-12′-carotenal) (14–17). These studies also show that xanthophylls (lutein, zeaxanthin, and astaxanthin) are not cleaved by BCO1.
In raw fruits and vegetables, β-carotene exists predominantly in the all-trans form, but processing leads to an increase of cis isomers, particularly 9-cis and 13-cis forms (18). These isomers have also been detected in human plasma (19, 20). Cleavage activity with these β-carotene isomers have been shown by incubation with intestine and liver homogenates (14, 21, 22).
However, the use of cell homogenates to study enzymatic oxidation of carotenoids in vitro is problematic. Carotenoids are relatively easy to oxidize, and even no-enzyme incubations carried out for relatively long periods (1 h) with β-carotene produces random cleavage products (23). The intestine contains other enzymes that could potentially oxidize carotenoids (reviewed by Hansen and Maret (23)). Indeed, early studies with intestine homogenates have detected random cleavage products in addition to retinal (11, 24). Although the use of an anti-oxidant such as α-tocopherol reduces the amount of random cleavage products (25), it is difficult to determine whether the enzymatic activity is modulated by the other components of the crude enzyme preparation.
The identification and cloning of BCO1 (5, 26) paved the way for the production and purification of recombinant BCO1. Two studies have used purified recombinant BCO1 preparations to study substrate specificity and, for the most part, agree with the suggestion of previous studies that BCO1 only cleaves carotenoids with at least one unsubstituted β-ionone ring (1, 7). Specifically, both studies showed that purified recombinant BCO1 does not cleave lycopene. Yan et al. (27) using extracts from Sf9 cells expressing recombinant human BCO1, also report that lycopene is not a substrate. These studies are in contrast with the study of Redmond et al., who observed that expression of murine BCO1 in lycopene-accumulating Escherichia coli results in bleaching of the red lycopene pigment, indicating cleavage of lycopene (4). Kim and Oh (7) also showed that purified recombinant chicken BCO1 cleaves β-apo-8′-carotenal but not β-apo-12′-carotenal, suggesting that BCO1 will not cleave the shorter β-apo-14′-carotenal.
In this work, we investigated the activity of purified recombinant human BCO1 with major dietary carotenoids and β-apocarotenals. We show that purified recombinant human BCO1 catalyzes the cleavage of lycopene to produce acycloretinal (apo-15-lycopenal) and β-apocarotenals as short as β-apo-14′-carotenal to produce retinal. We also find that it does not catalyze the cleavage of 9-cis-β-carotene.
EXPERIMENTAL PROCEDURES
Carotenoids and Retinoids
β-Carotene (≥97%), all-trans-retinal (≥98%), 9-cis-retinal (≥95%), 13-cis-retinal (≥85%), α-carotene (≥95%), β-cryptoxanthin (≥97%), zeaxanthin (≥95%), lutein (≥90%), and β-apo-8′-carotenal (≥96%) were purchased from Sigma-Aldrich. The lycopene standard (92% all-trans, 6% 5-cis, 2% other isomers) was a gift from Dr. Steven J. Schwartz of The Ohio State University. β-Apo-10′-carotenal, β-apo-12′-carotenal, and β-apo-14′-carotenal were synthesized according to published methods (28). The synthesis of acycloretinal (apo-15-lycopenal) used similar methods and will be reported in a future publication. The structures of synthesized substrates and standards were confirmed by mass spectrometry, ultraviolet-visible spectrophotometry, and nuclear magnetic resonance spectroscopy, and purity (≥95%) was further assessed by HPLC analysis. All experiments involving carotenoids and retinoids were done under amber lights.
Isolation of 9-cis-β-Carotene from Betatene®
Betatene® is a supplement of carotenoids extracted from the alga Dunaliella salina that is known to have relatively high amounts of 9-cis-β-carotene (29). Five capsules of Betatene® (Swanson Vitamins) were sliced open under methanol, and the resulting slurry of carotenoids was transferred to a conical glass centrifuge tube. The mixture was centrifuged, and the residue was washed three times with 5 ml of ethanol per wash. The residue was transferred to a separatory funnel with 1:1 (v/v) hexane-methanol. The volume of the mixture was then brought up to ∼100 ml of 1:1 (v/v) hexane-methanol, and 5 ml of water was added, producing two phases. The hexane layer was evaporated under argon, and the residue was dissolved in 75:25 (v/v) methanol-methyl t-butyl ether. The sample was separated using HPLC method C described below. The 9-cis-β-carotene fraction was collected, evaporated under argon, and stored at −80 °C until used. The product is ∼95% pure by HPLC.
Expression and Purification of Recombinant Human BCO1
A pET-28b plasmid vector containing the cDNA of human BCO1 with a C-terminal hexahistidine tag was a gift from Dr. William Blaner of Columbia University. The plasmid was transformed into E. coli BL21-Gold(DE3) (Stratagene) according to the manufacturer's instructions. The transformed bacterial cells were grown in LB broth (Sigma-Aldrich) to an A600 of 0.5–0.7 at 30 °C, and expression of the recombinant protein was induced by adding isopropyl β-d-1-thiogalactopyranoside (Gold Biotechnology) to a final concentration of 0.1 mm. Five liters of culture were grown for 24 h, and the cells were harvested by centrifugation. The cell pastes were frozen at −80 °C and thawed prior to lysis. The cells were lysed by gentle stirring with lysis buffer (50 mm phosphate, 50 mm NaCl, 10% (v/v) glycerol, 1% (v/v) Triton X-100, 1 mm β-mercaptoethanol, 2.5 mm imidazole, 10 mg/ml chicken egg white lysozyme (Sigma), 0.1 μl/ml benzonase nuclease HC (Novagen), 1 mm phenylmethanesulfonylfluoride, and 1 tablet of EDTA-free protease inhibitor mixture (Roche Diagnostics)/50 ml), using 5 ml of lysis buffer per gram of wet cell paste. The lysis was conducted in ice for 1 h. The lysate was centrifuged at 15,600 × g for 25 min, and the supernatant was filtered through a 0.22-μm syringe-driven filter. The NaCl concentration of the filtered supernatant was brought up to 300 mm using salt adjustment buffer (50 mm phosphate, 2,500 mm NaCl, 10% (v/v) glycerol, 1% (v/v) Triton X-100, 1 mm β-mercaptoethanol, 2.5 mm imidazole) and stirred with 0.5 ml of HisPur resin (Pierce) that has been previously washed with equilibration buffer (50 mm phosphate, 300 mm NaCl, 10% (v/v) glycerol, 1% (v/v) Triton X-100, 1 mm β-mercaptoethanol, 2.5 mm imidazole) for 1 h in an ice bath. The resin was harvested by centrifugation at 700 × g for 2 min and transferred to a gravity column. The resin was then eluted with the following solutions, which were prepared by combining the calculated amounts of equilibration buffer (2.5 mm imidazole) and elution buffer (same composition as equilibration buffer except for 150 mm imidazole): 4 ml of 2.5 mm imidazole (equilibration buffer), 1.5 ml of 10 mm imidazole, 1 ml each of 30, 60, 90, 120, and 150 mm imidazole (elution buffer). The fractions with pure protein were transferred into the final storage buffer (50 mm phosphate, 50 mm NaCl, 10% (v/v) glycerol, 1% (v/v) Triton X-100, 1 mm β-mercaptoethanol) using a PD-10 desalting column (GE Healthcare). SDS-PAGE was used to assess the purity of the protein throughout the purification process. The concentration of the protein solution was measured using the reducing agent-compatible bicinchoninic acid protein assay kit (Pierce). The protein was stored at −80 °C until used.
In Vitro BCO1 Activity Assay
The in vitro BCO1 enzyme assay was based on the method of During et al. (13) with the following exceptions. First, 500 μl of 1:1 (v/v) acetonitrile-isopropyl alcohol (instead of acetonitrile alone) was added after quenching the reaction with formaldehyde. Second, the resulting mixture was filtered through a 0.22-μm syringe filter instead of centrifugation to remove the insoluble particles. Third, only 100 μl of the sample, instead of 200 μl as in the original method, was injected into the HPLC. Five hundred ng of purified recombinant BCO1 are used per reaction, and the reaction time is 15 min, unless otherwise indicated. Standard curves were generated by dissolving various amounts of standard compounds (retinal or apo-15-lycopenal) in a solution of 200:0.3:50:250:250 (v/v/v/v) water, Tween 40, 37% formaldehyde acetonitrile, isopropyl alcohol, which has the same ratio of solvents as the samples obtained from the reaction mixtures.
The assay is sensitive to Triton X-100 concentration. We have found the optimum enzyme activity at 0.1% (v/v) Triton X-100, and all assays are carried out at this constant concentration of Triton X-100.
Analytical HPLC Methods
Method A
The reaction products were analyzed using an Agilent 1200 Series HPLC system and a Zorbax Eclipse XDB-C18 LC column (4.6 × 50 mm, 1.8 μm, Agilent) with a Zorbax Eclipse XDB-C18 guard column (4.6 × 12.5 mm, 5 μm, Agilent) or a Nova-Pak C18 reverse-phase analytical column (3.9 × 150 mm, 4 μm, Waters) with a Nova-Pak C18 guard column (3.9 × 20 mm, 4 μm, Waters). The column thermostat was set to 35 °C, and the flow rate was 1 ml/min. The following elution profile was used with 75:25 (v/v) acetonitrile and water with 0.1% NH4CH3COO (solvent A) and 72:18:10 (v/v) isopropyl alcohol and acetonitrile water with 0.1% NH4CH3COO (solvent B): gradient from 100% solvent A-100% solvent B over 10 min, 100% solvent B for 10 min, gradient from 100 solvent B-100% solvent A over 6 s, and 100% solvent A for 4 min. Elution was monitored at 380, 414, and 453 nm.
Method B
Retinal isomers were separated on a YMC Carotenoid S-3 column (4.6 × 250 mm, 3 μm). The following elution profile was used with water (solvent A) and 75:25 (v/v) acetonitrile, methyl tert-butyl ether (solvent B): 55% B for the first 5 min, followed by a gradient from 55% to 62.5% B over 25 min. The flow rate used was 1 ml/min, and the column thermostat was set to 35 °C. Elution was monitored at 380 nm.
Method C
β-Carotene isomers were analyzed using a YMC-carotenoid S-3 column (4.6 × 250 mm, 3 μm), with 75:25 (v/v) methanol-methyl t-butyl ether as mobile phase, a flow rate of 1.4 ml/min, and a column temperature of 35 °C. This was based on the HPLC method of Maeda et al. (31). Elution was monitored at 450 nm.
Method D
For multiple reaction monitoring analysis, the BCO1-lycopene reaction mixture was separated by an Agilent 1200 SL HPLC system (Agilent Technologies) using a YMC C30 column with dimensions 150 mm × 4.6 mm, 5 μm (Waters). The flow rate was 1.3 ml/min, and the column temperature was 35 °C. The composition of solvents was as follows: A = 80:20, methanol/water with 0.04% (w/v) ammonium acetate; B = 78:20:2 methanol-methyl t-butyl ether/methanol/water with 0.04% (w/v) ammonium acetate. A linear eluting gradient was applied as follows: 0–35.6% B for over 9 min, 35.6–100% B over 6.5 min, isocratic 100% B for 2.5 min, 100% B to 100% A over 0.1 min, and re-equilibrated for 3.4 min.
HPLC-MS/MS Method for Confirmation of Acycloretinal Produced from the Reaction of BCO1 and Lycopene
The BCO1-lycopene reaction mixture was separated using the HPLC method D described in the previous section. The HPLC was interfaced with a QTrap 5500 mass spectrometer (ABSciex), operated as a triple quadrupole, using an atmospheric pressure chemical ionization source in positive ion mode. Additional settings were as follows: nebulizer current, 5 μA; nebulizer temperature, 500 °C; declustering potential, 80 V. MS/MS transitions were optimized for acycloretinal using authentic standard prepared by organic synthesis. The multiple reaction monitoring sequence included transitions m/z 285 > 69, 119, 135, and 161 using collision energies of 28, 15, 15, and 14 eV, respectively.
Kinetic Data
Data from plots of reaction velocity versus substrate concentration were fit to the Michaelis-Menten equation using GraphPad Prism (version 4).
RESULTS
Expression and Purification of Recombinant Human BCO1
The pET-28b-human BCO1 plasmid was sequenced to verify the alignment with the sequence reported in PubMed (accession no. NM_017429.2, corresponding to a 547-amino acid peptide). The clone has an additional eight amino acids at the C terminus corresponding to the hexahistidine tag. The theoretical mass of the protein including the hexahistidine tag is 63,702 Da.
The protein tends to form inclusion bodies under conditions that favor high rate of expression (high isopropyl 1-thio-β-d-galactopyranoside and high growth temperature). Five liters of culture grown under the conditions described above yields 2–3 mg of purified protein. Sample purification gels are shown in Fig. 1. The enzyme is soluble in detergent-free phosphate buffer. However, we found greater activity if detergent is maintained through purification and storage. The average specific activity of our enzyme preparation is 132.1 ± 23.8 (mean ± S.D., n = 5) nmol retinal/mg BCO1/h, measured using 20 μm β-carotene and 500 ng of BCO1 per reaction. Our BCO1 preparation is active even without the addition of Fe2+, and all enzyme incubations are performed without addition of the latter.
FIGURE 1.
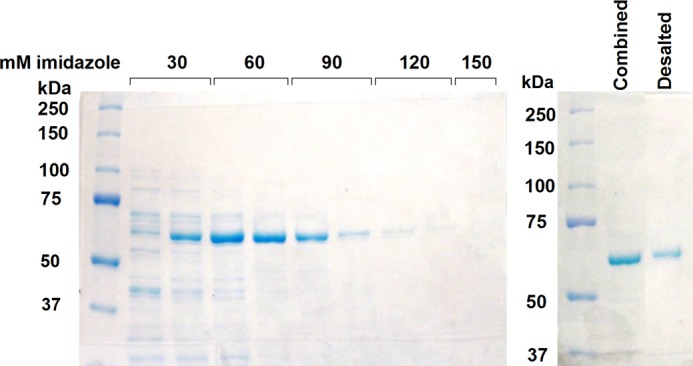
Purification of recombinant human BCO1 using cobalt affinity column chromatography. The SDS-PAGE gel on the left shows the elution of recombinant His-tagged human BCO1 (theoretical molecular mass = 63,702 Da) from the cobalt column by increasing concentrations of imidazole. Only the fractions that show a single band were combined and desalted into the final storage buffer (lanes 5–8). The SDS-PAGE gel on the right shows the combined fractions and the protein obtained after desalting.
The kinetic data for β-carotene is shown in Fig. 2. The production of retinal is linear up to 15 min and 500 ng of enzyme/200 μl reaction (Fig. 2, A and B).
FIGURE 2.
Kinetic data for purified recombinant BCO1 with β-carotene at 37 °C. Each data point represents the difference between the average of duplicate measurements at a given reaction time and the average of duplicate time zero controls. A, time course using 5 μm β-carotene incubated with 250 ng of BCO1. B, protein curve using 20 μm β-carotene and 15 min of reaction time. C, plot of reaction velocity as a function of β-carotene concentration using 500 ng of BCO1/200 μl reaction and 15 min of reaction time. The red trace is the best-fitting Michaelis-Menten curve generated by GraphPad Prism (version 4). Three independent substrate-velocity plots were generated to calculate the average kinetic parameters stated in Fig. 7.
Purified Recombinant Human BCO1 Cleaves β-Carotene Solely at the Central Bond
We found trace amounts of β-apocarotenals in the β-carotene-BCO1 reaction mixtures. We first conducted experiments to see whether BCO1 catalyzed cleavage of β-carotene at positions other than the 15–15′ bond. However, there was no evidence of enzyme-dependent formation of these β-apocarotenals (Fig. 3). Even at the higher protein concentrations tested when making the BCO1 protein curve (Fig. 2B), there was still no evidence that β-apocarotenals are formed enzymatically. Thus, BCO1 cleaves solely at the central 15–15′ bond.
FIGURE 3.

Purified recombinant BCO1 cleaves β-carotene solely at the central 15–15′ bond. A, chromatograms from the reaction mixture of β-carotene (20 μm) and BCO1 (500 ng/200 μl reaction) at 37 °C. Blue trace, 15 min reaction; red trace, time zero control. The product peak has the same retention time and UV-visible spectrum as standard all-trans-retinal. The following chromatograms are for standards all-trans-retinal (B), β-apo-14′-carotenal (C), β-apo-12′-carotenal (D), β-apo-10′-carotenal (E), and β-apo-8′-carotenal (F). Chromatography was performed using Zorbax Eclipse XDB-C18 LC-Column (4.6 × 50 mm, 1.8 μm, Agilent) and HPLC method A as described under “Experimental Procedures.” Signal was monitored at 380 nm.
Purified Recombinant BCO1 Cleaves β-Apocarotenals to Yield Retinal and Lycopene to Yield Acycloretinal
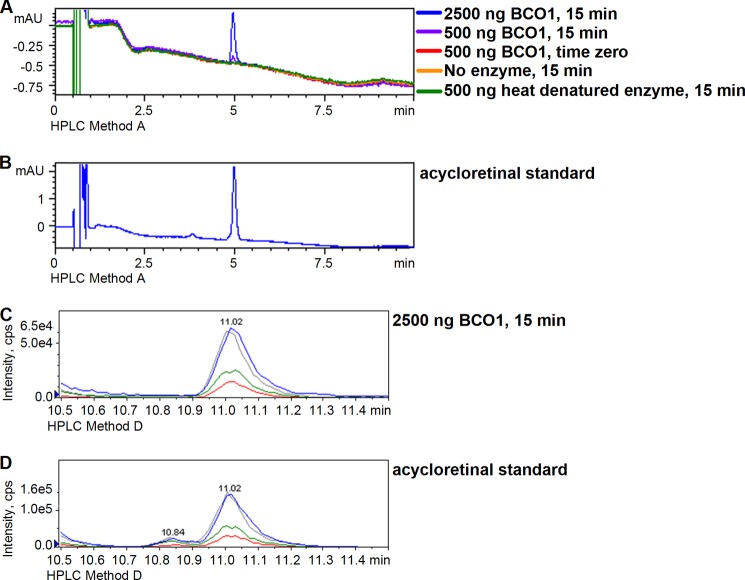
Six major dietary carotenoids (β-carotene, lycopene, α-carotene, β-cryptoxanthin, zeaxanthin, and lutein) as well as β-apocarotenals (β-apo-8′-carotenal, β-apo-10′-carotenal, β-apo-12′-carotenal, and β-apo-14′-carotenal) were tested for activity with BCO1, comparing peak areas from 15-min reactions with time zero, boiled enzyme, and no enzyme controls. We detected enzymatic cleavage activity with all of the test substrates except for zeaxanthin and lutein. For the asymmetrical provitamin A carotenoids α-carotene and β-cryptoxanthin, two product peaks were detected, one corresponding to all-trans-retinal, and the other corresponding to the non-retinal half of the molecule. However, we only quantified the amount of all-trans-retinal produced in the reaction. For lycopene, acycloretinal was detected as product (Fig. 4), and synthetic acycloretinal was used to generate standard curves for quantification.
FIGURE 4.
A, sample chromatograms for reaction mixtures of BCO1 (200-μl reaction) with lycopene (2.2 μm) at 37 °C. The chromatograms shown are for the reactions with 2500 ng of BCO1 at 15 min (blue), 500 ng of BCO1 at 15 min (violet), 500 ng of BCO1 at time zero (red), no enzyme at 15 min (orange) and 500 ng of heat-denatured BCO1 at 15 min (green). B, chromatogram for acycloretinal standard. Chromatography was performed using Zorbax Eclipse XDB-C18 LC column (4.6 × 50 mm, 1.8 μm, Agilent) and HPLC method A as described under “Experimental Procedures.” Signal was monitored at 380 nm. The acycloretinal product of the reaction of lycopene and BCO1 was confirmed by multiple reaction monitoring (MRM). Multiple reaction monitoring chromatograms are shown for the reaction of 2.2 μm lycopene with 2500 ng of BCO1/200 μl at 15 min and 37 °C (C) and the acycloretinal standard (D). The chromatography was performed using HPLC method D, and MS/MS conditions are as described under “Experimental Procedures.” Four transitions were monitored: m/z 285 > 69 (blue), 119 (red), and 135 (green), and 161 (gray). The matching retention times and relative intensities of the transitions confirm the identity of the product as acycloretinal. mAU, milli-absorbance units; cps, counts/s.
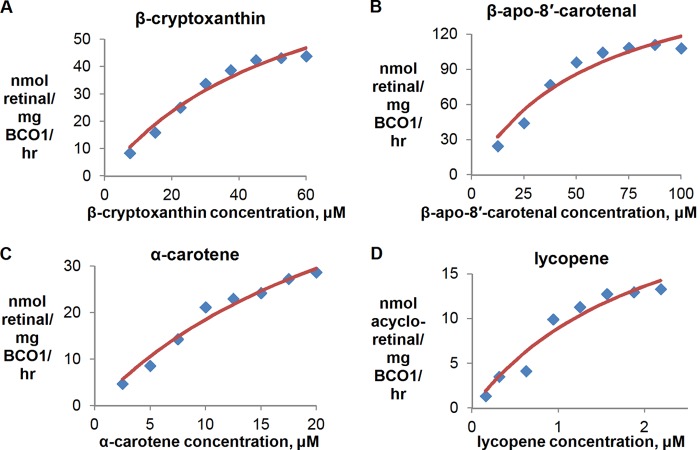
Time courses were run for all other substrates using a 500 ng of BCO1/200 μl reaction, and the production of retinal (or acyloretinal in the case of lycopene) was linear within the first 15–25 min (data not shown). The dependence of reaction velocity on substrate concentration is shown in Figs. 2C, 5, and 6. The carotenoids and β-apocarotenals vary in solubility, and this affects the range of concentrations that can be tested.
FIGURE 5.
Substrate-velocity plots for other substrates that display Michaelis-Menten behavior. The reactions were performed using 500 ng of BCO1/200 μl reaction and 15 min of reaction time at 37 °C. Each data point represents the difference between the average of duplicate measurements at a given reaction time and the average of duplicate time zero controls. The red traces are the best-fitting Michaelis-Menten curves generated by GraphPad Prism (version 4). Three independent substrate-velocity plots were generated to calculate the kinetic parameters stated in Fig. 7.
FIGURE 6.

Substrate-velocity plots for β-apocarotenals. The reactions were performed using 500 ng of BCO1/200 μl reaction and 15 min of reaction time at 37 °C. Each data point represents the average of three independent experiments. Only β-apo-8′-carotenal displays Michaelis-Menten kinetics (shown in Fig. 5) in the assay conditions used.
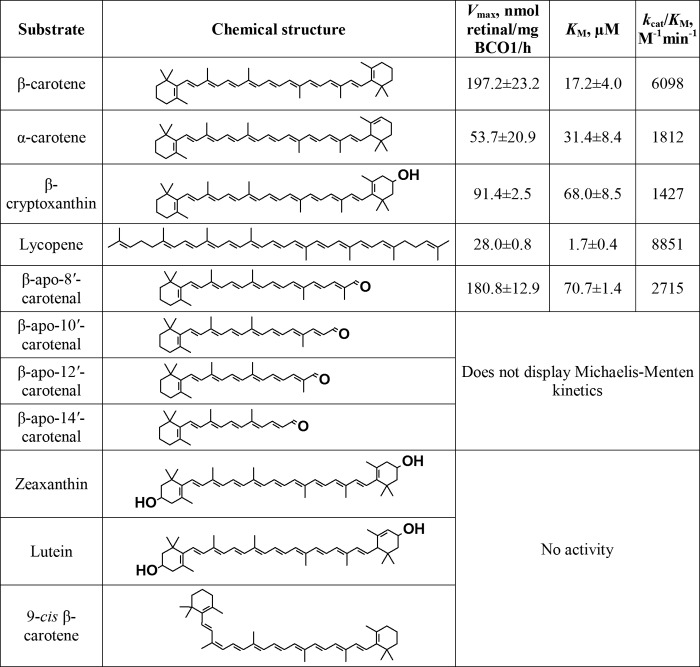
The kinetic data for all substrates were tested, and their values relative to β-carotene are shown in Fig. 7. With the exception of β-apo-8′-carotenal, the substrate curves for the β-apocarotenals do not fit well with the Michaelis-Menten equation (Fig. 6).
FIGURE 7.
Kinetic data for purified recombinant BCO1 and various carotenoids and apocarotenals. Results are calculated from three independent experiments. The Vmax and Km values shown are the means ± S.D.
The kinetic data with the full-length carotenoids suggest that a β-ionone ring conjugated to the rest of the polyene chain is essential for activity. Hydroxylation of the ring (as in β-cryptoxanthin) or loss of conjugation between the ring and the rest of the polyene chain (as in α-carotene) result in a decrease in catalytic activity. Hydroxylation of both rings (as in zeaxanthin and lutein) results in a loss of activity. Surprisingly, lycopene is cleaved by BCO1 at a catalytic efficiency similar to β-carotene. Although the Vmax of lycopene is low, the Km is also low, thus resulting in a relatively high kcat/Km value.
Purified Recombinant Human BCO1 Does Not Cleave 9-cis-β-Carotene
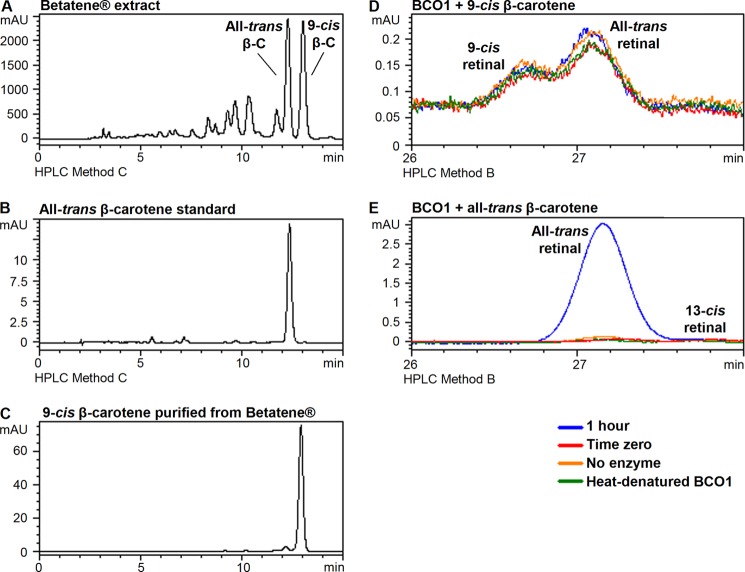
Fig. 8, A–C, shows the chromatograms for the Betatene® hexane extract, all-trans-β-carotene standard, and the purified 9-cis-β-carotene. We incubated 9-cis-β-carotene (20 μm) with BCO1 under standard conditions (500 ng/200 μl reaction, 15 min reaction time) but did not detect any difference between the amount of retinals in the 15-min reaction and time zero control (data not shown). We then incubated 9-cis-β-carotene (15 μm) with 2500 ng of BCO1 and 60 min of reaction time (Fig. 8D). There were trace amounts of 9-cis and all-trans-retinal detected in the reaction mixture, but their amounts are the same as those detected in the controls (time zero, no enzyme, heat-denatured enzyme). Incubation of all-trans-β-carotene under the same conditions yields a significant amount of retinal compared with the same controls (Fig. 8E). Thus, purified recombinant human BCO1 does not cleave 9-cis-β-carotene.
FIGURE 8.
Purified recombinant human BCO1 does not cleave 9-cis-β-carotene. Chromatograms for Betatene® extract obtained from liquid-liquid extraction with the β-carotene isomer peaks indicated (A), all-trans-β-carotene standard (B), 9-cis-β-carotene obtained using HPLC method C as described under “Experimental Procedures” (C). Signal was monitored at 450 nm. The test carotenoids (15 μm) were incubated with 2500 ng of purified recombinant human BCO1 for 1 h and analyzed using HPLC method B as described under “Experimental Procedures.” No enzyme-dependent production of retinal was observed for 9-cis-β-carotene (D), in contrast to all-trans-β-carotene (E).
DISCUSSION
Eccentric cleavage products of β-carotene (β-apocarotenoids) have been detected in foods such as melons (32) and peppers (33) and in human plasma (34). They have also been shown to have an effect on cell differentiation and proliferation (35, 36) and to modulate the activity of nuclear receptors (28, 37, 38). Thus, there is great interest in their origins and metabolism. Carotenoids are susceptible to non-enzymatic oxidation that yields β-apocarotenoids, but the assay system that we adapted from During et al. (13) has two features to avoid this. First, an antioxidant, α-tocopherol, is used, and this has been shown to reduce the occurrence of eccentric cleavage products in incubations of β-carotene with tissue homogenates (25). Second, the assay is much shorter because there is no liquid-liquid extraction of reaction products, evaporation of solvent, and reconstitution with an organic solvent. A mixture of acetonitrile and isopropyl alcohol is added to the aqueous reaction mixture, and the resulting solution is filtered and injected into the HPLC. This makes processing even faster than the original method, which calls for centrifugation for 10 min after addition of organic solvent. Filtration is made easier because of the much lower amounts of purified enzyme used (250–2500 ng) compared with the amounts of protein used in tissue homogenate incubations (0.5–1 mg) (13, 21). Our HPLC method was also able to separate the four β-apocarotenals that could be potentially produced by eccentric cleavage (Fig. 3). Thus, we easily identified the peaks in our chromatograms, compared the amounts present in the reaction mixture with three controls (time zero, no enzyme, and heat-denatured enzyme), and determined that purified recombinant human BCO1 does not produce β-apocarotenals, even with increased enzyme concentrations.
We also show that the β-apocarotenals of 22 carbons or greater are substrates for BCO1. The β-apocarotenals are more water-soluble than full-length carotenoids, which is why we were able to test higher concentrations for constructing substrate-reaction velocity curves. However, we did not determine the loading capacity of the Tween 40 micelles used in the assay. It is possible that at higher concentrations, some of the β-apocarotenal substrate molecules are in micelles, whereas the others are dissolved in water and thus introduces variability in the way the substrate is presented to the enzyme. This could explain why the substrate-reaction velocity curves for β-apocarotenals 27 carbons or shorter do not fit well with the Michaelis-Menten equation (Fig. 6).
The experiments reported here show that purified recombinant human BCO1 does not cleave 9-cis-β-carotene. Again, the shorter processing time of our assay and use of amber lights minimized non-enzymatic isomerization, and the use of purified protein and three controls make the interpretation of results straightforward. We expected some activity based on previous studies where 9-cis-β-carotene was incubated with supernatants of rat liver and intestine homogenates (21) and the extract from E. coli expressing recombinant murine BCO1 (31). Also, the incubation of 9-cis-β-carotene with the post-nuclear fraction from human intestinal mucosa was reported to yield 9-cis-retinoic acid, suggesting that 9-cis-β-carotene was cleaved, producing 9-cis-retinal, which was then oxidized to the acid (22). It is possible that because we are using purified enzyme, we are missing cofactors/coenzymes present in crude enzyme preparations, and we cannot discount the possibility that BCO1 might still cleave 9-cis-β-carotene in vivo. On the other hand, results with tissue homogenates should also be interpreted with caution, as the substrate might be acted on by enzymes other than BCO1.
It has been reported that intraperitoneal administration of 9-cis-β-carotene does not increase the amount of 9-cis-retinoids measured in the eyes and livers of WT and BCO1 knock-out mice (31). Several human studies have shown that oral 9-cis-β-carotene is absorbed poorly or not at all (19, 29, 39, 40). These studies suggest that 9-cis-β-carotene is poorly metabolized by humans, and our results are consistent with this picture. This calls into question the value of supplementation with Betatene® or other similar Dunaliella extracts, especially in the context of vitamin A supplementation. Furthermore, the inactivity of 9-cis-β-carotene with human BCO1 also suggests that the increase of cis isomers of β-carotene during food processing decreases its provitamin A value.
The discovery of 9-cis-retinoic acid as a ligand for retinoid X receptors (41, 42) has, of course, led to the question of its biosynthesis. The central cleavage of 9-cis-β-carotene to produce 9-cis-retinal and subsequent oxidation to 9-cis-retinoic acid has been proposed as a possible mechanism (43). However, our results argue against this, and it is more likely that the all-trans-retinoic acid/retinol that originated from all-trans-β-carotene is isomerized to the 9-cis form. It has been reported that all-trans-retinoic acid can be isomerized to 9-cis-retinoic acid by thiolate radicals (44, 45). Another study suggests that an isomerase converts all-trans-retinol to 9-cis-retinol (46).
Our results with the major dietary carotenoids are largely in agreement with other in vitro studies that used recombinant BCO1, with the notable exceptions of our detection of activity with lycopene and β-apocarotenoids shorter than β-apo-10′-carotenal (1, 7). During our initial attempts to generate a lycopene substrate curve, we used the same concentration range as β-carotene (2.5–20 μm). We observed the highest activity for the lowest substrate concentration, and above 10 μm, the substrate solutions were cloudy, meaning lycopene was not successfully micellarized. Thus, we decided to expand the low concentration range from 0.3–2.5 μm, and generated a substrate curve that shows maximum activity at 2.2 μm. It is possible that other in vitro studies were not able to detect activity with lycopene because they used relatively high concentrations that favored precipitation. We also found that exposure of the substrate solution to low temperatures at any point during the substrate preparation leads to a cloudy mixture. Examples of such conditions include evaporative cooling during removal of the organic solvent, and storage of the substrate solution in ice. Thus, in order to maximize activity as well as minimize non-enzymatic isomerization and oxidation, the substrate should be prepared just before use and be kept no lower than room temperature (∼22 °C) until used.
The cleavage of lycopene to produce acycloretinal suggests that it is possible to obtain acycloretinol and/or acycloretinoic acid in vivo. Acycloretinoic acid has been shown to induce apoptosis in human prostate cancer cell lines (47), activate the DR-5 retinoic acid response element in MCF-7 cells (Michigan Cancer Foundation-7 (a human mammary cancer cell line) (48), and stimulate gap junction communication (49). However, these studies show that supraphysiological concentrations are necessary for activity (47) or that acycloretinoic acid is less effective than retinoic acid (48) or lycopene (49).
However, acycloretinal and its alcohol and acid forms have not been detected in vivo (50–53). It is possible that very small amounts of acycloretinal are generated in vivo because of the low solubility of lycopene, an echo of our experience with the in vitro assays. Furthermore, the substrate that we used is 92% all-trans-lycopene, whereas a significant proportion (25–70%) of the lycopene in human body fluids and tissues are various cis isomers (54). Human BCO1 might cleave these cis isomers less efficiently or not at all.
Our results with lycopene show that the presence of an unmodified β-ionone ring is not crucial for activity with BCO1. For β-carotene and lycopene, there is a conjugated system starting from the 5-carbon to the 5′-carbon. In α-carotene, which is cleaved less efficiently than β-carotene and lycopene, the conjugated system is disrupted. Apparently, it is the structure of the conjugated system rather than the ring itself that is important for activity of BCO1 with the molecule. As of the time of writing, there is no crystal structure available for BCO1. There is only one structure of a retinal-forming carotenoid oxygenase known, that from the cyanobacterium Synechocystis sp. PCC 6803 (55). In this structure, the pocket where the carotenoid is held is lined with numerous nonpolar residues, mainly with aromatic side chains. It is thus possible that the conjugated system in the carotenoid forms π-π interactions with these side chains, and an alteration of the conjugated system, as in α-carotene, interferes with the binding. This is supported by the higher Km observed for α-carotene. Also, the α-ionone ring is free to rotate about the 6–7 bond, unlike the β-ionone ring. The loss of the planarity in this part of the molecule may also contribute to the lower binding observed. Hydroxylation of the ring significantly also affects the activity of BCO1 with the molecule, as shown by the lower activity with β-cryptoxanthin, and lack of activity with zeaxanthin and lutein. This might be due to unfavorable interaction of the hydroxyl group with the nonpolar residues in the substrate binding pocket.
As shown by this work and earlier studies, β-carotene is still the most effective provitamin A carotenoid in the context of BCO1 cleavage. However, there are other factors that will affect the effectiveness of provitamin A carotenoid to be converted into vitamin A in vivo. The molecular structure is only one of the many factors that influence the bioavailability and bioconversion of carotenoids (30, 56). Nevertheless, an in vitro substrate specificity study using purified enzyme is an important component of interpreting what is observed in vivo.
In summary, human BCO1 is, with the notable exception of its ability to cleave lycopene, mostly a provitamin A carotenoid oxygenase. It catalyzes the oxidative cleavage of major dietary provitamin A carotenoids and β-apocarotenals solely at the 15–15′ bond to produce retinal. Lutein and zeaxanthin, which will not yield retinal with central cleavage, do not react with BCO1. BCO1 does not catalyze the cleavage of 9-cis-β-carotene. The production of acycloretinal from lycopene by BCO1 is contrary to the majority of previous reports. This warrants a fresh look at acycloretinal and its alcohol and acid forms as metabolites of lycopene in future studies.
Acknowledgments
We thank Dr. William Blaner of Columbia University for the hBCO1-pET-28b plasmid and Morgan Cichon for the lycopene standard.
This work was supported by National Institutes of Health Grants R01-HL49879 and R01-DK44498 and a grant from the Ohio Agricultural Research Development Center.
- BCO1
- β-carotene 15,15′-oxygenase.
REFERENCES
- 1. Lindqvist A., Andersson S. (2002) Biochemical properties of purified recombinant human β-carotene 15,15′-monooxygenase. J. Biol. Chem. 277, 23942–23948 [DOI] [PubMed] [Google Scholar]
- 2. Paik J., During A., Harrison E. H., Mendelsohn C. L., Lai K., Blaner W. S. (2001) Expression and characterization of a murine enzyme able to cleave β-carotene. The formation of retinoids. J. Biol. Chem. 276, 32160–32168 [DOI] [PubMed] [Google Scholar]
- 3. Park C. S., Lee S. W., Kim Y. S., Kim E. J., Sin H. S., Oh D. K., Kim S. W., Um S. J. (2008) Utilization of the recombinant human β-carotene-15,15′-monooxygenase gene in Escherichia coli and mammalian cells. Biotechnol. Lett. 30, 735–741 [DOI] [PubMed] [Google Scholar]
- 4. Redmond T. M., Gentleman S., Duncan T., Yu S., Wiggert B., Gantt E., Cunningham F. X., Jr. (2001) Identification, expression, and substrate specificity of a mammalian β-carotene 15,15′-dioxygenase. J. Biol. Chem. 276, 6560–6565 [DOI] [PubMed] [Google Scholar]
- 5. Wyss A., Wirtz G., Woggon W., Brugger R., Wyss M., Friedlein A., Bachmann H., Hunziker W. (2000) Cloning and expression of β,β-carotene 15,15′-dioxygenase. Biochem. Biophys. Res. Commun. 271, 334–336 [DOI] [PubMed] [Google Scholar]
- 6. Lampert J. M., Holzschuh J., Hessel S., Driever W., Vogt K., von Lintig J. (2003) Provitamin A conversion to retinal via the β,β-carotene-15,15′-oxygenase (bcox) is essential for pattern formation and differentiation during zebrafish embryogenesis. Development 130, 2173–2186 [DOI] [PubMed] [Google Scholar]
- 7. Kim Y. S., Oh D. K. (2009) Substrate specificity of a recombinant chicken β-carotene 15,15′-monooxygenase that converts β-carotene into retinal. Biotechnol. Lett. 31, 403–408 [DOI] [PubMed] [Google Scholar]
- 8. Goodman D. S., Huang H. S. (1965) Biosynthesis of Vitamin A with Rat Intestinal Enzymes. Science 149, 879–880 [DOI] [PubMed] [Google Scholar]
- 9. Olson J. A., Hayaishi O. (1965) The enzymatic cleavage of β-carotene into vitamin A by soluble enzymes of rat liver and intestine. Proc. Natl. Acad. Sci. U.S.A. 54, 1364–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fidge N. H., Smith F. R., Goodman D. S. (1969) Vitamin A and carotenoids. The enzymic conversion of β-carotene into retinal in hog intestinal mucosa. Biochem. J. 114, 689–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang X. D., Tang G. W., Fox J. G., Krinsky N. I., Russell R. M. (1991) Enzymatic conversion of β-carotene into β-apo-carotenals and retinoids by human, monkey, ferret, and rat tissues. Arch. Biochem. Biophys. 285, 8–16 [DOI] [PubMed] [Google Scholar]
- 12. Devery J., Milborrow B. V. (1994) β-Carotene-15,15′-dioxygenase (EC 1.13.11.21) isolation reaction mechanism and an improved assay procedure. Br. J. Nutr. 72, 397–414 [DOI] [PubMed] [Google Scholar]
- 13. During A., Nagao A., Hoshino C., Terao J. (1996) Assay of β-carotene 15,15′-dioxygenase activity by reverse-phase high-pressure liquid chromatography. Anal. Biochem. 241, 199–205 [DOI] [PubMed] [Google Scholar]
- 14. Grolier P., Duszka C., Borel P., Alexandre-Gouabau M. C., Azais-Braesco V. (1997) In vitro and in vivo inhibition of β-carotene dioxygenase activity by canthaxanthin in rat intestine. Arch. Biochem. Biophys. 348, 233–238 [DOI] [PubMed] [Google Scholar]
- 15. Lakshmanan M. R., Chansang H., Olson J. A. (1972) Purification and properties of carotene 15,15′-dioxygenase of rabbit intestine. J. Lipid Res. 13, 477–482 [PubMed] [Google Scholar]
- 16. Lakshmanan M. R., Pope J. L., Olson J. A. (1968) The specificity of a partially purified carotenoid cleavage enzyme of rabbit intestine. Biochem. Biophys. Res. Commun. 33, 347–352 [DOI] [PubMed] [Google Scholar]
- 17. Wirtz G. M., Bornemann C., Giger A., Müller R. K., Schneider H., Schlotterbeck G., Schiefer G., Woggon W. (2001) The Substrate Specificity of β,β-Carotene 15,15′-Monooxygenase. Helv. Chim. Acta 84, 2301–2315 [Google Scholar]
- 18. Chandler L. A., Schwartz S. J. (1987) HPLC separation of cis-trans carotene isomers in fresh and processed fruits and vegetables. J. Food Sci. 52, 669–672 [Google Scholar]
- 19. Gaziano J. M., Johnson E. J., Russell R. M., Manson J. E., Stampfer M. J., Ridker P. M., Frei B., Hennekens C. H., Krinsky N. I. (1995) Discrimination in absorption or transport of β-carotene isomers after oral supplementation with either all-trans- or 9-cis-β-carotene. Am. J. Clin. Nutr. 61, 1248–1252 [DOI] [PubMed] [Google Scholar]
- 20. Krinsky N. I., Russett M. D., Handelman G. J., Snodderly D. M. (1990) Structural and geometrical isomers of carotenoids in human plasma. J. Nutr. 120, 1654–1662 [DOI] [PubMed] [Google Scholar]
- 21. Nagao A., Olson J. A. (1994) Enzymatic formation of 9-cis, 13-cis, and all-trans retinals from isomers of β-carotene. FASEB J. 8, 968–973 [DOI] [PubMed] [Google Scholar]
- 22. Wang X. D., Krinsky N. I., Benotti P. N., Russell R. M. (1994) Biosynthesis of 9-cis retinoic acid from 9-cis β-carotene in human intestinal mucosa in vitro. Arch. Biochem. Biophys. 313, 150–155 [DOI] [PubMed] [Google Scholar]
- 23. Hansen S., Maret W. (1988) Retinal is not formed in vitro by enzymatic central cleavage of β-carotene. Biochemistry 27, 200–206 [DOI] [PubMed] [Google Scholar]
- 24. Tang G. W., Wang X. D., Russell R. M., Krinsky N. I. (1991) Characterization of β-apo-13-carotenone and β-apo-14′-carotenal as enzymatic products of the excentric cleavage of β-carotene. Biochemistry 30, 9829–9834 [DOI] [PubMed] [Google Scholar]
- 25. Yeum K. J., dos Anjos Ferreira A. L., Smith D., Krinsky N. I., Russell R. M. (2000) The effect of α-tocopherol on the oxidative cleavage of β-carotene. Free Radic Biol. Med. 29, 105–114 [DOI] [PubMed] [Google Scholar]
- 26. von Lintig J., Vogt K. (2000) Filling the gap in vitamin A research. Molecular identification of an enzyme cleaving β-carotene to retinal. J. Biol. Chem. 275, 11915–11920 [DOI] [PubMed] [Google Scholar]
- 27. Yan W., Jang G. F., Haeseleer F., Esumi N., Chang J., Kerrigan M., Campochiaro M., Campochiaro P., Palczewski K., Zack D. J. (2001) Cloning and characterization of a human β, β-carotene-15,15′-dioxygenase that is highly expressed in the retinal pigment epithelium. Genomics 72, 193–202 [DOI] [PubMed] [Google Scholar]
- 28. Eroglu A., Hruszkewycz D. P., dela Sena C., Narayanasamy S., Riedl K. M., Kopec R. E., Schwartz S. J., Curley R. W., Jr., Harrison E. H. (2012) Naturally occurring eccentric cleavage products of provitamin A β-carotene function as antagonists of retinoic acid receptors. J. Biol. Chem. 287, 15886–15895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stahl W., Schwarz W., Sies H. (1993) Human serum concentrations of all-trans β- and α-carotene but not 9-cis β-carotene increase upon ingestion of a natural isomer mixture obtained from Dunaliella salina (Betatene). J. Nutr. 123, 847–851 [DOI] [PubMed] [Google Scholar]
- 30. de Pee S., West C. E. (1996) Dietary carotenoids and their role in combating vitamin A deficiency: a review of the literature. Eur. J. Clin. Nutr. 50, S38–S53 [PubMed] [Google Scholar]
- 31. Maeda T., Perusek L., Amengual J., Babino D., Palczewski K., von Lintig J. (2011) Dietary 9-cis-β, β-carotene fails to rescue vision in mouse models of leber congenital amaurosis. Mol. Pharmacol. 80, 943–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fleshman M. K., Lester G. E., Riedl K. M., Kopec R. E., Narayanasamy S., Curley R. W., Jr., Schwartz S. J., Harrison E. H. (2011) Carotene and novel apocarotenoid concentrations in orange-fleshed Cucumis melo melons: determinations of β-carotene bioaccessibility and bioavailability. J. Agric. Food Chem. 59, 4448–4454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mínguez-Mosquera M. I., Hornero-Méndez D., Garrido-Fernández J. (1995) Detection of bixin, lycopene, canthaxanthin, and β-apo-8′-carotenal in products derived from red pepper. J. AOAC Int. 78, 491–496 [PubMed] [Google Scholar]
- 34. Ho C. C., de Moura F. F., Kim S. H., Clifford A. J. (2007) Excentral cleavage of β-carotene in vivo in a healthy man. Am. J. Clin. Nutr. 85, 770–777 [DOI] [PubMed] [Google Scholar]
- 35. Suzuki T., Matsui M., Murayama A. (1995) Biological activity of (all-E)-β-apo-12′-carotenoic acid and the geometrical isomers on human acute promyelocytic leukemia cell line HL-60. J. Nutr. Sci. Vitaminol. 41, 575–585 [DOI] [PubMed] [Google Scholar]
- 36. Tibaduiza E. C., Fleet J. C., Russell R. M., Krinsky N. I. (2002) Excentric cleavage products of β-carotene inhibit estrogen receptor positive and negative breast tumor cell growth in vitro and inhibit activator protein-1-mediated transcriptional activation. J. Nutr. 132, 1368–1375 [DOI] [PubMed] [Google Scholar]
- 37. Ziouzenkova O., Orasanu G., Sukhova G., Lau E., Berger J. P., Tang G., Krinsky N. I., Dolnikowski G. G., Plutzky J. (2007) Asymmetric cleavage of β-carotene yields a transcriptional repressor of retinoid X receptor and peroxisome proliferator-activated receptor responses. Mol. Endocrinol. 21, 77–88 [DOI] [PubMed] [Google Scholar]
- 38. Eroglu A., Hruszkewycz D. P., Curley R. W., Jr., Harrison E. H. (2010) The eccentric cleavage product of β-carotene, β-apo-13-carotenone, functions as an antagonist of RXRα. Arch. Biochem. Biophys. 504, 11–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ben-Amotz A., Levy Y. (1996) Bioavailability of a natural isomer mixture compared with synthetic all-trans β-carotene in human serum. Am. J. Clin. Nutr. 63, 729–734 [DOI] [PubMed] [Google Scholar]
- 40. You C. S., Parker R. S., Goodman K. J., Swanson J. E., Corso T. N. (1996) Evidence of cis-trans isomerization of 9-cis-β-carotene during absorption in humans. Am. J. Clin. Nutr. 64, 177–183 [DOI] [PubMed] [Google Scholar]
- 41. Heyman R. A., Mangelsdorf D. J., Dyck J. A., Stein R. B., Eichele G., Evans R. M., Thaller C. (1992) 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell 68, 397–406 [DOI] [PubMed] [Google Scholar]
- 42. Levin A. A., Sturzenbecker L. J., Kazmer S., Bosakowski T., Huselton C., Allenby G., Speck J., Ratzeisen C., Rosenberger M., Lovey A., Grippo J. F. (1992) 9-Cis retinoic acid stereoisomer binds and activates the nuclear receptor RXRα. Nature 355, 359–361 [DOI] [PubMed] [Google Scholar]
- 43. Kane M. A. (2012) Analysis, occurrence, and function of 9-cis retinoic acid. Biochim. Biophys. Acta 1821, 10–20 [DOI] [PubMed] [Google Scholar]
- 44. Urbach J., Rando R. R. (1994) Thiol dependent isomerization of all-trans retinoic acid to 9-cis retinoic acid. FEBS Lett. 351, 429–432 [DOI] [PubMed] [Google Scholar]
- 45. Urbach J., Rando R. R. (1994) Isomerization of all-trans retinoic acid to 9-cis retinoic acid. Biochem. J. 299, 459–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lidén M., Eriksson U. (2005) Development of a versatile reporter assay for studies of retinol uptake and metabolism in vivo. Exp. Cell Res. 310, 401–408 [DOI] [PubMed] [Google Scholar]
- 47. Kotake-Nara E., Kim S. J., Kobori M., Miyashita K., Nagao A. (2002) Acyclo-retinoic acid induces apoptosis in human prostate cancer cells. Anticancer Res. 22, 689–695 [PubMed] [Google Scholar]
- 48. Ben-Dor A., Nahum A., Danilenko M., Giat Y., Stahl W., Martin H. D., Emmerich T., Noy N., Levy J., Sharoni Y. (2001) Effects of acyclo-retinoic acid and lycopene on activation of the retinoic acid receptor and proliferation of mammary cancer cells. Arch. Biochem. Biophys. 391, 295–302 [DOI] [PubMed] [Google Scholar]
- 49. Stahl W., von Laar J., Martin H. D., Emmerich T., Sies H. (2000) Stimulation of gap junctional communication: comparison of acyclo-retinoic acid and lycopene. Arch. Biochem. Biophys. 373, 271–274 [DOI] [PubMed] [Google Scholar]
- 50. Kopec R. E., Riedl K. M., Harrison E. H., Curley R. W., Jr., Hruszkewycz D. P., Clinton S. K., Schwartz S. J. (2010) Identification and quantification of apo-lycopenals in fruits, vegetables, and human plasma. J. Agric. Food Chem. 58, 3290–3296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gajic M., Zaripheh S., Sun F., Erdman J. W., Jr. (2006) Apo-8′-lycopenal and apo-12′-lycopenal are metabolic products of lycopene in rat liver. J. Nutr. 136, 1552–1557 [DOI] [PubMed] [Google Scholar]
- 52. Khachik F., Beecher G. R., Smith J. C., Jr. (1995) Lutein, lycopene, and their oxidative metabolites in chemoprevention of cancer. J. Cell Biochem. Suppl 22, 236–246 [DOI] [PubMed] [Google Scholar]
- 53. Khachik F., Spangler C. J., Smith J. C., Jr., Canfield L. M., Steck A., Pfander H. (1997) Identification, quantification, and relative concentrations of carotenoids and their metabolites in human milk and serum. Anal. Chem. 69, 1873–1881 [DOI] [PubMed] [Google Scholar]
- 54. Richelle M., Sanchez B., Tavazzi I., Lambelet P., Bortlik K., Williamson G. (2010) Lycopene isomerisation takes place within enterocytes during absorption in human subjects. Br. J. Nutr. 103, 1800–1807 [DOI] [PubMed] [Google Scholar]
- 55. Kloer D. P., Ruch S., Al-Babili S., Beyer P., Schulz G. E. (2005) The structure of a retinal-forming carotenoid oxygenase. Science 308, 267–269 [DOI] [PubMed] [Google Scholar]
- 56. West C. E., Castenmiller J. J. (1998) Quantification of the “SLAMENGHI” factors for carotenoid bioavailability and bioconversion. Int. J. Vitam. Nutr. Res. 68, 371–377 [PubMed] [Google Scholar]