Abstract
Background and Purpose
BDNF, a major neurotrophin and VEGF, an endothelial growth factor have a documented role in neurogenesis, angiogenesis and neuronal survival. In animal experiments they impact infarct size and functional motor recovery after an ischemic brain lesion. We sought to examine the association of serum BDNF and VEGF with the risk of clinical stroke or subclinical vascular brain injury in a community-based sample.
Methods
In 3440 stroke/TIA-free FHS participants (mean age 65±11yrs, 56%W), we related baseline BDNF and logVEGF to risk of incident stroke/TIA. In a subsample with brain MRI and with neuropsychological (NP) tests available (N=1863 and 2104, respectively; mean age 61±9yrs, 55%W, in each) we related baseline BDNF and logVEGF to log-white matter hyperintensity volume (lWMHV) on brain MRI, and to visuospatial memory and executive function tests.
Results
During a median follow-up of 10 years, 193 participants experienced incident stroke/TIA. In multivariable analyses adjusted for age-, sex- and traditional stroke risk factors, lower BDNF and higher logVEGF levels were associated with an increased risk of incident stroke/TIA (HR comparing BDNF Q1 versus Q2–4:1.47, 95%CI:1.09–2.00, p=0.012; and HR/SD increase in logVEGF:1.21, 95%CI:1.04–1.40, p=0.012). Persons with higher BDNF levels had less lWMHV (β±SE=−0.05±0.02; p=0.025), and better visual memory (β±SE=0.18±0.07; p=0.005).
Conclusions
Lower serum BDNF and higher VEGF concentrations were associated with increased risk of incident stroke/TIA. Higher levels of BDNF were also associated with less white matter hyperintensity and better visual memory. Our findings suggest that circulating BDNF and VEGF levels modify risk of clinical and subclinical vascular brain injury.
Keywords: BDNF, VEGF, Risk, Stroke, Brain MRI, Subclinical
INTRODUCTION
Cerebrovascular disease (overt and covert) accounts for a significant proportion of brain aging-related mortality and morbidity.1, 2 Whereas the burden of cerebrovascular disease may be largely attributed to well established stroke risk factors, genetic and lifestyle differences and structural and functional brain capacities (such as neural density, synaptic connectivity, and the compensatory ability of existing neural networks) may modulate risk and response to vascular brain injury.3, 4 Therefore, searching for novel pathways involved in cerebrovascular disease may improve our understanding of disease mechanisms and help facilitate discovery of new molecular targets for stroke therapy and prevention. Brain derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF), both present during early neuronal development, have also been found to have a role in maintaining neuronal plasticity and in neuronal repair by promoting neurogenesis and angiogenesis.5, 6
Low circulating BDNF concentrations have been observed in patients with coronary artery disease (CAD), type 2 DM, metabolic syndrome, acute coronary syndrome 7 and physical inactivity,8 and higher VEGF concentrations in patients with hypertension, type 2 DM, smoking, obesity, and CAD9, 10, all risk factors associated with stroke.11 Interestingly, under hypoxic and ischemic conditions, both BDNF and VEGF and their respective receptors are upregulated in neurons and vascular endothelial cells (ECs).12, 13
To our knowledge, there has to date been no prospective or clinical study that has systematically examined the role of BDNF and VEGF in determining the risk of clinical stroke or subclinical vascular brain injury in a community based population. We proposed to examine an association between serum BDNF and VEGF levels and i) risk of stroke/TIA, ii) brain MRI measures of subclinical vascular injury (WMHV) and iii) performance on executive function and visual memory tasks; the cognitive domains previously associated with vascular risk factors and WMHV.14
METHODS
Study Sample
The Framingham Study is a prospective, community-based cohort that enrolled 5209 participants in 1948; these participants have had biennial re-examinations.15 In 1971, the offspring of the Original cohort and their spouses were enrolled in an Offspring cohort, assessed once every four years.16 The participants from both cohorts have been under ongoing surveillance for incident stroke and TIA since the inception of the study.
Stroke- and TIA- free participants in the Original cohort who attended examination 23 [n=581, 80±4yrs, 64%Women(W)], and Offspring participants who attended examination 7 (n=2859, 62±9yrs, 54%W) were included if they also had available BDNF and VEGF levels with no missing covariate data (Study Sample 1; n=3,440); only 88 persons (3%) were excluded from Study sample 1 for missing covariate data.
Offspring participants who attended examination 7 were also invited to undergo cognitive testing and brain MRI (1999–2005). Among the 3020 persons who attended examination 7 and had available BDNF and VEGF levels, 68 were excluded for missing covariate data. Of the remaining 2952 persons, 2186 had a neuropsychological (NP) battery administered, and 1945 underwent brain MRI. 82 persons were excluded for having stroke, dementia or other neurological illness (such as multiple sclerosis, severe head trauma or brain tumor) that could affect MRI/NP measures; we did not exclude persons with TIA in this analysis. The rest were eligible for investigation of the association between BDNF and VEGF and a) WMHV and b) executive function and visual memory (Study samples 2a:N=1863, 61±9yrs, 55%W and 2b:N=2104, 61±9yrs, 55%W).
Laboratory Measurements of BDNF and VEGF
Serum BDNF and VEGF concentrations were measured using commercial ELISA assays obtained from R&D Systems (Minneapolis, MN) on previously frozen (stored at −70°C) blood samples drawn in the fasting state from persons who attended the 23rd Original cohort and the 7th Offspring examinations. The intra- and inter-assay coefficients of variation (CVs) for BDNF and VEGF were 4.8 and 7.6%, and for VEGF 3.9 and 8.4%, respectively.
The age and sex-adjusted Pearson correlation coefficient between BDNF and logVEGF was 0.15 (p<0.001) for the Original cohort, 0.23 (p<0.001) for the Offspring cohort, and 0.22 (p<0.001) for both cohorts combined.
Outcome Measures
Stroke/TIA (clinical outcome)
Our protocols for stroke surveillance and for establishing the diagnosis and type of stroke have been previously published.11 Stroke was defined as an acute onset focal neurological deficit of presumed or definite vascular etiology, persisting for more than 24 hours and TIA symptoms lasting for ≤24 hours. When available we also used imaging studies and other laboratory criteria, noninvasive vascular studies, cardiac evaluations for a source of embolus and also information from autopsy studies; these data were also used to categorize strokes as ischemic or hemorrhagic.
Brain Imaging Measures (subclinical outcome)
MRI acquisitions and measurement techniques and inter-rater reliability scores have been previously published.17 In summary, digital MRI information once acquired on a 1 or 1.5 Tesla Siemens Magnetom and transferred to the centralized imaging center at the University of California-Davis Medical center, was analyzed by operators blinded to the participant’s demographics, exposure to stroke risk factors, and BNDF and VEGF levels. The volume of abnormal WMH was determined by using previously described semi-automated methods, adjusted for total cranial volume (TCV) and log-transformed (log-WMHV). Participants whose log-WMHV was more than 1 SD above the age-specific mean were categorized as having extensive WMH volume (ext-WMHV).
Cognitive Testing
Subjects were administered (typically on the same day as their MRI) a half-hour NP battery using standard administration protocols and trained examiners. Details of the tests administered and normative values for the Framingham Original and Offspring cohorts have been previously published.18, 19 In this study we selected cognitive domains previously associated with vascular risk factors and vascular brain injury; visuospatial memory [measured by delayed recall on the visual reproduction test (VR-d) from the Wechsler Memory Scale] and executive function [measured by Trail Making Test B-A]. We transformed Trails B-A using a log-transformation to normalize its distribution and changed its sign so that smaller numbers indicate poorer performance.
Definition of Covariates
We used previously described and validated components of the Framingham Stroke Risk Profile (FSRP),11 as baseline covariates. Educational achievement was defined as: no high-school (HS) degree, HS degree only, or > HS degree (college degree)]. Depressive symptoms were evaluated with the Center for Epidemiological Studies Depression scale (CES-D). 20 The physical activity index (PAI) was calculated as a composite score based on information collected from a structured questionnaire.21 Both CES-D and PAI were log-transformed for analysis.
Statistical Analysis
BDNF and VEGF were analyzed as continuous variables. VEGF values were log-transformed to normalize the skewed distribution of VEGF. We used Cox-proportional hazards models to relate baseline serum BDNF and logVEGF levels (separately) to risk of incident stroke/TIA in Study Sample 1. We first examined the linear effect of a 1-standard deviation (SD) unit increase/decrease in BDNF and logVEGF on risk of stroke/TIA. We also explored threshold effects comparing the risk across quartiles of BDNF and VEGF. We observed a threshold effect with BDNF only. Therefore, we ran threshold models for BDNF using the lowest quartile, Q1, as the reference and also assessed a threshold model comparing risk for persons in Q1 to persons in Q2–4. We performed a similar set of analyses on a Study Sample 1b with stroke alone as the outcome. Using multivariable linear and logistic regression models we examined the cross-sectional associations between BDNF and logVEGF and markers of brain aging (WMHV, and VR-d and Trails B-A transformed as previously described) using Study Samples 2a and 2b. Results are given as the effect of a 1 SD increase (β/SD or OR/SD) or the difference between Q1 and Q2–4 (β/Q1 or OR/SD).
All primary analyses were adjusted for age as a continuous variable and sex (Model A). In Model A, for the MRI and NP analyses we also adjusted for the time interval between baseline (time when BDNF and VEGF were measured) and date of MRI acquisition and/or NP testing; for the NP analyses we also adjusted for education. In Model B, we additionally adjusted for vascular covariates that are components of the FSRP (systolic blood pressure, antihypertensive therapy, diabetes, smoking status, CVD and AF).
In secondary analyses, we ran Model C examining the association of BDNF and VEGF with MRI and NP after adjusting for total plasma homcysteine (tHcy) and total cholesterol which are biomarkers related to these measures in some but not all studies; these data were available at our baseline examination only for the Offspring, hence we could not adjust the stroke/TIA related analyses for these covariates. In Model D, we adjusted for lifestyle factors adding education level, depression status and physical activity index scores (PAI) to Model B.
To examine the incremental utility of BDNF and VEGF over age-, sex- and components of the FSRP in predicting stroke/TIA, we calculated net reclassification improvement (NRI) in 4 different models adding data on whether or not serum BDNF was in the lowest (Q1) quartile, SD units of change in BDNF and in logVEGF and finally when information on both BDNF and VEGF levels was utilized. We used clinically meaningful risk categories of <5%, 8% and >8% 10-year stroke risks,11, 22 defined a priori as risks experienced by the average person, at the baseline age of the current sample, in the presence of zero (low risk), one (intermediate risk), or more than one (high risk) of the stroke risk factors included in the FSRP.23
All analyses were performed using Statistical Analyses System (SAS® Institute Inc).
RESULTS
Study Sample
In Table 1 we present the distribution of clinical and biomarker characteristics for Study Samples 1, 2a and 2b. In supplementary tables (Table I–III) we also provide the distribution of baseline characteristics based on BDNF quartiles (Q1 vs. Q2–4) for the stroke/TIA, the brain MRI and the NP study samples. In the stroke/TIA sample, as compared to those in Q2–Q4, participants in Q1 were significantly older and predominantly women, and had a higher prevalence of atrial fibrillation and lower prevalence of smoking. Similarly, in the brain MRI and the NP samples, participants in the Q1 subset were significantly older and predominantly women, and had a lower prevalence of smoking (significant only in the brain MRI sample); they also had a higher prevalence of diabetes mellitus, but had lower cholesterol levels.
Table 1.
Baseline Characteristics of the Study Samples
| Clinical Characteristics | Incident Stroke/TIA Gen 1 and Gen 2 N=3440 |
Neuropsychological Test Sample Gen 2 N=2104 |
MRI Sample Gen 2 N=1863 |
|---|---|---|---|
| Women,% | 56% | 55% | 55% |
| Age, years* | 65±11 | 61±9 | 61±9 |
| Systolic Blood Pressure, mm Hg* | 130±20 | 126±19 | 126±19 |
| Anti-hypertensive medication, % | 36% | 31% | 30% |
| Current smokers, % | 11% | 11% | 11% |
| Diabetes mellitus, % | 12% | 11% | 12% |
| Prevalent cardiovascular disease, % | 14% | 10% | 9% |
| Atrial fibrillation, % | 4% | 3% | 2% |
| Total cholesterol, mg/dl* | NA | 201±37 | 201±37 |
| Total homocysteine, μmol/L* | NA | 7.8 [6.5,9.5] | 7.8 [6.5,9.5] |
| College Degree | NA | 39% | 39% |
| Depression (CES-D score)** | NA | 3 [1,7] | 3 [1,7] |
| Physical activity index (PAI) | NA | 37 [33,41] | 37 [33,41] |
| Growth Factors range | |||
| BDNF, ng/mL | 23297 [17959,28841] | 23861 [18220,29347] | 23853 [18225,29321] |
| VEGF, ng/mL | 290 [165,454] | 281 [158,440] | 277 [158,432] |
| Brain MRI Measures | |||
| WMHI (Log)* | N/A | N/A | −3 ± 1 |
| Extensive WMH | N/A | N/A | 12% |
| Cognitive Measures | |||
| VRd | N/A | 8±3 | N/A |
| TrB-TrA | N/A | 0.67 [0.45–0.98] | N/A |
mean+SD. Values for BDNF and VEGF, tHcy, CES-D, PAI, and TrB-TrA represent median (25th %ile, 75th %ile)
CES-D=Center for Epidemiological Studies Depression scale
Association of BDNF and VEGF and Stroke/TIA
BDNF and Stroke/TIA (Table 2)
Table 2.
Association of serum BDNF and logVEGF and risk of stroke/TIA
| Case/N | Stroke/TIA (193/3440) | |||
|---|---|---|---|---|
| HR [95% CI] | P | |||
| BDNF | Per SD | Model A | 0.87 [0.75–1.01] | 0.07 |
| Model B | 0.87 [0.75–1.01] | 0.07 | ||
| Q1 | Model A | 1.00 | REF | |
| Q2 | 0.78 [0.55–1.12] | 0.18 | ||
| Q3 | 0.51 [0.34–0.78] | 0.002 | ||
| Q4 | 0.69 [0.47–1.03] | 0.07 | ||
| Q1vsQ2–Q4 | Model A | 1.51 [1.11–2.03] | 0.008 | |
| Model B | 1.47 [1.09–2.00] | 0.012 | ||
| logVEGF | Per SD | Model A | 1.21 [1.05–1.40] | 0.009 |
| Model B | 1.21 [1.04–1.40] | 0.012 | ||
Model A: adjusted for age and sex
Model B: adjusted for systolic blood pressure, prevalent CVD, diabetes mellitus, atrial fibrillation, current smoking
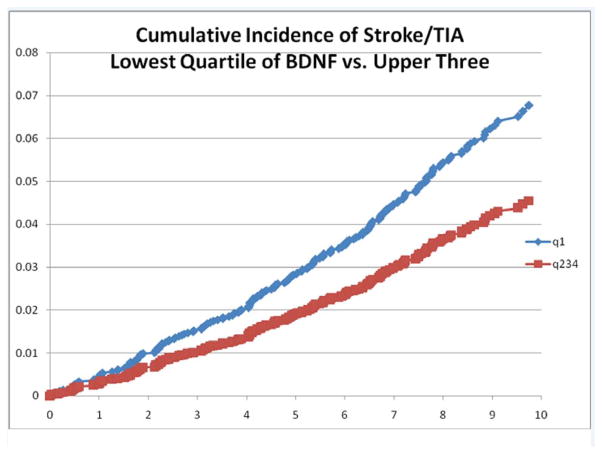
Over the 10 year follow-up period (median 8 years) we observed 193 incident events [130 strokes (including 111 ischemic, 18 hemorrhagic, and one clinically definite stroke of uncertain subtype) and 63 TIAs] in persons initially free of both stroke and TIA. In multivariable age- and sex- adjusted analysis (Model A) and in analysis adjusted for traditional stroke risk factors (Model B) we observed statistically non significant trends towards lower risk of stroke/TIA with higher BDNF levels (Model A: HR per SD increase in BDNF=0.87/SD, p=0.07). In quartile-based analysis adjusted for age and sex, we observed that lower serum BDNF levels were associated with an increased risk of stroke/TIA (HR comparing Q1 to Q2–4=1.51; 95%CI:1.11–2.03, p=0.008); this was also true after adjustment for traditional risk factors (p=0.012). In Figure 1 we show the cumulative incidence of stroke/TIA in Q1 vs. Q2–4. The results relating BDNF to stroke alone are shown in the online Supplementary table 4.
Figure 1.
Kaplan-Meier Curves of the Cumulative Probability of Incident Stroke within BDNF quartiles (Q1 vs Q2–4).
VEGF and Stroke/TIA (Table 2)
In multivariable analyses we observed statistically significant associations between higher serum logVEGF levels and increased risk of stroke/TIA (HR =1.21/SD; 95%CI:1.04–1.40, p=0.012) or stroke alone (HR =1.23/SD; 95%CI:1.05–1.46, p=0.013) as shown in the online Supplementary table 4.
Net Reclassification Improvement (NRI)
In reclassification analyses (supplementary table 5), BDNF and logVEGF alone and the combination of BDNF and logVEGF, when added to a risk assessment model based on traditional stroke risk factors alone (as identified by the FSRP) resulted in significant improvement of risk prediction (NRI for BDNFQ1vsQ2–4 =0.199, 95%CI: 0.023–0.384; NRI for logVEGF=0.234, 95%CI:0.067–0.401, and NRI for BDNF+logVEGF=0.274, 95%CI:0.090–0.449
Association of BDNF and VEGF and White Matter Hyperintensities (Table 3)
Table 3.
Association of BDNF and VEGF and Brain MRI Measures
| Log(WMHIV) | EXT-WMH | |||||
|---|---|---|---|---|---|---|
| β±SE | P | OR [95% CI] | P | |||
| BDNF | Per SD | Model A | −0.04±0.02 | 0.034 | 0.84[0.72–0.96] | 0.014 |
| Model B | −0.05±0.02 | 0.025 | 0.83[0.71–0.95] | 0.009 | ||
| Model C†† | −0.05±0.02 | 0.020 | 0.83[0.72–0.96] | 0.013 | ||
| Model D††† | −0.04±0.02 | 0.042 | 0.83[0.72–0.97] | 0.015 | ||
| Q1vsQ2–Q4 | Model A | 0.09±0.05 | 0.071 | 1.28[0.93–1.76] | 0.132 | |
| Model B | 0.09±0.05 | 0.067 | 1.29[0.93–1.78] | 0.126 | ||
| Model C†† | 0.09±0.05 | 0.060 | 1.26[0.91–1.74] | 0.169 | ||
| Model D††† | 0.09±0.05 | 0.078 | 1.26[0.90–1.77] | 0.171 | ||
| logVEGF | Per SD | Model A | −0.02±0.02 | 0.352 | 0.95[0.83–1.10] | 0.506 |
| Model B | −0.02±0.02 | 0.313 | 0.95[0.82–1.09] | 0.439 | ||
| Model C†† | −0.02±0.02 | 0.313 | 0.95[0.82–1.09] | 0.465 | ||
| Model D††† | −0.02±0.02 | 0.316 | 0.93[0.80–1.08] | 0.341 | ||
Model A: adjusted for age and sex and time from baseline to MRI
Model B: Model A additionally adjusted for SBP, CVD, DM, AF and SMK
Model C: Model B additionally adjusted for total cholesterol and total homocysteine (available only in Offspring; N=1862)
Model D: Model B additionally adjusted for education, PAI and CESD (Offspring only; N=1954)
The median time interval between BDNF and VEGF collection and brain MRI and NP measures was 0.6 years. In our sample, the mean log-WMHV was −3±1 ml and the prevalence of ext-WMHV was 12%. In Table 3 we present results of multivariable analyses relating serum BDNF and VEGF levels individually to the log-WMHV and to the prevalence of ext-WMH on brain MRI respectively. After adjustment for traditional stroke risk factors we observed that higher serum BDNF levels were strongly associated with less log-WMHV and with lower prevalence of ext-WMH (Model B:β=−0.05±0.02, p=0.025 and OR=0.83, p=0.009, respectively). This was also true after adjusting for tHcy and total cholesterol levels (Model C) or education, PAI and CESD (Model D). We did not observe an association between logVEGF and WMH measures.
Association of BDNF and VEGF and Cognitive Measures (Table 4)
Table 4.
Association of BDNF and VEGF and NP Measures
| VRd | Log (TrB-TrA) | |||||
|---|---|---|---|---|---|---|
| β±SE | P | β±SE | P | |||
| BDNF | Per SD | Model A | 0.17±0.07 | 0.010 | −0.001±0.004 | 0.742 |
| Model B | 0.18±0.07 | 0.005 | −0.001±0.004 | 0.831 | ||
| Model C†† | 0.18±0.07 | 0.005 | −0.001±0.004 | 0.810 | ||
| Model D††† | 0.14±0.07 | 0.036 | −0.002±0.004 | 0.631 | ||
| Q1vsQ2–Q4 | Model A | −0.40±0.15 | 0.010 | −0.015±0.010 | 0.156 | |
| Model B | −0.40±0.15 | 0.010 | −0.014±0.010 | 0.165 | ||
| Model C†† | −0.40±0.15 | 0.010 | −0.014±0.010 | 0.165 | ||
| Model D††† | −0.32±0.16 | 0.041 | −0.016±0.010 | 0.118 | ||
| logVEGF | Per SD | Model A | −0.02±0.07 | 0.707 | 0.002±0.004 | 0.635 |
| Model B | −0.01±0.07 | 0.829 | 0.003±0.004 | 0.532 | ||
| Model C†† | −0.01±0.07 | 0.852 | 0.003±0.004 | 0.512 | ||
| Model D††† | −0.01±0.07 | 0.909 | 0.004±0.004 | 0.383 | ||
Model A: adjusted for age and sex, time from baseline to NP, and education
Model B: Model A additionally adjusted for SBP, CVD, DM, AF and SMK
Model C: Model B additionally adjusted for total cholesterol and total homocysteine (available only in Offspring; N=1862)
Model D: Model B additionally adjusted for PAI and CESD (Offspring only; N=1954)
The mean cognitive test scores were 8±3 on VR-d and 0.9±0.9 for Trails B-A. In all models lower serum BDNF levels were associated with poorer visual memory, in both continuous and quartile-based analyses (Model B: β/SD=0.18±0.07, p=0.005 and β/Q1 vs Q2–4 =−0.40±0.15, p=0.010, respectively). We did not observe any association between BDNF and performance on the executive function test. We also did not observe any association between logVEGF levels and either of the cognitive measures.
DISCUSSION
In our community-based, stroke-free middle-aged to elderly sample of 3440 Framingham study participants lower serum BDNF and higher serum VEGF concentrations were associated with increased risk of incident stroke/TIA over a 10 year observation period. Lower serum BDNF levels were also associated with greater white matter hyperintensities (log-WMHV and ext-WMHV) and with poorer visual memory performance (VR-d) in a subset of otherwise stroke-and dementia-free middle-aged persons. The associations were present after adjustment for putative stroke risk factors; suggesting that novel mechanisms associated with circulating levels of BDNF and VEGF and perhaps mediated via BDNF and VEGF pathways modify clinical stroke/TIA risk and the extent of covert vascular brain disease.
BDNF and VEGF levels and Stroke/TIA
To the best of our knowledge this is the first community-based study to report an association between serum BDNF and VEGF levels and risk of stroke/TIA. These associations are novel, but not unexpected.
As mentioned earlier, low BDNF and high circulating VEGF levels have been observed in patients with vascular risk factors associated with atherosclerosis and cerebrovascular disease.7–10, 12, 13, 23, 24 Thus, BDNF and VEGF may be biomarkers of these processes or may modify the risk of stroke/TIA by modulating the conventional risk factor pathways. Animal studies have established a positive effect of BDNF and VEGF on infarct size and on long-term potentiation, neuronal remodeling and functional motor recovery after induction of an ischemic brain lesion.25, 26 In addition to being a potent angiogenic factor, VEGF has also demonstrated neuroprotective properties: inhibition of apoptosis and stimulation of astrocytes, microglia and cortical neurons growth during embryogenesis and adult neurogenesis.27 However, higher VEGF levels have been observed in persons with obesity, metabolic syndrome, and markers of atherosclerorsis such as increased carotid intimo-medial thickness, perhaps as a compensation to their subclinical disease.28
Possible Mechanisms for Association of BDNF and Stroke/TIA
There is experimental evidence that neurons and glial cells act as endogenous sources of BDNF after ischemic and other brain injuries.25, 29 It has been shown that BDNF regulates homeostatic interactions between neurons, glial cells and the vasculature, jointly referred to as “neurovascular unit”. Dysfunction of cerebral vascular BDNF signaling therefore may contribute to disruption of the neurovascular unit, hence to an alteration of tissue responses to vascular injury.30 A small molecule BDNF mimetic (LM22A-4) when administered immediately after an ischemic stroke in adult mice lead to increased neurogenesis and improved functional motor recovery.31 Studies have also found that exogenous BDNF affects vasodilatation via upregulation of prostacyclin synthesis, and protects against vasoconstriction-related injury and thrombus formation in the walls of an isolated cerebral artery.13 Therefore BDNF could reduce stroke risk through its neurotrophic or its vascular effect.
Possible Mechanisms for Association of VEGF and Stroke/TIA
Upregulation of VEGF expression has been observed in the neurons, ECs and astrocytes within border zones of infracted brain tissue in patients after ischemia.32 Two small clinical studies related higher VEGF levels in patients with acute stroke to better outcomes.23, 24 However, this upregulation could result in higher VEGF levels serving as a marker of chronic cerebral ischemia, and hence of increased stroke risk. Whereas there are no prior community-based studies to evaluate the effect of VEGF levels on the risk of incident stroke/TIA, our findings parallel prior observations that higher VEGF levels are associated with other CVD risk factors and outcomes.9, 10, 12, 13 The biological mechanisms by which VEGF may play a role in cerebrovascular disease are thus complex and deserving of further investigation. While VEGF promotes neurogenesis and beneficial angiogenesis, angiogenesis is also a component in the progression of atherosclerosis.9, 33 Moreover, in the acute stroke setting the major source of the circulating VEGF may not be the ischemic brain tissue, but rather the transient peripheral leukocytosis. 33 Therefore, the net impact of the proatherogenic and angiogenic roles of VEGF in cerebrovascular disease warrants further investigation.
BDNF and White Matter Hyperintensities
To our knowledge, this is also the first study to examine an association between BDNF and WMH in a community-based, middle-aged, stroke- and dementia-free sample. Although the pathogenesis of WMH is largely unknown, based on available literature our findings have several possible explanations.
WMHs are highly prevalent in persons with vascular risk factors and stroke.34, 35 These radiographic lessons are thought to be a consequence of neuronal and/or vascular injury; resulting either from chronic hypoperfusion and ischemia, demyelination, axonal loss and gliosis in the territory of deep, small perforating cerebral arteries, or through neurodegenerative processes involved in usual brain aging and dementia.14, 34 In prior reports, WMHs were strongly related to stroke risk factors 35 and incident stroke, MCI and dementia.14, 36 As mentioned earlier, BDNF is highly expressed in neuronal and vascular tissue.25, 27, 37 Low levels have been observed in patients with vascular risk factors,7 and in the present study with increased risk of stroke/TIA. However, only a few studies have explored in humans, the association between BDNF (studying genetic polymorphism rather than gene expression or circulating levels) and white matter abnormalities on brain MRI; these studies were restricted to patients with various psychiatric conditions.38 In a cross-sectional study of older subjects with depression, a specific BDNF genotype (the Val66Met allele) was associated with greater WMH volume,38 whereas in a prospective study the same polymorphism appeared to modify the association between stroke and depression.39 In a primate model of lacunar stroke, the production of BDNF was stimulated in activated glial cells at the ischemic border zones. The activation of astrocytes in the white-matter ipsilateral to the injury appeared to contribute to tissue repair and regeneration.40 It is likely that the state of hypoperfusion and chronic ischemia in oligemic/ischemic tissue that leads to manifestation of WMH on brain MRI also acts as a trigger for upregulation of BDNF expression. Further experimental and clinical studies are warranted to evaluate the role of BDNF in subclinical vascular brain disease.
BDNF and Cognitive Performance
BDNF plays a critical role in mediating synaptic transmission and synaptic plasticity, a molecular basis for processing new learning and consolidation of short and long term memories.41 A decline in BDNF levels precedes and parallels the observed decrease in choline acetyltransterase enzyme activity in aging brains, preclinical AD and AD.41, 42 In prior reports, higher serum BDNF levels were found to be associated with better performance on neuropsychological testing in healthy older adults.43 Recently, in a prospectively followed aging population (57–79 years), decreased levels of BDNF were also associated with poorer performance on global cognitive testing and in several specific cognitive domains such as verbal memory and language, but not in executive function tests. 44 Although different cognitive test batteries were used in this prior study (MMSE and CERAD score), our current findings are largely in agreement with their observations. Decline in cognitive function has been associated with higher FSRP and with the presence of ext-WMH.14,45 In the present study we also observed an association between low BDNF levels and a) stroke/TIA and b) WMHs, both previously associated with decline in cognitive function, MCI and dementia.36 In conclusion, our data are consistent with the idea that BDNF pathways are involved in processes of brain aging and cognitive function.
Strengths and Limitations
The prospective community-based setting of our study, ability to adjust for concomitant and pre-stroke levels of vascular risk factors, the ability to study a middle-aged to elderly sample free of stroke and dementia, and the fact that all outcome assessment was done blinded to biomarkers levels and to clinical/subclinical outcomes are some strengths of our study. However, the predominantly European origin of our sample limits the generalizability of our findings. In addition, participants included in the study were not representative of the general population; they had less vascular disease burden then those who were excluded or declined MRI or NP testing. Due to the small number of events in each stroke subtype categories, we used a composite outcome of all strokes as a clinical outcome. A single-occasion measurement of brain MRI and NP outcomes prevents us from examining the association with change in structural and functional measures. There was no CSF data available for BDNF and VEGF, although serum and CSF levels have been shown to be moderately correlated in other studies. Finally, whereas these are exciting and novel observations, but our exploratory analyses needs to be replicated in other prospective studies since the models used (such as the threshold effect for BDNF) were based on our own data and that could result in a risk of model overfitting.
SUMMARY
The results of our study suggest that BDNF and VEGF as novel risk markers may improve stratification of patients at risk for stroke/TIA. Second, as a marker of neuronal function, BDNF may serve as an intermediate biomarker for subclinical vascular disease. More importantly, BDNF may have biological potential to serve as a therapeutic target for primary and secondary prevention of clinical and subclinical vascular brain disease.
Supplementary Material
Acknowledgments
Funding Sources:
This work was supported by National Institutes of Health/National Heart, Lung, and Blood Institute Contract (N01-HC-25195, N01HV28178, R01HL093029, U01 HL096917 and 2K24HL04334), the National Institute on Aging (R01 AG16495; AG08122, AG033193, AG031287), the National Institute of Neurological Disorders and Stroke (R01 NS17950), the American Heart Association (Award #11CRP4930020) and the National Center for Research Resources and the National Center for Advancing Translational Science-NIH through Boston University-CTSI Grant # UL1 TR000157. The content is solely the responsibility of the authors and does not necessarily represent the official views of NINDS, NHLBI, NIA, NIH or AHA.
Footnotes
Financial Disclosures:
None
Conflict of Interests:
None
References
- 1.Ivan CS, Seshadri S, Beiser A, Au R, Kase CS, Kelly-Hayes M, et al. Dementia after stroke: The framingham study. Stroke; a journal of cerebral circulation. 2004;35:1264–1268. doi: 10.1161/01.STR.0000127810.92616.78. [DOI] [PubMed] [Google Scholar]
- 2.Gresham GE, Kelly-Hayes M, Wolf PA, Beiser AS, Kase CS, D’Agostino RB. Survival and functional status 20 or more years after first stroke: The framingham study. Stroke; a journal of cerebral circulation. 1998;29:793–797. doi: 10.1161/01.str.29.4.793. [DOI] [PubMed] [Google Scholar]
- 3.Christensen H, Anstey KJ, Parslow RA, Maller J, Mackinnon A, Sachdev P. The brain reserve hypothesis, brain atrophy and aging. Gerontology. 2007;53:82–95. doi: 10.1159/000096482. [DOI] [PubMed] [Google Scholar]
- 4.Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Annals of neurology. 2008;63:272–287. doi: 10.1002/ana.21393. [DOI] [PubMed] [Google Scholar]
- 5.Emanueli C, Schratzberger P, Kirchmair R, Madeddu P. Paracrine control of vascularization and neurogenesis by neurotrophins. British journal of pharmacology. 2003;140:614–619. doi: 10.1038/sj.bjp.0705458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pucci S, Mazzarelli P, Missiroli F, Regine F, Ricci F. Neuroprotection: Vegf, il-6, and clusterin: The dark side of the moon. Progress in brain research. 2008;173:555–573. doi: 10.1016/S0079-6123(08)01138-2. [DOI] [PubMed] [Google Scholar]
- 7.Golden E, Emiliano A, Maudsley S, Windham BG, Carlson OD, Egan JM, et al. Circulating brain-derived neurotrophic factor and indices of metabolic and cardiovascular health: Data from the baltimore longitudinal study of aging. PloS one. 2010;5:e10099. doi: 10.1371/journal.pone.0010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferris LT, Williams JS, Shen CL. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Medicine and science in sports and exercise. 2007;39:728–734. doi: 10.1249/mss.0b013e31802f04c7. [DOI] [PubMed] [Google Scholar]
- 9.Blann AD, Belgore FM, McCollum CN, Silverman S, Lip PL, Lip GY. Vascular endothelial growth factor and its receptor, flt-1, in the plasma of patients with coronary or peripheral atherosclerosis, or type ii diabetes. Clinical science. 2002;102:187–194. [PubMed] [Google Scholar]
- 10.Lieb W, Safa R, Benjamin EJ, Xanthakis V, Yin X, Sullivan LM, et al. Vascular endothelial growth factor, its soluble receptor, and hepatocyte growth factor: Clinical and genetic correlates and association with vascular function. European heart journal. 2009;30:1121–1127. doi: 10.1093/eurheartj/ehp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: A risk profile from the framingham study. Stroke; a journal of cerebral circulation. 1991;22:312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 12.Lennmyr F, Ata KA, Funa K, Olsson Y, Terent A. Expression of vascular endothelial growth factor (vegf) and its receptors (flt-1 and flk-1) following permanent and transient occlusion of the middle cerebral artery in the rat. Journal of neuropathology and experimental neurology. 1998;57:874–882. doi: 10.1097/00005072-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Santhanam AV, Smith LA, Katusic ZS. Brain-derived neurotrophic factor stimulates production of prostacyclin in cerebral arteries. Stroke; a journal of cerebral circulation. 2010;41:350–356. doi: 10.1161/STROKEAHA.109.564492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Au R, Massaro JM, Wolf PA, Young ME, Beiser A, Seshadri S, et al. Association of white matter hyperintensity volume with decreased cognitive functioning: The framingham heart study. Archives of neurology. 2006;63:246–250. doi: 10.1001/archneur.63.2.246. [DOI] [PubMed] [Google Scholar]
- 15.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: The framingham study. American journal of public health and the nation’s health. 1951;41:279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The framingham offspring study. American journal of epidemiology. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 17.DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, et al. Measures of brain morphology and infarction in the framingham heart study: Establishing what is normal. Neurobiology of aging. 2005;26:491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Farmer ME, White LR, Kittner SJ, Kaplan E, Moes E, McNamara P, et al. Neuropsychological test performance in framingham: A descriptive study. Psychological reports. 1987;60:1023–1040. doi: 10.1177/0033294187060003-201.1. [DOI] [PubMed] [Google Scholar]
- 19.Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, et al. National institute of neurological disorders and stroke-canadian stroke network vascular cognitive impairment harmonization standards. Stroke; a journal of cerebral circulation. 2006;37:2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 20.Beekman AT, Deeg DJ, Van Limbeek J, Braam AW, De Vries MZ, Van Tilburg W. Criterion validity of the center for epidemiologic studies depression scale (ces-d): Results from a community-based sample of older subjects in the netherlands. Psychological medicine. 1997;27:231–235. doi: 10.1017/s0033291796003510. [DOI] [PubMed] [Google Scholar]
- 21.Kannel WB, Sorlie P. Some health benefits of physical activity. The framingham study. Archives of internal medicine. 1979;139:857–861. [PubMed] [Google Scholar]
- 22.Pencina MJ, D’Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Statistics in medicine. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slevin M, Krupinski J, Slowik A, Kumar P, Szczudlik A, Gaffney J. Serial measurement of vascular endothelial growth factor and transforming growth factor-beta1 in serum of patients with acute ischemic stroke. Stroke; a journal of cerebral circulation. 2000;31:1863–1870. doi: 10.1161/01.str.31.8.1863. [DOI] [PubMed] [Google Scholar]
- 24.Lee SC, Lee KY, Kim YJ, Kim SH, Koh SH, Lee YJ. Serum vegf levels in acute ischaemic strokes are correlated with long-term prognosis. European journal of neurology: the official journal of the European Federation of Neurological Societies. 2010;17:45–51. doi: 10.1111/j.1468-1331.2009.02731.x. [DOI] [PubMed] [Google Scholar]
- 25.Kleim JA, Jones TA, Schallert T. Motor enrichment and the induction of plasticity before or after brain injury. Neurochemical research. 2003;28:1757–1769. doi: 10.1023/a:1026025408742. [DOI] [PubMed] [Google Scholar]
- 26.Schabitz WR, Steigleder T, Cooper-Kuhn CM, Schwab S, Sommer C, Schneider A, et al. Intravenous brain-derived neurotrophic factor enhances poststroke sensorimotor recovery and stimulates neurogenesis. Stroke; a journal of cerebral circulation. 2007;38:2165–2172. doi: 10.1161/STROKEAHA.106.477331. [DOI] [PubMed] [Google Scholar]
- 27.Tsukahara T, Yonekawa Y, Tanaka K, Ohara O, Wantanabe S, Kimura T, et al. The role of brain-derived neurotrophic factor in transient forebrain ischemia in the rat brain. Neurosurgery. 1994;34:323–331. doi: 10.1227/00006123-199402000-00016. discussion 331. [DOI] [PubMed] [Google Scholar]
- 28.Zachary I. Neuroprotective role of vascular endothelial growth factor: Signalling mechanisms, biological function, and therapeutic potential. Neuro-Signals. 2005;14:207–221. doi: 10.1159/000088637. [DOI] [PubMed] [Google Scholar]
- 29.Sandhofer A, Tatarczyk T, Kirchmair R, Iglseder B, Paulweber B, Patsch JR, et al. Are plasma vegf and its soluble receptor sflt-1 atherogenic risk factors? Cross-sectional data from the saphir study. Atherosclerosis. 2009;206:265–269. doi: 10.1016/j.atherosclerosis.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 30.Guo S, Kim WJ, Lok J, Lee SR, Besancon E, Luo BH, et al. Neuroprotection via matrix-trophic coupling between cerebral endothelial cells and neurons. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7582–7587. doi: 10.1073/pnas.0801105105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han J, Pollak J, Yang T, Siddiqui MR, Doyle KP, Taravosh-Lahn K, et al. Delayed administration of a small molecule tropomyosin-related kinase b ligand promotes recovery after hypoxic-ischemic stroke. Stroke; a journal of cerebral circulation. 2012;43:1918–1924. doi: 10.1161/STROKEAHA.111.641878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Issa R, Krupinski J, Bujny T, Kumar S, Kaluza J, Kumar P. Vascular endothelial growth factor and its receptor, kdr, in human brain tissue after ischemic stroke. Laboratory investigation; a journal of technical methods and pathology. 1999;79:417–425. [PubMed] [Google Scholar]
- 33.Khurana R, Simons M, Martin JF, Zachary IC. Role of angiogenesis in cardiovascular disease: A critical appraisal. Circulation. 2005;112:1813–1824. doi: 10.1161/CIRCULATIONAHA.105.535294. [DOI] [PubMed] [Google Scholar]
- 34.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: Systematic review and meta-analysis. Bmj. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeerakathil T, Wolf PA, Beiser A, Massaro J, Seshadri S, D’Agostino RB, et al. Stroke risk profile predicts white matter hyperintensity volume: The framingham study. Stroke; a journal of cerebral circulation. 2004;35:1857–1861. doi: 10.1161/01.STR.0000135226.53499.85. [DOI] [PubMed] [Google Scholar]
- 36.Debette S, Beiser A, DeCarli C, Au R, Himali JJ, Kelly-Hayes M, et al. Association of mri markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: The framingham offspring study. Stroke; a journal of cerebral circulation. 2010;41:600–606. doi: 10.1161/STROKEAHA.109.570044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujimura H, Altar CA, Chen R, Nakamura T, Nakahashi T, Kambayashi J, et al. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thrombosis and haemostasis. 2002;87:728–734. [PubMed] [Google Scholar]
- 38.Kim JM, Stewart R, Kim SW, Yang SJ, Shin IS, Kim YH, et al. Bdnf genotype potentially modifying the association between incident stroke and depression. Neurobiology of aging. 2008;29:789–792. doi: 10.1016/j.neurobiolaging.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 39.Sato Y, Chin Y, Kato T, Tanaka Y, Tozuka Y, Mase M, et al. White matter activated glial cells produce bdnf in a stroke model of monkeys. Neuroscience research. 2009;65:71–78. doi: 10.1016/j.neures.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 40.Waterhouse EG, Xu B. New insights into the role of brain-derived neurotrophic factor in synaptic plasticity. Molecular and cellular neurosciences. 2009;42:81–89. doi: 10.1016/j.mcn.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Durany N, Michel T, Kurt J, Cruz-Sanchez FF, Cervos-Navarro J, Riederer P. Brain-derived neurotrophic factor and neurotrophin-3 levels in alzheimer’s disease brains. International journal of developmental neuroscience: the official journal of the International Society for Developmental Neuroscience. 2000;18:807–813. [PubMed] [Google Scholar]
- 42.Peng S, Wuu J, Mufson EJ, Fahnestock M. Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of alzheimer’s disease. Journal of neurochemistry. 2005;93:1412–1421. doi: 10.1111/j.1471-4159.2005.03135.x. [DOI] [PubMed] [Google Scholar]
- 43.Gunstad J, Benitez A, Smith J, Glickman E, Spitznagel MB, Alexander T, et al. Serum brain-derived neurotrophic factor is associated with cognitive function in healthy older adults. Journal of geriatric psychiatry and neurology. 2008;21:166–170. doi: 10.1177/0891988708316860. [DOI] [PubMed] [Google Scholar]
- 44.Komulainen P, Pedersen M, Hanninen T, Bruunsgaard H, Lakka TA, Kivipelto M, et al. Bdnf is a novel marker of cognitive function in ageing women: The dr’s extra study. Neurobiology of learning and memory. 2008;90:596–603. doi: 10.1016/j.nlm.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 45.Seshadri S, Wolf PA, Beiser A, Elias MF, Au R, Kase CS, et al. Stroke risk profile, brain volume, and cognitive function: The framingham offspring study. Neurology. 2004;63:1591–1599. doi: 10.1212/01.wnl.0000142968.22691.70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.