Abstract
The hyperpolarization-activated cation current (Ih) plays an important role in determining membrane potential and firing characteristics of neurons and therefore is a potential target for regulation of intrinsic excitability. Here we show that an increase in AMPA-receptor-dependent synaptic activity induced by α-latrotoxin or glutamate application as well as direct depolarization results in an increase in Ih recorded from cell-attached patches in hippocampal CA1 pyramidal neurons. This mechanism requires Ca2+ influx but not increased levels of cAMP. Artificially increasing Ih by using a dynamic clamp during whole-cell current clamp recordings results in reduced firing rates in response to depolarizing current injections. We conclude that modulation of somatic Ih represents a previously uncharacterized mechanism of homeostatic plasticity, allowing a neuron to control its excitability in response to changes in synaptic activity on a relatively short-term time scale.
The intrinsic excitability of neurons determines the translation from synaptic input to axonal output. Regulation of intrinsic excitability may therefore constitute a form of cellular plasticity that controls the dynamic range of the input-output relationship. Such a mechanism of cellular plasticity may exist in parallel to synapse-specific mechanisms of plasticity like long-term potentiation and long-term depression. There is increasing evidence for the existence of mechanisms of plasticity that are not synapse-specific but act at the cellular level (1-3). Long-lasting changes in synaptic activity over several days have been shown to modulate the intrinsic voltage-gated ionic conductances that shape neuronal firing patterns (4-6). Modulation of voltage-gated conductances occurring at a time scale of hours has also been reported (7, 8). However, under physiological conditions, where the level of synaptic activity can change quickly, modulation of somatic voltage-gated conductances may be a potent mechanism to regulate excitability. It is unknown, however, whether such a mechanism of plasticity exists on a relatively short-term time scale.
Hyperpolarization-activated cation channels (Ih) are a subset of voltage-gated channels that are important in determining intrinsic excitability. Ih channels, which are permeable to both Na+ and K+ ions, operate in the subthreshold voltage range where they influence membrane potential, firing threshold, and firing patterns, as well as synaptic integration (9-14). Here we show that somatic Ih channels in rat hippocampal CA1 pyramidal neurons are subject to modulation by an enhancement of synaptic activity on a time scale of tens of minutes and that this modulation reduces the excitability of these neurons.
Methods
Slice Preparation and Electrophysiology. Parasaggital hippocampal slices (250 μm) were prepared from 14- to 28-day-old male Wistar rats (Harlan, Zeist, The Netherlands). Experiments were conducted according to the ethics committee guidelines for animal experimentation of the University of Amsterdam. After decapitation, the brain was rapidly removed and placed in ice-cold artificial cerebrospinal fluid (ACSF) containing 120 mM NaCl, 3.5 mM KCl, 2.5 mM CaCl2, 1.3 mM MgSO4, 1.25 mM NaH2PO4, 25 mM glucose, and 25 mM NaHCO3, equilibrated with 95% O2 and 5% CO2 (pH 7.4). Subsequently, slices were cut by using a vibroslicer (752 M, Campden Instruments, Lough-borough, U.K.) and allowed to recover for 1 h at 31°C. CA1 pyramidal neurons were visualized with an upright Zeiss Axioskop with Hoffman modulation contrast optics and a VX44 charge-coupled device camera (PCO, Kelheim, Germany). During voltage clamp experiments, slices were continuously super-fused with ACSF supplemented with 1 μM tetrodotoxin (Latoxan, Valence, France). Patch clamp recordings were made at room temperature.
For whole-cell voltage and current clamp experiments, patch pipettes were pulled from borosilicate glass and had a resistance of 2-4 MΩ when filled with 140 mM potassium gluconate, 10 mM Hepes, 5 mM EGTA, 0.5 mM CaCl2, 2 mM Mg-ATP, and 10 mM sucrose (pH 7.4 with KOH). In whole-cell voltage clamp experiments, Ih channels in the somatic compartment (defined as the soma together with the basal dendrites and the initial segment of the apical dendrite) were isolated from the majority of dendritic Ih channels by placing a cut in stratum radiatum parallel to the CA1 pyramidal cell layer, under the visual guidance of a dissecting microscope. The distance of the cut from the pyramidal cell layer was determined once slices were placed into the recording chamber and ranged from 80 to 120 μm. Currents were activated by hyperpolarizing voltage steps (1,000 ms) from a holding potential of -50 mV. Series resistance was compensated for 80%. Current signals in voltage clamp were filtered at 333 Hz and sampled at 1 kHz by using an EPC9 amplifier and PULSE 8.31 software (HEKA Electronik, Lambrecht, Germany). Voltage signals in current clamp were filtered at 3.33 kHz and sampled at 10 kHz.
For experiments in the cell-attached configuration, patch pipettes were pulled from borosilicate glass and had a resistance of 1.5-3 MΩ when filled with 120 mM NaCl, 10 mM Hepes, 3 mM KCl, 1 mM MgCl2, and 1 μM tetrodotoxin (pH 7.4 with NaOH). Note that in the cell-attached configuration, the pipette potential and the resting membrane potential are positioned in series to form the local transmembrane potential over the patch, i.e., a pipette potential of 0 mV refers to the resting membrane potential. In this recording configuration, applying a positive pipette potential results in membrane hyperpolarization, and applying a negative pipette potential results in membrane depolarization, exactly opposite to sign conventions in the whole-cell configuration. In the text and figures, we give membrane potentials relative to the resting membrane potential, but we use the standard sign convention that negative potentials indicate a hyperpolarization and positive potentials indicate a depolarization. Currents were evoked by hyperpolarizing voltage steps (1,000 ms) from a relative membrane potential of +20 mV.
To enhance synaptic activity, α-latrotoxin (α-LTX; dissolved in 50% glycerol, final concentration 0.15 nM in ACSF) was bath-applied for 15-25 min (15). The enhancement of synaptic activity by α-LTX was confirmed by separate experiments in which miniature synaptic events were recorded in whole-cell voltage clamp mode. Glutamate (100 μM), γ-aminobutyric acid (GABA) (100 μM), ACSF, or KCl (50 mM) were locally applied from a second pipette connected to a picospritzer (General Valve, Fairfield, NJ) by short and repeated pressure applications of 100 ms for 4 s at 5 Hz at 10-s intervals. Hyperpolarization-activated currents were recorded in the 10-s intervals.
To modulate intracellular Ca2+ levels, slices were incubated for 1 h with the membrane-permeable Ca2+ chelator BAPTA-AM (dissolved in DMSO and diluted to a concentration of 50 μM in ACSF; final DMSO concentration 0.05%). To block both low- and high-voltage-activated Ca2+ channels, Ni2+ (100 μM) and Cd2+ (100 μM) were bath-applied. Glutamatergic AMPA and NMDA receptors were blocked with 50 μM 6-cyano-7-nitroquinoxaline-2,3-dione disodium (CNQX) and 100 μM of d(-)-2-amino-5-phosphonopentanoic acid. To assess the involvement of cAMP, slices were incubated for 30 min with 8-bromoadenosine 3′,5′-cyclic monophosphate (8-Br-cAMP, 500 μM) and 8-(4-chlorophenylthio) adenosine 3′,5′-cyclic monophosphate (500 μM). To block Ih channels, ZD7288 (50 μM) was bath-applied during whole-cell experiments and included in the pipette solution during cell-attached experiments. Chemicals were purchased from Sigma, Tocris, or Molecular Probes.
Dynamic Clamp. The functional consequences of changes in Ih at the soma were investigated in whole-cell current clamp recordings by using a dynamic clamp to artificially manipulate the amplitude of Ih. A personal computer with data acquisition card (National Instruments, Austin, TX) sampled membrane voltage at 5 kHz and injected Ih with the same sampling rate. The voltage dependence of Ih was taken from the fit to Ih from our experiments (with V in mV): Ih(V) = Ih max/{1 + exp[(V + 141)/20.5]}. The amplitude (Ih max) was controlled by the external stimulus input of the HEKA amplifier. Rate constants α(V) = 0.071/{1 + exp[(V + 108)/8.3]} and β(V) = 0.24/{1 + exp[(V + 26.5)/-23]} determined the time constants τ(V) = 1/α(V) + β(V) for activation and deactivation. They were fit to the voltage range given in ref. 13 and, after correction for temperature and Na+ concentration, matched to our own data set. To attain a fast and uniform response time of the real-time computer model, the functions were tabulated with the 12-bit input and output resolution of the hardware system. The properties of Ih in the dynamic clamp model were verified in voltage clamp mode.
Analysis. Ih recorded in the cell-attached configuration was leak corrected by using an online leak substraction protocol (P/4) in which leak pulses were determined from a relative holding potential of +20 mV to a range of relative membrane potentials between +22.5 and -20 mV. Current amplitude was determined as the mean value at 750-850 ms after the start of hyperpolarizing voltage steps. The time constant of activation of Ih was determined by fitting a single exponential function to the start of current traces. All values are given as mean ± SEM. Statistical differences were tested by nonparametric Mann-Whitney test or by Student's t test. P < 0.05 was used to indicate a significant difference.
Results
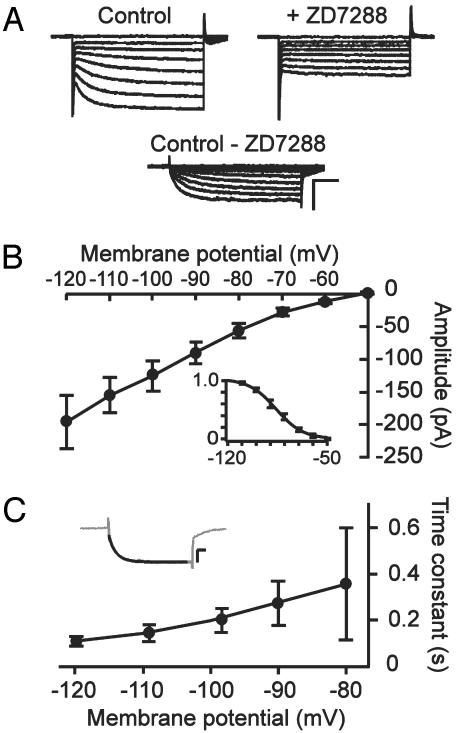
Characterization of Ih in the Somatic Compartment of CA1 Pyramidal Neurons. Hyperpolarization-activated currents were recorded in the whole-cell voltage clamp configuration from CA1 pyramidal neurons in hippocampal slices. Because of the high density of Ih channels in the distal dendrites of these neurons (13, 14, 16, 17), somatic Ih was isolated from the majority of dendritic Ih by cutting off the dendrites in the slices (see Methods). Step hyperpolarizations from a holding potential of -50 mV resulted in slowly activating inward currents (Fig. 1A), which were blocked in the presence of 50 μM ZD7288 (Fig. 1 A). Ih was isolated by subtracting current traces before and after application of ZD7288 (Fig. 1 A). The I-V curve of somatic Ih displayed inward rectification and a threshold of activation of around -60 mV (Fig. 1B). The activation curve for somatic Ih was generated by determining tail current amplitudes and fitting the normalized amplitudes with a Boltzmann equation (Fig. 1B Inset). The voltage-dependency of activation showed a voltage of half-maximal activation of -86 ± 2 mV and a slope parameter of 9.3 ± 0.9 mV (n = 4). Somatic Ih showed a typical slow activation time course (100-400 ms) that became faster with deeper hyperpolarization (Fig. 1C), and Ih did not inactivate over a period of 1,000 ms.
Fig. 1.
Characterization of somatic Ih in CA1 pyramidal neurons. (A) Hyperpolarization-activated currents recorded in whole-cell mode with the majority of the dendrites cut off. Currents were evoked by stepping from a holding potential of -50 mV to membrane potentials ranging from -60 to -120 mV in 10 mV decrements. Bath application of 50 μM ZD7288 blocked slow hyperpolarization-activated currents, and Ih was isolated by subtracting current traces before and after addition of ZD7288. (Scale bars, 250 pA and 200 ms.) (B) I-V relationship of somatic Ih. (Inset) Boltzmann fit to the normalized current activation as a function of membrane potential generated from tail current amplitudes measured 20 ms after step repolarization. (C) Time constant of activation of Ih, as determined by fitting a single exponential function to the rising phase of current traces (Inset). (Scale bars, 100 pA and 100 ms.) Data represent mean ± SEM of four to five cells.
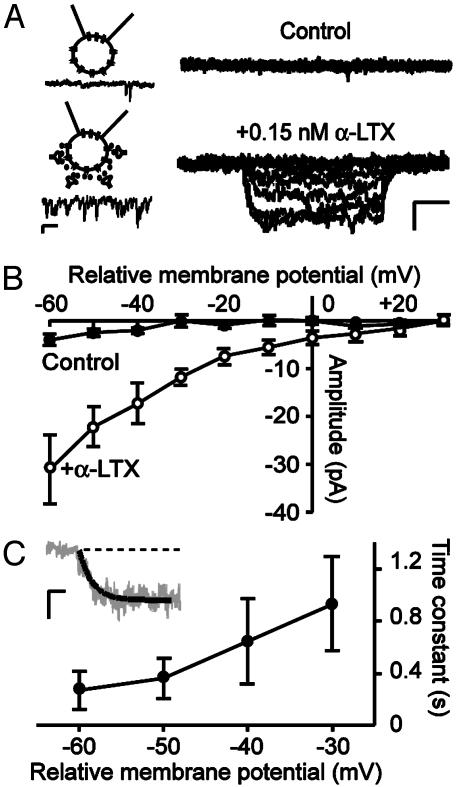
Enhanced Synaptic Activity Increases Somatic Ih. To study whether synaptic activity can modulate Ih, a nonselective increase in synaptic activity in intact slices was induced by bath application of 0.15 nM α-LTX in the presence of 1 μM tetrodotoxin. Because Ih is highly sensitive to modulation by intracellular components (11, 12), Ih was recorded from somatic cell-attached patches of CA1 pyramidal neurons. Hyperpolarizing voltage steps were applied from a relative membrane potential of +20 mV. Under control conditions, stepping to a relative membrane potential of -60 mV resulted in little inward current (4 ± 1 pA, Fig. 2B). Bath application of α-LTX for 15-25 min enhanced the synaptic activity in the slice throughout the duration of recordings (Fig. 2 A Inset). In 10 of 13 somatic patches, the enhanced synaptic activity increased the amplitude of Ih at a relative membrane potential of -60 mV ≈8-fold to a mean value of 31 ± 7 pA (P < 0.05, compared with control). In the other three patches, no change in Ih amplitude was seen. The I-V curve of Ih in the presence of α-LTX displayed inward rectification and a threshold for activation at a relative membrane potential of ≈0 mV, which corresponds to the cell's resting membrane potential (Fig. 2B). Tail current amplitudes of cell-attached Ih were too small to accurately determine the voltage dependence of activation. The cell-attached Ih had a slow time course of activation (300-900 ms), which became faster with deeper hyperpolarization (Fig. 2C) and Ih showed no inactivation. These results demonstrate that a period of enhanced synaptic activity in the slice increases Ih on the soma of CA1 pyramidal neurons.
Fig. 2.
Enhancement of synaptic activity by α-LTX induces an increase in somatic Ih. (A) Ih before (Upper) and after (Lower) bath application of 0.15 nM α-LTX for 15-25 min in the same cell. Currents were recorded from somatic cell-attached patches of CA1 pyramidal neurons with intact dendrites and were evoked by step hyperpolarizations from a relative membrane potential of +20 mV to potentials ranging from +30 to -60 in 10-mV decrements. (Scale bars, 25 pA and 250 ms.) Cartoons on the left are schematic representations of the recording configuration in the absence (Upper) and presence (Lower) of α-LTX. Below cartoons, example traces of miniature synaptic events recorded in a separate whole-cell experiment at a holding potential of -60 mV. (Scale bars, 5 pA and 200 ms.) (B) I-V curve of Ih before (filled symbols) and after (open symbols) bath application of α-LTX. (C) Activation kinetics of Ih. A single exponential function (solid line in inset) was fitted to the rising phase of Ih. (Inset) Example trace recorded at a relative membrane potential of -60 mV. (Scale bars, 20 pA and 100 ms.) Data points represent mean ± SEM of 6-10 cells.
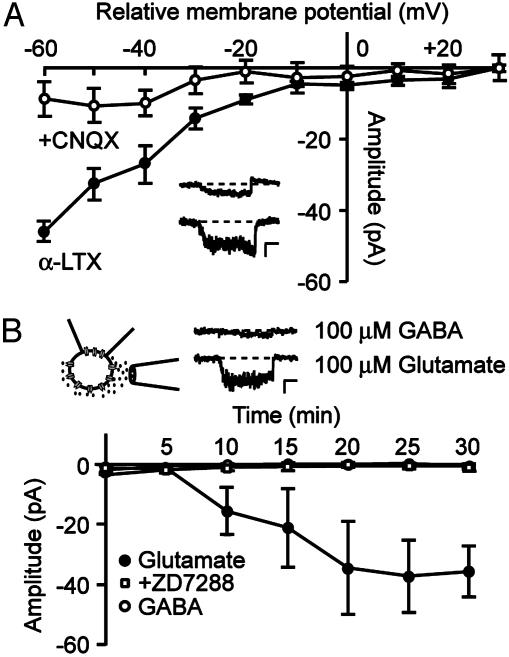
Enhanced Glutamatergic, but not GABAergic, Activity Increases Somatic Ih. Because the majority of synaptic terminals targeting CA1 pyramidal neurons are glutamatergic, we determined whether glutamate mediates the activity-induced increase in Ih. Bath application of the AMPA receptor antagonist CNQX (50 μM), subsequent to inducing the α-LTX effect on Ih, reversed the increase in Ih amplitude (Fig. 3A), suggesting a requirement for prolonged enhanced glutamatergic activity via AMPA receptors in the α-LTX-induced effect. Therefore, we studied whether direct pressure application of glutamate could mimic the effect of α-LTX on Ih. Glutamate (100 μM) was applied by using short and repetitive pressure application from a second pipette near the soma of the neuron under investigation. Within 10 min of application, in seven of nine patches, glutamate induced a gradual increase in Ih amplitude recorded at a relative membrane potential of -60 mV from 1 ± 1 pA to 36 ± 8 pA at 30 min after the start of glutamate application (P < 0.05 compared with control, Fig. 3B). In two patches, no change in Ih amplitude was seen. The gradual increase in Ih was prevented by including 50 μM ZD7288 in the pipette solution (1 ± 1 pA at t = 30, n = 7; Fig. 3B). Control recordings in which ACSF was pressure-applied did not show an increase in Ih (5 ± 4 pA at t = 30, n = 5; data not shown). Pressure application of the inhibitory neurotransmitter GABA (100 μM) had no detectable effect on Ih (1 ± 1 pA at t = 30, n = 5; Fig. 3B). This result suggests that up-regulation of Ih is specifically related to enhanced excitatory glutamatergic but not to inhibitory GABAergic activity, although decreases in Ih could escape detection.
Fig. 3.
Enhanced glutamatergic activity increases Ih. (A) I-V curve of cell-attached Ih recorded in the presence of 0.15 nM α-LTX (filled symbols) and after subsequent bath application of the AMPA receptor antagonist CNQX (50 μM, open symbols). (Inset) Current traces, recorded by stepping to a relative membrane potential of -60 mV in the presence of α-LTX (lower) and after addition of CNQX (upper). (Scale bars, 20 pA and 250 ms.) (B) Glutamate or GABA was applied near the soma by short and repeated pressure application. Pressure application of 100 μM glutamate (filled circles) gradually increased the amplitude of Ih, whereas application of 100 μM GABA (open circles) had no detectable effect. Including 50 μM ZD7288 (open squares) in the pipette solution prevented the increase in Ih. The cartoon is a schematic representation of the recording configuration. Example current traces are shown that were recorded by stepping to a relative membrane potential of -60 mV after glutamate (lower) and GABA (upper) application. (Scale bars, 10 pA and 250 ms.) Data points represent mean ± SEM of four to seven cells.
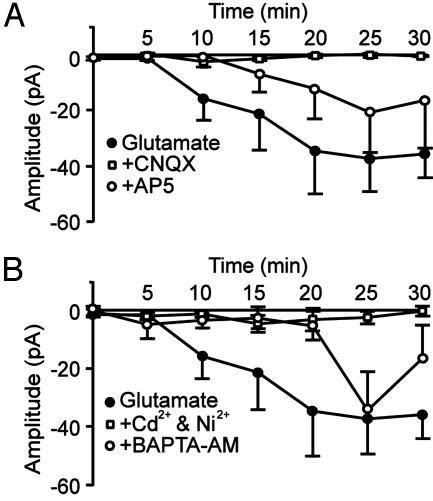
AMPA-Receptor Mediated Depolarization and a Rise in Intracellular Ca2+ Concentration ([Ca2+]i) Are Required for the Increase in Ih. The underlying mechanism of the glutamate-induced increase in Ih was further investigated. In the continuous presence of 50 μM CNQX, the glutamate-induced increase in Ih amplitude was entirely prevented (Fig. 4A). In the presence of 100 μM d(-)-2-amino-5-phosphonopentanoic acid, the glutamate-induced increase in Ih was delayed and reduced in amplitude to ≈30-50% (Fig. 4A). These findings indicate that AMPA receptor activation alone can initiate the glutamate-induced increase in Ih, and that NMDA receptor activation has an additive effect.
Fig. 4.
AMPA receptor activation and a rise in [Ca2+]i are required for the glutamate-induced increase in Ih. (A) Time course of the amplitude of Ih, recorded at a relative membrane potential of -60 mV during application of 100 μM glutamate (filled circles). In the continuous presence of 50 μM CNQX (open squares), the glutamate-induced increase in Ih was absent, whereas in the continuous presence of 100 μM d(-)-2-amino-5-phosphonopentanoic acid (AP5, open circles), the increase in Ih was reduced and its onset delayed. (B) The continuous presence of 100 μMNi2+ and 100 μMCd2+ (open squares) prevented the increase in Ih. The effect of glutamate on Ih was prevented for up to 20 min after the start of glutamate application by loading the cells with 50 μM BAPTA-AM to buffer intracellular Ca2+(open circles). Data points represent mean ± SEM of four to seven cells.
Application of glutamate will depolarize the cell, a finding confirmed by separate whole-cell recordings showing that glutamate puffs induced a transient depolarization of 19 ± 3 mV (n = 4). To test whether direct depolarization also modulates Ih, we applied puffs of KCl (50 mM) near the soma of neurons, causing in whole-cell experiments a transient depolarization of 18 ± 3 mV (n = 4). In four of five cell-attached patches, puffs of KCl increased Ih with a similar time course as the glutamate puffs. The mean amplitude of Ih at a relative membrane potential of -60 mV after 30 min of KCl application was 120 ± 12 pA (n = 5; data not shown).
Because AMPA receptor-mediated depolarization and direct depolarization are likely to cause Ca2+ influx through voltage-gated Ca2+ channels, we studied the role of intracellular Ca2+ in the modulation of Ih. In the presence of Ni2+ (100 μM) and Cd2+ (100 μM), which block both low- and high-voltage-activated Ca2+ channels, glutamate application failed to induce an increase in Ih amplitude (Fig. 4B), suggesting that a rise in intracellular Ca2+ via voltage-gated Ca2+ channels is required for the modulation of Ih. This result was corroborated by the finding that buffering of [Ca2+]i by incubating slices with the membrane-permeable analogue of the high affinity Ca2+ chelator BAPTA (50 μM) prevented the increase in Ih for up to 20 min after the start of glutamate application (Fig. 4B).
Ih channels are susceptible to modulation by cAMP (18-21). To investigate the involvement of cAMP in the increase in Ih, we incubated slices with the membrane-permeable analogues 8-bromoadenosine 3′,5′-cyclic monophosphate (8-Br-cAMP, 500μM) and 8-(4-chlorophenylthio) adenosine 3′,5′-cyclic monophosphate (500 μM). After slices were incubated for 30 min with either 8-Br-cAMP or 8-(4-chlorophenylthio) adenosine 3′,5′-cyclic monophosphate, recordings from cell-attached patches showed a mean Ih amplitude at a relative membrane potential of -60 mV of 3 ± 1 pA (8-Br-cAMP, n = 8) and 2 ± 1 pA (8-(4-chlorophenylthio) adenosine 3′,5′-cyclic monophosphate; n = 3), which is not significantly different from control amplitudes, suggesting that an elevation of cAMP is not sufficient to induce an increase in cell-attached Ih and that the 8-fold increase in Ih, induced by glutamate is likely to be mediated by a cAMP-independent mechanism.
Increased Ih Reduces Intrinsic Excitability. Given the pivotal role of Ih in determining intrinsic excitability, we set out to determine the functional consequences of changes in somatic Ih on the firing behavior of CA1 pyramidal neurons. To effectively control the amplitude of Ih at the soma during whole-cell current clamp recordings, we used a dynamic clamp to artificially change Ih. Under control conditions, hyperpolarizing current injections evoked a hyperpolarization with a characteristic depolarizing sag reflecting the slow activation of Ih (Fig. 5A). In response to increasing depolarizing current injections, neurons fired with rates of up to 29 spikes per s. When the endogenous Ih was counteracted by the dynamic clamp, the depolarizing sag was obliterated, and firing rates tended to increase (Fig. 5A). Conversely, increasing Ih with the dynamic clamp resulted in a more pronounced depolarizing sag on hyperpolarization and strongly decreased firing rates in response to depolarizing current injections (Fig. 5A).
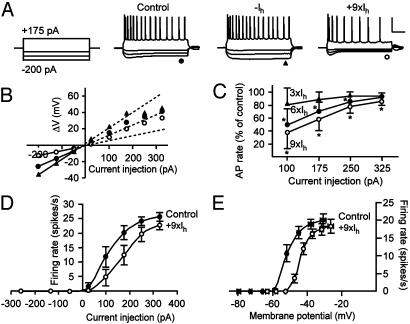
Fig. 5.
Increased Ih reduces intrinsic excitability of CA1 pyramidal neurons. (A) Firing responses to a depolarizing (175 pA) current injection and hyperpolarizing (-50, -125, and -200 pA) current injections. Counteracting Ih by using a dynamic clamp abolished the depolarizing sag and tended to increase firing rate, whereas increasing Ih resulted in a more prominent sag and decreased firing rate. A dc was used to set the resting membrane potential at -60 mV. (Scale bars, 50 mV and 200 ms.) (B) Input resistance was calculated from the linear range (-200 to -50 pA) of I-V relationships determined at the end of voltage traces in control situation (filled circles), with counteracted Ih (triangles), and with increased Ih (open circles). (C) Firing rates in response to depolarizing current injections expressed as the percentage of control firing rates. Three different levels of Ih were used: 3× (triangles), 6× (filled circles), and 9× (open circles) the average somatic Ih as determined from separate whole-cell voltage clamp recordings (see Fig. 1). Asterisks indicate significant differences from control. (D) Firing rate as a function of depolarizing current injection for 9× Ih. Compared with control (filled symbols), the input-output relationship is shifted to the right with increased Ih (open symbols). (E) Firing rate as a function of the membrane potential in a separate set of experiments in which no dc was applied in control situation (closed symbols) and with increased Ih (open symbols). With increased Ih, the input-output relationship is shifted toward more depolarized potentials. Data points represent mean ± SEM of five to six cells.
Changes in Ih will affect the input resistance of the neuron; therefore, the input resistance was determined from hyperpolarizing current injections (-200 to -50 pA) by using the slope of the I-V relationship in the linear range. Artificially counteracting the endogenous somatic Ih increased the input resistance from 100 ± 13 MΩ to 144 ± 28 MΩ (n = 3), whereas increasing Ih decreased the input resistance (Fig. 5B). The glutamate-induced increase in Ih found in the cell-attached recordings was ≈2-8 fold over the entire voltage range (Fig. 2B). Therefore, we increased the amplitude of Ih by a factor of 3, 6, or 9 times the mean Ih amplitude in the somatic compartment as recorded in separate whole-cell voltage clamp recordings (Fig. 1). In the control situation, the average input resistance was 102 ± 9 MΩ, which decreased to 74 ± 5 MΩ with 3× Ih, to 65 ± 3 MΩ with 6× Ih, and to 58 ± 2MΩ with 9× Ih (P < 0.05, n = 6 in all cases).
The effect of increased Ih on firing rate was expressed as the percentage of control. Fig. 5C shows that increasing Ih six or nine times decreased firing rates over the entire range of current injections. The largest effect was found with the smallest current injections, indicating that Ih most strongly affects firing rates at membrane potentials that are relatively close to resting membrane potential. The relationship between firing rate and injected current, the input-output relationship, is shifted to the right as illustrated in Fig. 5D for the condition with 9× Ih. These results indicate that because of the reduced input resistance with increased Ih, a larger current injection is required to achieve a certain firing rate.
In all of these experiments, a DC current was injected to keep resting membrane potentials at -60 mV. However, under physiological conditions, increased Ih will also affect resting membrane potential and cause a small depolarization. A separate set of experiments was performed in which neurons were allowed to depolarize as a result of the increase of Ih. The depolarization amounted to 5 ± 1 mV for 3× Ih, 9 ± 1 mV for 6× Ih, and 12 ± 1 mV for 9× Ih (P < 0.05, n = 5 in all cases). Plotting the firing rate as a function of the voltage (the sum of the membrane potential and the depolarization induced over the decreased input resistance) showed that the dynamic range of the input-output relationship was shifted toward more depolarized potentials (Fig. 5E). Thus, the two parameters that are associated with an increase in Ih (a decreased input resistance and a depolarization) together shift the output range of CA1 pyramidal neurons in such a way that a higher level of excitation is required to reach a certain firing rate. The increase in Ih on enhanced excitatory synaptic activity can therefore serve as a homeostatic mechanism to control intrinsic excitability.
Discussion
Our experiments demonstrate that an enhancement of glutamatergic synaptic activity gradually increases the amplitude of Ih at the soma of hippocampal CA1 pyramidal neurons on a time scale of tens of minutes. This modulation depends on AMPA-receptor-mediated depolarization and on a rise of [Ca2+]i, and it appears to be cAMP-independent. Increasing Ih by using the dynamic clamp showed that increased Ih shifts the input-output relationship toward more depolarized potentials and that this mechanism can therefore serve as a homeostatic mechanism in response to enhanced excitatory synaptic activity. It has previously been shown that long-lasting changes in synaptic activity modulate voltage-gated conductances at a time scale of hours to days (4-8). Together with other recent studies (22-24), the results described here suggest that more rapid mechanisms of cellular plasticity also exist. To our knowledge, however, this is the first study that reports direct evidence for a homeostatic modulation of voltage-gated conductances on a relatively short-term time scale.
The effect of enhanced synaptic activity on Ih amplitude was specifically related to glutamatergic excitatory activity, whereas GABAergic inhibitory activity had no detectable effect on Ih amplitude (Fig. 3B). AMPA-receptor-mediated depolarization as well as direct depolarization by KCl increased Ih, and preventing a rise in [Ca2+]i by blocking Ca2+ influx or by buffering [Ca2+]i abolished or reduced the increase of Ih (Fig. 4B). Because AMPA receptors on CA1 pyramidal neurons have a low Ca2+ permeability (25), we hypothesize that AMPA receptor-mediated depolarization results in Ca2+ influx through NMDA receptors and voltage-gated Ca2+ channels. It therefore seems likely that depolarization-induced Ca2+ influx is the primary requirement for up-regulation of Ih. Because of technical limitations, it proved difficult to test the bidirectionality of this effect. However, once induced, the increase in Ih could be reversed by blockade of AMPA receptors (Fig. 4A), indicating that factors that directly or indirectly reduce intracellular Ca2+ could potentially decrease Ih.
A rise in [Ca2+]i can trigger several intracellular messenger pathways. The relatively slow time-course of Ih modulation suggests that it is not Ca2+ itself but rather the activation of downstream intracellular pathways that leads to modulation of Ih. In line with this hypothesis, we could not reproduce the large effect of α-LTX or glutamate application on Ih in whole-cell recordings (increase in Ih amplitude of 9.4 ± 0.2%, n = 6; data not shown). The large increase in Ih could not be mimicked by elevating cAMP levels, suggesting a Ca2+-dependent but cAMP-independent modulation. It therefore seems likely that the modulation of Ih requires the convergent action of several factors, including AMPA receptor-mediated depolarization, a rise in [Ca2+]i, and the activation of signaling pathways downstream of Ca2+.
Although the previously reported studies on the modulation of intrinsic voltage-gated conductances after long-lasting changes in activity (4-8) may involve the synthesis and incorporation of new ion channels, the more rapid time course of the effects observed here suggests the modulation of existing ion channels or alternatively the insertion of spare ion channels into the membrane. In experiments using α-LTX, 3 of 13 patches did not show an increase in Ih amplitudes, and with glutamate application, 2 of 9 patches also did not show an increased Ih. Because of the cell-attached configuration, the sampling of currents is restricted to a relatively small fraction of the somatic membrane. Regional differences in either existing or inserted channel densities may therefore underlie the large variation found in the increase of Ih in somatic patches.
Irrespective of the precise mechanism underlying the modulation of somatic Ih, the results of this study suggest that this form of modulation may be a potent mechanism to regulate intrinsic excitability and therefore represents a mechanism of cellular plasticity that acts on a relatively short-term time scale. Several forms of cellular plasticity exist in addition to, and in cooperation with, synapse-specific forms of plasticity (1-3). Interestingly, synapse-specific mechanisms of plasticity commonly thought to underlie learning and memory, such as long-term potentiation and long-term depression, take place on a time scale similar to that of the effects described in this study and also involve the concurrent action of glutamate receptors and [Ca2+]i. Presynaptic Ih channels have been suggested to play an important role in several forms of long-term synaptic plasticity (refs. 26 and 27, but see ref. 28). Whether the modulation we describe here also affects presynaptic Ih channels remains to be determined.
Ih channels are present in much lower densities at the soma than at the dendrites of CA1 pyramidal neurons (13, 14, 16, 17). Dendritic Ih channels play an important functional role in determining the integrative properties of dendrites by dampening dendritic excitability and normalizing temporal summation (13, 14, 16). It is yet unknown whether dendritic Ih channels are also modulated in response to changes in synaptic activity or whether the mechanism we describe here is typically confined to the somatic compartment. In CA1 pyramidal neurons, the levels of Ih increase ≈7-fold from the soma toward the distal dendrites (13). If the mechanism we describe here is typically confined to the somatic compartment, it could function to locally regulate and control action potential generation. If the increase in Ih in response to excitatory synaptic activity is uniformly present in all neuronal compartments, it most likely serves as a general homeostatic cellular mechanism to dampen excitability and thus to scale the output of CA1 pyramidal neurons according to the level of excitatory input they receive.
Acknowledgments
We thank Sascha du Lac for discussions and comments on the manuscript, Marian Joëls and Fernando Lopes da Silva for their comments on an earlier version of the manuscript, and Oscar van den Bosch for writing the dynamic clamp model. J.A.v.H. is supported by a fellowship from the Royal Netherlands Academy of Arts and Sciences.
Abbreviations: Ih, hyperpolarization-activated cation current; α-LTX, α-latrotoxin; ACSF, artificial cerebrospinal fluid; CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione disodium; GABA, γ-aminobutyric acid; [Ca2+]i, intracellular Ca2+ concentration.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Marder, E., Abbott, L. F., Turrigiano, G. G., Liu, Z. & Golowasch, J. (1996) Proc. Natl. Acad. Sci. USA 93, 13481-13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turrigiano, G. G. (1999) Trends Neurosci. 22, 221-227. [DOI] [PubMed] [Google Scholar]
- 3.Burrone, J., O'Byrne, M. & Murthy, V. N. (2002) Nature 420, 414-418. [DOI] [PubMed] [Google Scholar]
- 4.Turrigiano, G. G., LeMasson, G. & Marder, E. (1995) J. Neurosci. 15, 3640-3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desai, N. S., Rutherford, L. C. & Turrigiano, G. G. (1999) Nat. Neurosci. 2, 515-520. [DOI] [PubMed] [Google Scholar]
- 6.Baines, R. A., Uhler, J. P., Thompson, A., Sweeney, S. T. & Bate, M. (2001) J. Neurosci. 21, 1523-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golowasch, J., Abbot, L. F. & Marder, E. (1999) J. Neurosci. 19, RC33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aizenman, C. D., Akerman, C. J., Jensen, K. R. & Cline, H. T. (2003) Neuron 39, 831-842. [DOI] [PubMed] [Google Scholar]
- 9.Hille, B. (2001) Ion Channels of Excitable Membranes (Sinauer, Sunderland, MA).
- 10.Macaferri, G., Mangoni, M., Lazzari, A. & DiFrancesco, D. (1993) J. Neurophysiol. 69, 2129-2136. [DOI] [PubMed] [Google Scholar]
- 11.Pape, H. C. (1996) Annu. Rev. Physiol. 58, 299-327. [DOI] [PubMed] [Google Scholar]
- 12.Robinson, R. B. & Siegelbaum, S. A. (2002) Annu. Rev. Physiol. 65, 29.1-29.28. [DOI] [PubMed] [Google Scholar]
- 13.Magee, J. C. (1998) J. Neurosci. 18, 7613-7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magee, J. C. (1999) Nat. Neurosci. 2, 508-514. [DOI] [PubMed] [Google Scholar]
- 15.Capogna, M., Gahwiler, B. H. & Thompson, S. M. (1996) J. Neurophysiol. 76, 3149-3158. [DOI] [PubMed] [Google Scholar]
- 16.Poolos, N. P., Migliore, M. & Johnston, D. (2002) Nat. Neurosci. 5, 767-774. [DOI] [PubMed] [Google Scholar]
- 17.Lorinz, A., Notomi, T., Tamas, G., Shigemoto, R. & Nusser, Z. (2002) Nat. Neurosci. 5, 1185-1193. [DOI] [PubMed] [Google Scholar]
- 18.McCormick, D. A. & Pape, H. C. (1990) J. Physiol. (London) 431, 319-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ingram, S. L. & Williams, J. T. (1996) J. Physiol. (London) 492, 97-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luthi, A. & McCormick, D. A. (1999) Nat. Neurosci. 2, 634-641. [DOI] [PubMed] [Google Scholar]
- 21.Pedarzani, P. & Storm, J. F. (1995) Proc. Natl. Acad. Sci. USA 92, 11716-11720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aizenman, C. D. & Linden, D. J. (2000) Nat. Neurosci. 3, 109-111. [DOI] [PubMed] [Google Scholar]
- 23.Egorov, A. V., Hamam, B. N., Fransen, E., Hasselmo, M. E. & Alonso A. A. (2002) Nature 420, 173-178. [DOI] [PubMed] [Google Scholar]
- 24.Nelson, A. B., Krispel, C. M., Sekirnjak, C. & du Lac, S. (2003) Neuron 40, 609-620. [DOI] [PubMed] [Google Scholar]
- 25.Burnashev, N., Monyer, H., Seeburg, P. H. & Sakmann, B. (1992) Neuron 8, 189-198. [DOI] [PubMed] [Google Scholar]
- 26.Beaumont, V., Zhong, N., Froemke, R. C., Ball, R. W. & Zucker, R. S. (2002) Neuron 33, 601-613. [DOI] [PubMed] [Google Scholar]
- 27.Mellor, J., Nicoll, R. A. & Schmitz, D. (2002) Science 295, 143-147. [DOI] [PubMed] [Google Scholar]
- 28.Chevaleyre, V. & Castillo, P. E. (2002) Proc. Natl. Acad. Sci. USA 99, 9538-9543. [DOI] [PMC free article] [PubMed] [Google Scholar]