Abstract
Growing evidence indicates that women with a history of common pregnancy complications, including fetal growth restriction and preterm delivery (often combined as low birth weight), hypertensive disorders of pregnancy, and gestational diabetes, are at increased risk for cardiovascular disease later in life. The purpose of this paper was to review the associations of parity and these 4 pregnancy complications with cardiovascular morbidity and mortality; to review the role of cardiovascular risk factors before, during, and after pregnancy complications in explaining these associations; and to explore the implications of this emerging science for new research and policy. We systematically searched for relevant cohort and case-control studies in Medline through December 2012 and used citation searches for already published reviews to identify new studies. The findings of this review suggest consistent and often strong associations of pregnancy complications with latent and future cardiovascular disease. Many pregnancy complications appear to be preceded by subclinical vascular and metabolic dysfunction, suggesting that the complications may be useful markers of latent high-risk cardiovascular trajectories. With further replication research, these findings would support the utility of these prevalent pregnancy complications in identifying high-risk women for screening, prevention, and treatment of cardiovascular disease, the leading cause of morbidity and mortality among women.
Keywords: birth weight; cardiovascular disease; diabetes, gestational; preeclampsia; pregnancy; premature birth; women's health
INTRODUCTION
It has long been understood that pregnancy complications are important for the lifelong health of offspring but much less appreciated that these complications also have key implications for the long-term health of the mother. An accumulating body of research has shown that common pregnancy complications, including gestational diabetes mellitus (GDM), preeclampsia, fetal growth restriction, and preterm delivery, predict the future risk of chronic diseases in women, including cardiovascular disease, diabetes, and breast cancer (1). We focus this review on the implications of pregnancy history for cardiovascular disease (CVD), a leading cause of female mortality (2, 3).
Globally, 1 of 3 women dies from CVD (4, 5). We do a worse job of recognizing and predicting CVD in women than in men, in part because CVD presents itself differently between the sexes (6, 7). This has important implications for the prevention of CVD. Primary prevention, if applied to high-risk populations early enough to avert the cumulative damage of chronic disease, can reduce CVD incidence (8–10). In response to the growing appreciation that many preventive efforts start too late to be effective, there has been a call for “primordial prevention”— prevention of the major CVD risk factors themselves (11, 12). In this context, pregnancy complications have the potential to be effective CVD risk “stress tests” to identify women who would most benefit from primordial or primary prevention efforts to reduce CVD risk (13).
On average, more than 80% of women in high-income countries bear at least 1 child (14, 15), as do upwards of 90% of women in most lower- and middle-income nations (16). A high proportion of women will, in the course of their reproductive career, have a pregnancy complicated by GDM, a hypertensive disorder of pregnancy, fetal growth restriction, macrosomia, or preterm delivery. The prevalence of any one of these conditions in any given pregnancy ranges from 2% to greater than 12%. In 1 study from the United Kingdom, 36% of singleton pregnancies were complicated by at least one of these factors (17). In the US national Nurses’ Health Study 2, we estimate that 29% of parous study participants have had one of the above pregnancy complications. As reviewed below, each of these complications has been associated with roughly a 2-fold increase in the risk of CVD events. If 80% of women are parous and 30% of them have had a pregnancy complication predictive of CVD, then roughly 20%–30% of women are unwittingly carrying a potent predictor of their future CVD risk.
The aim of this paper is to review the evidence for associations of parity and common pregnancy complications (low birth weight, fetal growth restriction, preterm delivery, hypertensive disorders of pregnancy, and GDM) with future CVD risk. We first present the evidence for the associations of parity and pregnancy complications with a woman's future CVD risk. Then we explore the physiological mechanisms that might explain these associations. Finally, we discuss the implications of these findings for future research and for health-care design and policy.
MATERIALS AND METHODS
We conducted Medline searches for English-language cohort and case-control studies published in the peer-reviewed literature through December 2012 that included combinations of the following terms: “parity”, “birth weight”, “birthweight”, “fetal growth”, “preterm”, “GDM”, “gestational hyperglycemia”, “macrosomia”, “preeclampsia”, “gestational hypertension”, “pregnancy-induced hypertension”, “hypertensive disorder of pregnancy”, “pregnancy complications”, “cardiovascular disease”, “coronary heart disease”, “coronary artery disease”, and “stroke”. We did not restrict by country of origin. The bibliographies of articles identified by the above search were also searched for relevant articles, and we performed citation searches on the retrieved articles as well as review articles. We included original papers as well as systematic reviews and meta-analyses.
We presented figures for the association of each of the main pregnancy complications with risk of maternal coronary heart disease (where available) and CVD (where specific coronary heart disease estimates were not available). Where possible, we presented crude or age-adjusted estimates in the figures to improve comparability across studies and to reflect this review's emphasis on disease prediction instead of etiology. In the case of preeclampsia, 3 studies had provided only estimates stratified by preeclampsia severity or timing, for which Bellamy et al. (18) had provided global preeclampsia estimates in their meta-analysis; for the sake of brevity and comparability across studies, we presented the “Bellamy estimates” for these 3 studies (19–21) in the figures.
ASSOCIATIONS OF PARITY AND PREGNANCY COMPLICATIONS WITH CVD RISK IN MOTHERS
Parity and CVD
Most (22, 23), but not all (24), studies have found a positive association between parity (number of children) and later CVD. In the largest study to date, the association was examined in 1.3 million women with a median follow-up time of 9.5 years (range, 0–24) by using Swedish registry data (25). Parity was associated with CVD in a J-shaped fashion, with 2 births representing the nadir of risk. Compared with women with 2 births, women with 0 and ≥5 births had multivariable-adjusted hazard ratios of 1.11 (95% confidence interval (CI): 1.09, 1.14) and 1.57 (95% CI: 1.52, 1.64), respectively.
Desired family size may affect the shape of the parity–CVD risk distribution in different societies. In Sweden, the modal family size (2 children) coincides with the nadir of maternal cardiovascular risk (25). This suggests that many women who bore only 1 child suffered from secondary infertility, first pregnancy complications that precluded further pregnancies, or severe neonatal outcomes that discouraged further childbearing. To the extent that subfertility and severe pregnancy complications predict future CVD risk, they may explain the low parity “hook” of the J-shaped association of parity and maternal CVD. The increase in CVD risk with increasing parity after 2 children may be the result of different phenomena. These include rival, but not mutually exclusive, theories that 1) adverse physiological change accumulates over pregnancies; 2) adverse lifestyle habits accrue with more children; and/or 3) selection bias, in which women at higher CVD risk opt for larger families. Thus, it is unclear whether the association of higher parity with CVD risk is causal or correlational.
Some insight into the association of parity with maternal CVD risk may be gleaned by examining the association of number of children with paternal CVD risk. Similar associations for mothers and fathers would suggest that the association between parity and maternal CVD is not causal but is more likely a result of confounding by socioeconomic position and/or behaviors related to child rearing. Three reports examined associations of number of children with CVD in fathers. In general, men who have fathered the most children appear to have small increased CVD risk, though this association is not always statistically significant and is weaker than the associations observed among mothers (26–28). Adjustment for lifestyle factors tends to reduce the associations in both mothers and fathers (27). These results suggest that the association between high parity and CVD in later life may be largely the result of socioeconomic position and/or behavioral risk factors associated with child rearing that are shared by both parents.
Common pregnancy complications and CVD in mothers
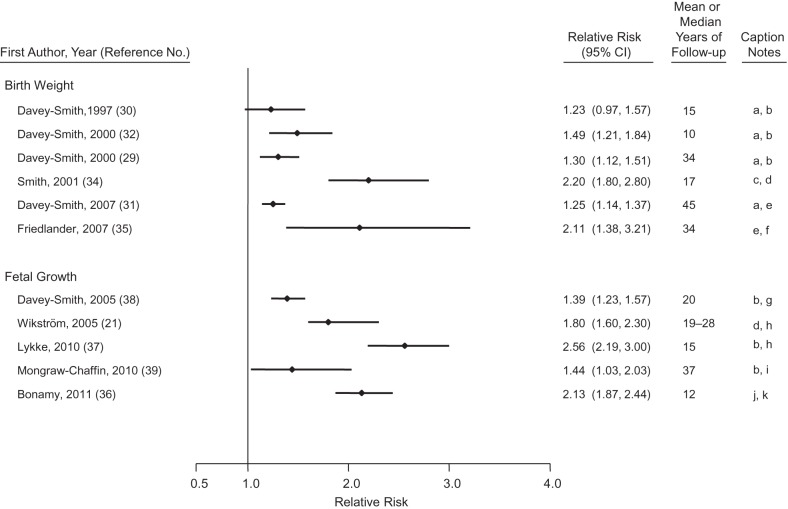
Offspring birth weight predicts maternal life span (29–33). Figure 1 presents the findings from studies that have examined associations of offspring birth weight or fetal growth (a function of birth weight and gestational length) with maternal CVD risk (18, 21, 29–32, 34–39). One meta-analysis has calculated that, for every standard deviation (roughly 500 g) higher birth weight of the firstborn child, maternal CVD mortality is decreased by 25% (31). It is unclear whether the inverse association of offspring birth weight with mortality is constant across the entire range of birth weight, as the association of high birth weight with maternal CVD risk varies by study. In some populations, the mothers of the largest infants (>4,000 or >4,500 g) have the lowest risks of CVD (30, 32), while in other populations there is an uptick in CVD risk for the mothers of macrosomic newborns (31, 35, 36, 40). Given the strong associations of macrosomia with GDM and later type 2 diabetes (41), the presence and magnitude of the association of large birth weight with future CVD risk may depend on the population prevalence of GDM and chronic diabetes during pregnancy (in other words, the extent to which large infant size is pathological). Indeed, the association of macrosomia with CVD risk is attenuated by adjustment for GDM (36), indicating that a substantial portion of the association of macrosomia and CVD is explained by metabolic risk.
Figure 1.
Results from studies of offspring birth weight or fetal growth and relative risk of maternal cardiovascular disease. Caption notes: a, per 1-standard deviation lower birth weight; b, cardiovascular disease mortality; c, lowest birth weight quintile compared with all others; d, coronary heart disease events; e, coronary heart disease mortality; f, birth weight <2,500 g compared with 1,500–3,999 g; g, per 1-standard deviation lower birth weight, adjusted for gestational age; h, small for gestational age; i, intrauterine growth restriction; j, ∼2 standard deviations below the mean birth weight adjusted for gestational age; k, cardiovascular disease events. CI, confidence interval.
It is abundantly clear, however, that the 8% of deliveries that are low birth weight (<2,500 g) are associated with twice the maternal CVD incidence and mortality of other deliveries (29–32). Associations of offspring birth weight with maternal CVD are only modestly diminished by adjustment for cigarette smoking and not affected by control for prepregnancy body mass index (36, 38).
Birth weight is the product of fetal growth rate and gestational length. Fetal growth, represented as birth weight corrected for gestational length, predicts maternal CVD risk (31, 36, 40), as does gestational length (reviewed below). In fact, the coincidence of restricted fetal growth and prematurity yields a more than 3-fold increased CVD risk (36). The curvilinear association of offspring birth weight with maternal CVD risk observed in many populations may be the product of competing pathological phenomena. At one end of the birth weight spectrum, the association of macrosomia with maternal CVD risk may be explained by underlying metabolic risk; at the other end of the spectrum, the association of low birth weight with maternal CVD risk may be driven by endothelial dysfunction and other pathologies associated with restricted fetal growth and preterm birth.
First offspring birth weight also predicts paternal CVD, although the magnitude of the positive association of offspring birth weight with paternal CVD risk is less than a third of that for the infant's mother (31). The fact that the birth weight of their first child predicts CVD events in both parents suggests that shared lifestyle or environmental factors, such as cigarette smoking, might influence both the growth of the fetus and CVD risk in the parents and/or that pleiotropic genetic variants affect both growth and CVD risk. Birth weight is passed down through maternal and paternal lines (42), opening the possibility that paternal CVD/fetal growth genes could affect both the pregnancy outcome and long-term chronic disease risk in the father (43). However, the stronger association in mothers than in fathers suggests either parent-specific genomic imprinting or—as seems more parsimonious—that maternal health during pregnancy affects fetal growth and is a marker of her future CVD risk.
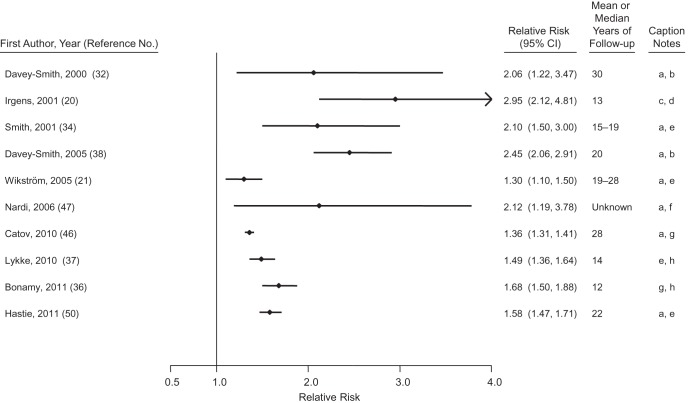
Preterm delivery (<37 weeks’ gestation) accounts for 6%–12% of deliveries in the developed world (44). The hazard ratios for CVD associated with total preterm delivery are depicted in Figure 2 and are on the order of 1.3–2.6 for births with <37 completed weeks compared with term births (20, 21, 32, 34, 36–38, 45–49). There is a greater range of relative risk when distinct preterm phenotypes are examined separately. Although most preterm deliveries follow spontaneous labor or preterm premature rupture of membranes, a significant and growing fraction result from medically induced labor or cesarean section without labor. The chief reasons for these medically indicated deliveries include preeclampsia and fetal growth restriction, both of which have been associated with increased maternal CVD risk. In studies that have distinguished them, hypertensive preterm deliveries consistently have a stronger association with maternal CVD outcomes than do normotensive preterm deliveries, though the latter are still associated with a 1.2- to 3-fold increased risk compared with term deliveries (20, 46). In the 2 studies that have contrasted CVD risk among mothers with spontaneous versus indicated preterm deliveries (49, 50), indicated delivery was associated with higher risks of CVD mortality than was spontaneous preterm delivery. Nevertheless, spontaneous preterm delivery (compared with term delivery) was associated with doubling of CVD risk (49, 50).
Figure 2.
Results from studies of preterm delivery and relative risk of maternal cardiovascular disease. Caption notes: a, <37 gestation weeks compared with term; b, cardiovascular disease mortality; c, <37 weeks’ gestation length compared with term normotensive pregnancies; d, cardiovascular disease mortality, excluding stroke mortality; e, coronary heart disease events; f, myocardial infarction; g, cardiovascular disease events; h, 32–36 weeks’ gestation length compared with term. CI, confidence interval.
Unlike the associations of parity or birth weight with paternal CVD risk, 2 studies (20, 38) have reported that preterm delivery is not associated with paternal risk of CVD, implying that the association of preterm delivery with maternal CVD risk is not the product of a high CVD risk lifestyle or genetic variants shared between both parents and their offspring. Of relevance, preterm birth risk appears to be passed only through the maternal line (51). These observations suggest that maternal intrauterine environment and health determine the risk of preterm delivery and explain its association with maternal CVD risk, rather than shared lifestyle or environment of mother and father.
Gestational diabetes mellitus is a common and growing pregnancy complication that affects as many as 5% of pregnancies. It is well established that women with GDM are at increased risk of developing diabetes later in life (52); between 3% and 70% of women with a history of GDM will develop type 2 diabetes within 3 decades of the pregnancy (53), with a meta-analysis of 675,455 women finding a 7-fold increase in risk of later type 2 diabetes (52). Type 2 diabetes is an important CVD risk factor, having a markedly higher relative and absolute association with CVD in women than it does in men (54). Given these associations, it seems self-evident that a history of GDM would be associated with increased CVD risk. However, largely because of the fact that GDM screening during pregnancy was neither routine nor standardized until recent decades, there are few cohorts with long enough follow-up of screened populations to detect CVD incidence or mortality among women with a history of GDM (55, 56). These are displayed in Figure 3. The only large population-based study of this topic is a record linkage study conducted in Ontario, Canada, with a median follow-up of 11.5 years (55). In that study, a history of GDM was associated with a greater risk of hospital admission for acute myocardial infarction, coronary bypass, coronary angioplasty, stroke, or carotid endarterectomy (hazard ratio (HR) = 1.71, 95% CI: 1.08, 2.69). Upon adjustment for diabetes after pregnancy, the association was attenuated toward the null (adjusted HR = 1.13, 95% CI: 0.67, 1.89). A smaller, cross-sectional study found that women with a history of GDM had a higher CVD risk than women without a history of GDM (adjusted odds ratio = 1.85, 95% CI: 1.21, 2.82) and experienced CVD events 7 years earlier, on average (56).
Figure 3.

Results from studies of gestational diabetes mellitus and relative risk of maternal cardiovascular disease. Caption notes: a, self-reported coronary artery disease; b, cardiovascular disease events. CI, confidence interval.
Lesser degrees of antepartum hyperglycemia have also been associated with an elevated risk of subsequent diabetes and CVD. In the Ontario study, women with evidence of elevated glycemia short of GDM criteria were at an increased risk of diabetes (adjusted HR = 2.56, 95% CI: 2.28, 2.87) (57) and CVD (adjusted HR = 1.19, 95% CI: 1.02, 1.39) (58) compared with normoglycemic women.
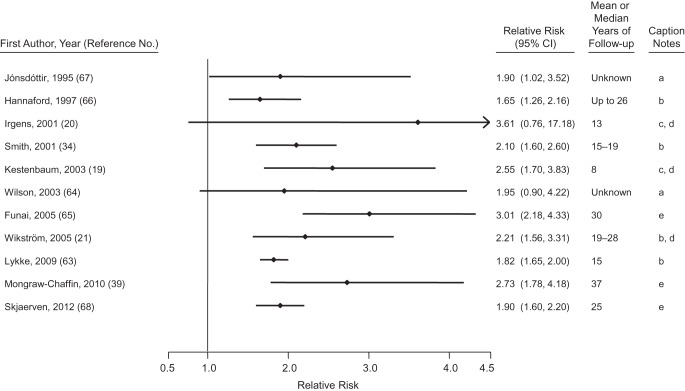
Hypertensive disorders of pregnancy are common pregnancy complications that presage CVD. Preeclampsia, the combination of hypertension and proteinuria, affects approximately 2%–5% of pregnancies, with a predominance among first pregnancies (17, 59). Estimates of the prevalence of gestational hypertension, new-onset hypertension without proteinuria, vary from 3% to 14% (17, 59, 60). Women with a history of preeclampsia have roughly 4-fold higher incidence of hypertension and 2-fold elevated risks of heart disease, stroke, and venous thromboembolism (18, 61). Two systematic reviews, one of cohort studies (n = 25) and the other of both cohort (n = 10) and case-control (n = 5) studies, have both reported a doubling of risk for different measures of CVD comparing women with preeclampsia with normotensive women over a median of 10–12 years of follow-up (18, 61). Figure 4 depicts the relative risk of coronary heart disease and CVD outcomes among mothers with a history of preeclampsia (19–21, 34, 39, 62–67).
Figure 4.
Results from studies of hypertensive disorders of pregnancy and relative risk of maternal cardiovascular disease. Caption notes: a, coronary heart disease mortality; b, coronary heart disease events; c, cardiovascular disease events; d, composite estimate provided by the Bellamy et al. review (18); e, cardiovascular disease mortality. A 2011 study by Lin et al. (62) reported a relative risk of 23.0 (95% confidence interval: 5.1, 103.7) for cardiovascular disease events (except stroke) during pregnancy and up to 3 years after delivery. We omitted that study from the figure so that we could keep the relative risk scale consistent across figures.
Publications from 3 cohort studies published since those reviews give some insight into the onset and duration of CVD risk following hypertensive disorders of pregnancy (34, 39, 62, 63). In a short-term follow-up of over 1,000,000 pregnancies in Taiwan, women with preeclampsia/eclampsia were at double the risk of major CVD from the third trimester of pregnancy up to 3 years postpartum, with particularly high unadjusted relative risks for stroke (HR = 21.0, 95% CI: 2.5, 174.0) and myocardial infarction (HR = 22.6, 95% CI: 8.7, 58.4) (62). Although these results suggest a high relative risk immediately following hypertensive disorders of pregnancy, the confidence intervals are wide and the absolute risk of CVD events is very small at this age, so that this immediate risk is unlikely to account for a large number of CVD events. The Child Health and Development Study in California has provided some of the longer follow-up; over 37 years after pregnancy, women with a history of preeclampsia in any pregnancy had double the risk of CVD death (adjusted HR = 2.14, 95% CI: 1.29, 3.57) (39). This doubling of risk is consistent with studies with shorter duration of follow-up. Consideration of the exponential increase in the absolute numbers of CVD events with increasing age suggests that the elevated risk of CVD among women with a history of hypertensive disorders of pregnancy is not limited to the early years postpartum.
Thus, studies repeatedly report a doubling of CVD risk among women with a history of preeclampsia and suggest lesser degrees of excess risk among women with a history of gestational hypertension, despite the strong association of gestational hypertension with development of chronic hypertension (63). The combination of preterm delivery and preeclampsia—a likely marker of the severity of preeclampsia—is a particularly potent predictor of CVD risk. Compared with normotensive term pregnancies, women delivering preterm preeclamptic pregnancies have very high relative risks of future CVD ranging from 2.5 to 9.5 (34, 39, 63, 68).
Recurrent pregnancy complications, last pregnancy complications, and maternal CVD risk
Much of the above literature is based on first pregnancies, precluding examination of the association of recurring pregnancy complications with CVD risk. There is evidence that recurrent preeclampsia (63) and preterm delivery (45, 46) are associated with a greater risk of CVD than a single complicated pregnancy in multiparous women. Although the association of recurrent GDM with CVD risk has not been studied, after a first GDM pregnancy, each subsequent GDM pregnancy has been associated with a modestly increased risk of type 2 diabetes (adjusted HR = 1.16, 95% CI: 1.01, 1.34) and each non-GDM pregnancy with a reduced risk of diabetes (adjusted HR = 0.34, 95% CI: 0.27, 0.41) (69). In fact, this highlights an intriguing pattern that is emerging with respect to last births: Having preeclampsia (68), preterm delivery (49), or GDM (69) in the last pregnancy appears to be associated with especially high risk of future CVD in mothers. Perhaps reflecting the same phenomenon, women who have 1 preterm delivery and 1 term delivery in their first 2 births appear to be at higher risk of coronary heart disease if the preterm delivery was the second birth (45, 46). This suggests that pregnancy complications severe enough to contraindicate or discourage a subsequent pregnancy may be particularly potent predictors of future CVD risk.
PHYSIOLOGICAL MECHANISMS LINKING PREGNANCY COMPLICATIONS TO MATERNAL CVD RISK
Pathways that link pregnancy exposures to later life CVD are not well understood. Considerable evidence supports the existence of common predisposing factors for both pregnancy complications and CVD risk. There have been almost no studies examining the alternative, that pregnancy complications might cause increased CVD risk. To address this issue, we summarize evidence that compares CVD risk before, during, and after pregnancies with and without complications.
Cardiovascular risk factors preceding pregnancy complications
Subtle prepregnancy blood pressure elevation is evident before many pregnancies complicated by preeclampsia and fetal growth restriction. Chronic hypertension has a well established relation to increased risk of preeclampsia (known as “superimposed” preeclampsia). Even within the normotensive range, there is a positive dose-response association of prepregnancy systolic and/or diastolic blood pressure with preeclampsia (70). Preexisting hypertension has also been associated with growth restriction, especially in cases that were also preterm (71). Risks for these complications also rise with increasing maternal age, suggesting that the aging endothelium may less successfully adapt to the profound vascular demands of pregnancy.
Prepregnancy lipid concentrations are also associated with pregnancy complications and offspring birth weight; the nature of the association varies with the pregnancy outcome in question. Lipid profiles consistent with elevated CVD risk, including higher prepregnancy triglyceride levels, total cholesterol, and lower high density lipoprotein cholesterol, have been associated with preeclampsia and preterm delivery in the study in Norway (70, 72).
The US Coronary Artery Risk Development in Young Adults (CARDIA) Study found a curvilinear association of prepregnancy cholesterol levels with risk of delivering preterm (73). With respect to fetal growth, women with a more atherogenic lipid profile may bear larger infants (74); this suggests that the association of low birth weight—at least the fetal growth component of low birth weight—with maternal CVD risk may not operate via dyslipidemia.
Prepregnancy adiposity and glucose/insulin dysregulation are strongly implicated in the etiology of GDM, based on the observation that women with GDM tend to have family history of type 2 diabetes and higher body mass index before pregnancy (75), as well as higher levels of glucose, insulin, and lower levels of adiponectin before the onset of the midpregnancy hyperglycemia that defines GDM (76–80). Higher body mass index and family history of diabetes are also associated with increased risk of preeclampsia (81, 82). The risk of preeclampsia doubles with every 5–7 kg/m2 increase in body mass index before pregnancy (82).
Thus, subclinical elevations in the classic CVD risk factors of blood pressure, lipid levels, elevated body mass index, and glucose/insulin dysregulation appear to predate both preeclampsia and GDM. Less clear is the extent to which CVD risk factors precede spontaneous preterm deliveries or fetal growth restriction in normotensive pregnancies. To our knowledge, the roles of prepregnancy inflammatory and coagulation factors have not been studied with respect to pregnancy complications, despite the importance of these systems for both reproduction and CVD risk (83).
Cardiovascular risk factors during pregnancy
Cardiovascular adaptation in normal pregnancy
In normal gestation, maternal blood volume increases progressively from 6 to 8 weeks’ gestation, peaking at an increase of 45% by 32 weeks (84). Cardiac output increases by 30%–50%, with half of this increase occurring very early in gestation. Pulse rate increases 17%, and there are striking alterations in renal physiology. Although the insulin response to glucose is augmented in early pregnancy, insulin resistance emerges in the second half of pregnancy (85). In addition, cholesterol and triglyceride profiles change after gestation week 9 to support steroid synthesis and fetal growth (86). In uncomplicated pregnancy, there is a tendency for low density lipoproteins (LDLs) to shift across gestation from large, buoyant particles to smaller, denser, and more atherogenic particles (87). Fat is accumulated during the second trimester and then mobilized to support the dramatic fetal growth of the third trimester (88).
Cardiovascular risk factors during pregnancy complications
Vascular and endothelial dysfunction is characteristic of pregnancies complicated by preeclampsia or growth restriction. Placental underperfusion is common, and there are elevated markers of endothelial dysfunction in the maternal circulation. Women with hypertensive disorders of pregnancy demonstrate increased resistance in the uterine arteries (89, 90), vascular stiffness, and impaired endothelial response (91, 92). In addition, placental vascular lesions indicative of failed spiral artery remodeling, ischemia, or hemorrhage have also been reported in cases of both medically indicated and spontaneous preterm birth (93).
During pregnancy, lipid aberrations accompany several pregnancy complications. Again, the direction of the associations appears to depend on the nature of the pregnancy complication. The dylipidemias associated with atherosclerosis (hypertriglyceridemia, hypercholesterolemia, elevated free fatty acids, and excess oxidized LDLs) are frequently seen during preeclampsia (94–97). There is also emerging evidence to suggest that this atherogenic lipid profile is associated with both spontaneous and indicated preterm births (98, 99). Similarly, women with GDM exhibit elevations in triglycerides and, less consistently, total cholesterol and LDLs during pregnancy (100). On the other hand, low maternal total and LDL cholesterol concentrations appear in the third trimester in pregnancies complicated by fetal growth restriction (101). Placental studies are conflicting, with some suggesting reduced expression of lipoprotein receptors in placentas from fetal growth restriction versus appropriate weight-for-gestational-age births (102) and others suggesting overexpression of these receptors (103). Fetal growth restriction studies are hampered by nonstandard phenotyping, and thus findings may represent different levels of severity. Despite these limitations, these data suggest that extremes of lipid concentrations are associated with adverse pregnancy outcomes. Longitudinal studies are needed to better understand how the relative contributions of low or high cholesterol are related to failed or compensatory lipid adaptation required to optimize fetal growth.
Metabolic dysregulation in pregnancy defines GDM and is a strong risk factor for preeclampsia; there is considerable overlap of the 2 conditions, with twice the rate of preeclampsia in diabetic versus nondiabetic pregnancies (104). However, GDM has only a modest association with spontaneous preterm birth (105). Higher early pregnancy body mass index is associated with increased risk of hypertensive disorders of pregnancy and GDM (75, 82, 106) but with reduced risk of small for gestational age and spontaneous preterm birth in most studies (107).
Systemic inflammation during pregnancy may be important in the pathogenesis of several pregnancy complications. Elevated serum levels of C-reactive protein and/or leukocytes have been detected in women who experience GDM, fetal growth restriction, and both spontaneous and indicated preterm deliveries (108–112). However, neither midgestation circulating levels of C-reactive protein nor proinflammatory cytokines have proven to have prognostic value for specific pregnancy outcomes (113, 114).
Normal pregnancy is a state of hypercoagulability, and complications such as preeclampsia and preterm birth are characterized by particularly high biomarkers of an activated fibrinolytic cascade, as well as perhaps an impaired ability to mount this response appropriately (115–117). It has been hypothesized that aberrations in the cross-talk between inflammation and the coagulation cascades could contribute to the pathophysiology of these pregnancy complications (118).
Cardiovascular risk factors after pregnancy
Enduring cardiovascular impact of normal pregnancy
Most of the cardiovascular adaptations to normal pregnancy resolve in the postpartum period, although there are some detectable and lasting pregnancy effects. Blood pressure is modestly decreased in the postpartum period after a first uncomplicated pregnancy (119). However, other lingering effects are not as salutary. Importantly, women retain, on average, 0.5–5.0 kg of weight following each pregnancy (120, 121). Lactation may help resolve the cardiometabolic adaptations and fat accumulation associated with pregnancy (122–124).
The first birth may be a sentinel marker for complications in later pregnancies and future CVD risk (125–127). Several factors distinguish first births. First, longitudinal studies suggest that the lasting blood pressure and lipid changes associated with pregnancy occur after first, but not subsequent, births (119). In addition, first births are at higher risk for the major obstetric complications of preterm delivery, hypertensive disorders of pregnancy, fetal growth restriction, and stillbirth. Women with any of these complications are at higher risk in subsequent pregnancies for recurrence of the same complication as well as the onset of other complications. Importantly, complications during a first pregnancy impact the likelihood of having a subsequent pregnancy. As noted above, complications in a last pregnancy appear to be associated with especially high relative risks of CVD events. Thus, health status of the first and last pregnancies may be particularly telling of future maternal health.
The cumulative effect of these adaptations and resolutions and risks may contribute to the above-noted J-shaped association between parity and maternal CVD risk, with lowest risk for women who have delivered 2 infants. It is not clear whether pregnancies exert a cumulative cardiovascular burden with increasing parity, whether higher-order pregnancies at more advanced maternal age exert more cardiovascular risk, or whether women at high cardiovascular risk bear more children.
Cardiovascular risk after pregnancy complications
The association of vascular and endothelial dysfunction with pregnancy complications continues after delivery. Women with preeclampsia have impaired endothelial function after pregnancy (128). This may also be true, although to a lesser extent, of women who deliver small babies due to fetal growth restriction or preterm delivery. For example, lower offspring birth weight is associated with higher maternal blood pressure in the years after pregnancy (129). Some (130), but not all (131), studies report higher blood pressure and atherosclerotic carotid vessel remodeling among women who have delivered a fetal growth restriction neonate. Although studies are not unanimous (132, 133), women with a history of GDM are more likely to have hypertension (134, 135), vascular dysfunction (136), impaired endothelium-dependent vasodilatation (137), and higher carotid artery intima-media thickness (138). These differences are not fully explained by the higher body mass index typical of women with a history of GDM.
Studies of lipid profiles after pregnancies complicated by preeclampsia are consistent with increased atherogenesis risk, including consistently reported higher total cholesterol, LDL cholesterol, and triglycerides, although these differences are not always statistically significant (17, 139–145). Associations of reduced high density lipoprotein cholesterol after preeclampsia have been reported by some (17, 140, 144, 145), but not all (139, 141, 142), studies. One study has reported dyslipidemia among women with a history of spontaneous and indicated preterm births (131). Some (133–135, 146), but not all (17, 136), have reported elevated total cholesterol, LDL cholesterol, and/or triglycerides in women with a history of GDM. As with the studies of lipid concentrations before and during pregnancy, studies of lipid concentrations in women in the years after fetal growth restriction are conflicting, with some reporting hyperlipidemia (147) and others reporting no differences compared with women with uncomplicated births (131).
It is now firmly established that women with a history of GDM have a manifold higher risk of developing type 2 diabetes than women with normoglycemic pregnancies (52). It is less widely appreciated that women with a history of preeclampsia are also at high risk of type 2 diabetes. After preeclampsia, mothers are 3 times more likely to develop diabetes within 16 years (63), an observation bolstered by evidence of dysregulated glucose and insulin, as well as insulin resistance as early as 2 years after preeclamptic pregnancy (17, 139, 140, 142, 145, 148). However, not all pregnancy complications are associated with risk of future metabolic disorder: In the Nurses’ Health Study 2, although the 2% of women who delivered a very preterm infant (<32 weeks’ gestation) had a 35% higher risk of developing type 2 diabetes, moderate preterm delivery was not associated with increased diabetes risk (149).
After pregnancy, plasma C-reactive protein is elevated among women with prior preeclampsia and indicated preterm births, suggesting that systemic low grade inflammation may link some adverse pregnancy outcomes and later CVD (150, 151). Several studies have documented higher C-reactive protein levels among women with a history of GDM (136, 146, 152). Although inflammation seems a likely culprit to explain the association of spontaneous preterm delivery with CVD risk, the only study to date that has examined this question has reported no differences in plasma C-reactive protein levels of women with a history of spontaneous preterm delivery compared with term delivery (150).
Women with a history of pregnancies complicated by preeclampsia may maintain a procoagulation state in the years after pregnancy, predisposing them to vascular and thrombotic events (153), although this pathway is less studied than others linking pregnancy complications to maternal CVD risk.
Thus, the associations of pregnancy complications with future CVD events in women are likely explained, at least in part, by their associations with classic CVD risk factors of hypertension, dyslipidemia, type 2 diabetes, and perhaps inflammation and thrombosis, which are evident before, during, and after such complicated pregnancies. The pregnancy complications may serve as an early indication that a woman is on a high CVD risk trajectory, before these classic CVD risk factors are clinically detected.
IMPLICATIONS
Research implications
The bulk of the research associating pregnancy history to CVD risk is derived from the linkage of large, often national, vital statistics registries for birth, hospitalization, and mortality statistics. These exercises have yielded consistent associations of pregnancy complications with CVD risk. However, as most registries were founded in the 1950s or 1960s, the longest running have been able to follow women only into the early postmenopausal years. The data on GDM are further limited by the lack of consistent methods for screening and diagnosing GDM. Further follow-up will determine the extent to which the associations of pregnancy complications are maintained into the age range at which CVD events are most common in women. In the meantime, the stratification of risk by time since pregnancy is a helpful way to examine the extent to which risk associated with pregnancy complications changes over time (50). Although relative risk of CVD events may weaken with time, the absolute risks associated with a history of pregnancy complications are likely to grow with time since pregnancy, as women age.
To understand the trajectory of CVD risk after pregnancy, we need more studies to measure established CVD risk factors among participants before, during, and after pregnancy. In addition, we should incorporate pregnancy history data into existing CVD cohorts with decades of follow-up. By illuminating the timing with which particular CVD risk factors emerge in the wake of specific pregnancy complications, we may be able to leverage the information contained by pregnancy history to predict CVD risk earlier than by conventional risk screening protocols.
We need to establish whether pregnancy complications and history act as stress tests to unmask women who are already at risk, or whether there is some additional causal stress of the pregnancy experience itself. Irrespective of causality, a key question is the extent to which pregnancy history can be used to improve CVD risk-scoring systems for women, such as the Framingham Risk Score. At present, these scoring systems are of debatable utility for women under the age of 70 years (154), and the addition of pregnancy complications to prediction at these relatively younger ages may be particularly important.
If these complications are useful for early CVD risk identification, the next question is whether earlier risk identification—as early as at the time of pregnancy—is a cost-effective way of reducing future risk. We would need to test the extent to which lifestyle or pharmacological prevention is effective at preventing future CVD in young or middle-aged women with a history of pregnancy complications. Key to this is identifying stages in the life course when women are (or are not) receptive to CVD prevention, including the postpartum year. It is also important to examine the extent to which standard CVD primordial and primary prevention is effective in women with a history of pregnancy complications, or whether new screening, prevention, and therapy protocols can be optimized on the basis of a woman's particular pregnancy history.
Taking all of this together, future research requires large data sets that have prospectively collected accurate data on cardiovascular risk factors before, during, and after pregnancy, into middle age and beyond, when disease begins to emerge. Data on pregnancy complications are also required. Only with such detailed information can we determine the extent to which specific pregnancy complications are related to future CVD, over and above prepregnancy risk factors, and whether they add to established risk factor scores calculated in middle age. With large birth cohorts increasingly recognizing the importance of long-term follow-up of mothers as well as their infants, the potential for this research is increasing. Ultimately, randomized controlled trials will be necessary to establish whether pregnancy advice and/or continued monitoring and early treatment of women identified as at risk during pregnancy are cost-effective ways of reducing CVD risk in women.
Methods for improving our understanding of whether pregnancy complications are causally related to later maternal health need to go beyond conventional multivariable approaches in prospective cohorts. For example, if it is found that genetic variants associated with high blood pressure and glucose intolerance/type 2 diabetes in general populations of men and nonpregnant women are also associated with hypertensive disorders of pregnancy and GDM, this would lend some support to the hypothesis of a common etiology and pregnancy unmasking a preexisting (genetic) risk. There is some evidence that several type 2 diabetes mellitus variants from genome-wide association studies show robust associations with GDM (155–158).
Although it is not feasible to randomize women to pregnancy complications, long-term follow-up of women who have been in randomized controlled trials that have effectively treated the pregnancy complication will also address some of the research questions above. Finally, experimental induction of pregnancy complications in animal models and following the mothers after delivery to examine whether vascular damage was sustained or metabolic risk increased are important for examining the question of a pregnancy causal effect (159). However, the generalizability of the animal models depends on the fidelity with which the human pregnancy complications, such as preeclampsia, can be mimicked in other species, where they may not occur naturally.
Implications for prenatal care and CVD prevention
The associations of pregnancy complications with CVD events are remarkably consistent.
Although untested, the use of pregnancy complication history to screen women for targeted CVD prevention has potential to improve public health, given the magnitude of the associations, the prevalence of the pregnancy complications, and the importance of CVD in women. Several pregnancy complications are more common among racial minority groups, who are also at higher risk of metabolic and cardiovascular disease. Pregnancy complications occur early enough in a woman's life course to offer a significant meaningful “runway” for primordial CVD prevention by lifestyle intervention and primary prevention by statins and antihypertensive drugs. In 2011, both the American Heart Association and the European Society of Cardiology included histories of preeclampsia and (in the case of the American Heart Association) GDM as part of CVD risk assessment that would trigger closer monitoring and control of CVD risk factors (7, 160).
We are just at the beginning of this research and clinical agenda. First, we need to establish the clinical relevance of pregnancy complications for maternal chronic disease risk and our ability to change the health trajectories of women with histories of complicated pregnancy; then we will have to consider the many issues of integrating the findings into clinical and public health systems. Some potential clinical implications have been addressed elsewhere, including the need to link prenatal with primary care medical records, development of clinical screening, prevention and treatment protocols after pregnancy complications, and increasing awareness among clinicians of these associations that span typical clinical silos between obstetrics and medicine (161).
CONCLUSION
The stress test of pregnancy provides glimpses into the otherwise silent early adult years in which chronic disease trajectories are set. Research to characterize the ways in which pregnancy complications inform us about subclinical and clinical vascular and metabolic risk in the mother is in its infancy. This research requires integration across such diverse specialties including obstetrics, primary care, pediatrics, endocrinology, and cardiology. This broader perspective may yield novel insights into the determinants of pregnancy outcomes and lifelong health, perhaps creating a large shift in the ways in which we promote the health of women and children.
ACKNOWLEDGMENTS
Author affiliations: Connors Center for Women's Health and Gender Biology, Brigham and Women's Hospital, Boston, Massachusetts (Janet W. Rich-Edwards); Harvard Medical School, Boston, Massachusetts (Janet W. Rich-Edwards); Harvard School of Public Health, Boston, Massachusetts (Janet W. Rich-Edwards); Medical Research Council, Centre for Causal Analysis in Translational Epidemiology, School of Social and Community Medicine, University of Bristol, Bristol, United Kingdom (Abigail Fraser, Deborah A. Lawlor); Departments of Obstetrics and Gynecology and of Epidemiology, University of Pittsburgh, Pittsburgh, Pennsylvania (Janet M. Catov); and Magee-Womens Research Institute, Pittsburgh, Pennsylvania (Janet M. Catov).
This work was supported by the Maternal and Child Health Lifecourse Research Network. A. F. and D. A. L. work in a center that receives infrastructure funding from the United Kingdom Medical Research Council (G0600705), and A. F. is funded by a United Kingdom Medical Research Council fellowship (G0701594). A grant to D. A. L. from the Wellcome Trust also supports this collaborative work (WT094529MA). J. M. C. is funded by RO1HL103825 and K12HD43441. J. R. E. is supported by an American Heart Association Founder's Grant (13GRNT17070022).
Conflict of interest: none declared.
REFERENCES
- 1.Rich-Edwards JW. Reproductive health as a sentinel of chronic disease in women. Womens Health (Lond Engl) 2009;5(2):101–105. doi: 10.2217/17455057.5.2.101. [DOI] [PubMed] [Google Scholar]
- 2.Decline in deaths from heart disease and stroke—United States, 1900–1999. MMWR Morb Mortal Wkly Rep. 1999;48(30):649–656. [PubMed] [Google Scholar]
- 3.Yusuf S, Reddy S, Ôunpuu S, et al. Global burden of cardiovascular diseases. Circulation. 2001;104(22):2746–2753. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 4.Shah RU, Klein L, Lloyd-Jones DM. Heart failure in women: epidemiology, biology and treatment. Womens Health (Lond Engl) 2009;5(5):517–527. doi: 10.2217/whe.09.50. [DOI] [PubMed] [Google Scholar]
- 5.Mathers C, Boerma JT, Fat DM, et al. The Global Burden of Disease: 2004 Update. Geneva, Switzerland: World Health Organization; 2008. [Google Scholar]
- 6.Shaw LJ, Bairey Merz CN, Pepine CJ, et al. Insights from the NHLBI-sponsored Women's Ischemia Syndrome Evaluation (WISE) Study: Part I: gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J Am Coll Cardiol. 2006;47(suppl 3):S4–S20. doi: 10.1016/j.jacc.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 7.Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: a guideline from the American Heart Association. Circulation. 2011;123(11):1243–1262. doi: 10.1161/CIR.0b013e31820faaf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shay CM, Ning H, Allen NB, et al. Status of cardiovascular health in US adults: clinical perspective prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2003–2008. Circulation. 2012;125(1):45–56. doi: 10.1161/CIRCULATIONAHA.111.035733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chronic disease reports in the Morbidity and Mortality Weekly Report (MMWR) MMWR Morb Mortal Wkly Rep. 1989;38(suppl 1):1–8. [PubMed] [Google Scholar]
- 10.Scarborough P, Weissberg P. Trends in Coronary Heart Disease, 1961–2011. London, United Kingdom: British Heart Foundation; 2011. [Google Scholar]
- 11.Labarthe DR. Prevention of cardiovascular risk factors in the first place. Prev Med. 1999;29(6 Pt 2):S72–S78. doi: 10.1006/pmed.1999.0539. [DOI] [PubMed] [Google Scholar]
- 12.Weintraub WS, Daniels SR, Burke LE, et al. Value of primordial and primary prevention for cardiovascular disease: a policy statement from the American Heart Association. Circulation. 2011;124(8):967–990. doi: 10.1161/CIR.0b013e3182285a81. [DOI] [PubMed] [Google Scholar]
- 13.Sattar N, Greer IA. Pregnancy complications and maternal cardiovascular risk: opportunities for intervention and screening? BMJ. 2002;325(7356):157–160. doi: 10.1136/bmj.325.7356.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez G, Daniels K, Chandra A. Fertility of men and women aged 15–44 years in the United States: National Survey of Family Growth, 2006–2010. Natl Health Stat Report. 2012;51:1–28. [PubMed] [Google Scholar]
- 15.Social Policy Division, Organisation for Economic Co-operation and Development; Paris, France: 2010. SF2.5 report on childlessness. www.oecd.org/social/family/database. Accessed June 24, 2013. [Google Scholar]
- 16.World Fertility Report 2009. New York, NY: Department of Economic and Social Affairs, Population Division, United Nations; 2011. [Google Scholar]
- 17.Fraser A, Nelson SM, Macdonald-Wallis C, et al. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age. Circulation. 2012;125(11):1367–1380. doi: 10.1161/CIRCULATIONAHA.111.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellamy L, Casas JP, Hingorani AD, et al. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335(7627):974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kestenbaum B, Seliger SL, Easterling TR, et al. Cardiovascular and thromboembolic events following hypertensive pregnancy. Am Jf Kidney Dis. 2003;42(5):982–989. doi: 10.1016/j.ajkd.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Irgens HU, Reisaeter L, Irgens LM, et al. Long term mortality of mothers and fathers after pre-eclampsia: population based cohort study. BMJ. 2001;323(7323):1213–1217. doi: 10.1136/bmj.323.7323.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wikström AK, Haglund B, Olovsson M, et al. The risk of maternal ischaemic heart disease after gestational hypertensive disease. BJOG. 2005;112(11):1486–1491. doi: 10.1111/j.1471-0528.2005.00733.x. [DOI] [PubMed] [Google Scholar]
- 22.Green A, Beral V, Moser K. Mortality in women in relation to their childbearing history. BMJ. 1988;297(6645):391–395. doi: 10.1136/bmj.297.6645.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ness RB, Harris T, Cobb J, et al. Number of pregnancies and the subsequent risk of cardiovascular disease. N Engl J Med. 1993;328(21):1528–1533. doi: 10.1056/NEJM199305273282104. [DOI] [PubMed] [Google Scholar]
- 24.Steenland K, Lally C, Thun M. Parity and coronary heart disease among women in the American Cancer Society CPS II population. Epidemiology. 1996;7(6):641–643. doi: 10.1097/00001648-199611000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Parikh NI, Cnattingius S, Dickman PW, et al. Parity and risk of later-life maternal cardiovascular disease. Am Heart J. 2010;159(2):215–221. doi: 10.1016/j.ahj.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 26.Ness RB, Cobb J, Harm T, et al. Does number of children increase the rate of coronary heart disease in men? Epidemiology. 1995;6(4):442–445. doi: 10.1097/00001648-199507000-00023. [DOI] [PubMed] [Google Scholar]
- 27.Lawlor DA, Emberson JR, Ebrahim S, et al. Is the association between parity and coronary heart disease due to biological effects of pregnancy or adverse lifestyle risk factors associated with child-rearing? Circulation. 2003;107(9):1260–1264. doi: 10.1161/01.cir.0000053441.43495.1a. [DOI] [PubMed] [Google Scholar]
- 28.Dekker JM, Schouten EG. Number of pregnancies and risk of cardiovascular disease. N Engl J Med. 1993;329(25):1893–1894. author reply 1894–1895. [PubMed] [Google Scholar]
- 29.Davey-Smith G, Harding S, Rosato M. Relation between infants’ birth weight and mothers’ mortality: prospective observational study. BMJ. 2000;320(7238):839–840. doi: 10.1136/bmj.320.7238.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davey-Smith G, Hart C, Ferrell C, et al. Birth weight of offspring and mortality in the Renfrew and Paisley study: prospective observational study. BMJ. 1997;315(7117):1189–1193. doi: 10.1136/bmj.315.7117.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davey-Smith G, Hypponen E, Power C, et al. Offspring birth weight and parental mortality: prospective observational study and meta-analysis. Am J Epidemiol. 2007;166(2):160–169. doi: 10.1093/aje/kwm054. [DOI] [PubMed] [Google Scholar]
- 32.Davey-Smith G, Whitley E, Gissler M, et al. Birth dimensions of offspring, premature birth, and the mortality of mothers. Lancet. 2000;356(9247):2066–2067. doi: 10.1016/S0140-6736(00)03406-1. [DOI] [PubMed] [Google Scholar]
- 33.Catov J, Newman A, Roberts J, et al. Association between infant birth weight and maternal cardiovascular risk factors in the Health, Aging, and Body Composition Study. Ann Epidemiol. 2007;17(1):36–43. doi: 10.1016/j.annepidem.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Smith GC, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: a retrospective cohort study of 129,290 births. Lancet. 2001;357(9273):2002–2006. doi: 10.1016/S0140-6736(00)05112-6. [DOI] [PubMed] [Google Scholar]
- 35.Friedlander Y, Paltiel O, Manor O, et al. Birthweight of offspring and mortality of parents: the Jerusalem perinatal study cohort. Ann Epidemiol. 2007;17(11):914–922. doi: 10.1016/j.annepidem.2007.07.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonamy AK, Parikh NI, Cnattingius S, et al. Birth characteristics and subsequent risks of maternal cardiovascular disease: effects of gestational age and fetal growth. Circulation. 2011;124(25):2839–2846. doi: 10.1161/CIRCULATIONAHA.111.034884. [DOI] [PubMed] [Google Scholar]
- 37.Lykke JA, Langhoff-Roos J, Lockwood CJ, et al. Mortality of mothers from cardiovascular and non-cardiovascular causes following pregnancy complications in first delivery. Paediatr Perinat Epidemiol. 2010;24(4):323–330. doi: 10.1111/j.1365-3016.2010.01120.x. [DOI] [PubMed] [Google Scholar]
- 38.Davey-Smith G, Sterne J, Tynelius P, et al. Birth weight of offspring and subsequent cardiovascular mortality of the parents. Epidemiology. 2005;16(4):563–569. doi: 10.1097/01.ede.0000164790.96316.c0. [DOI] [PubMed] [Google Scholar]
- 39.Mongraw-Chaffin ML, Cirillo PM, Cohn BA. Preeclampsia and cardiovascular disease death: prospective evidence from the Child Health and Development Studies cohort. Hypertension. 2010;56(1):166–171. doi: 10.1161/HYPERTENSIONAHA.110.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lykke JA, Paidas MJ, Triche EW, et al. Fetal growth and later maternal death, cardiovascular disease and diabetes. Acta Obstet Gynecol Scand. 2012;91(4):503–510. doi: 10.1111/j.1600-0412.2011.01355.x. [DOI] [PubMed] [Google Scholar]
- 41.Metzger BE, Cho NH, Roston SM, et al. Prepregnancy weight and antepartum insulin secretion predict glucose tolerance five years after gestational diabetes mellitus. Diabetes Care. 1993;16(12):1598–1605. doi: 10.2337/diacare.16.12.1598. [DOI] [PubMed] [Google Scholar]
- 42.Lie RT, Wilcox AJ, Skjaerven R. Maternal and paternal influences on length of pregnancy. Obstet Gynecol. 2006;107(4):880–885. doi: 10.1097/01.AOG.0000206797.52832.36. [DOI] [PubMed] [Google Scholar]
- 43.Freathy RM, Weedon MN, Bennett A, et al. Type 2 diabetes TCF7L2 risk genotypes alter birth weight: a study of 24,053 individuals. Am J Hum Genet. 2007;80(6):1150–1161. doi: 10.1086/518517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beck S, Wojdyla D, Say L, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ. 2010;88(1):31–38. doi: 10.2471/BLT.08.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lykke J, Paidas M, Damm P, et al. Preterm delivery and risk of subsequent cardiovascular morbidity and type-II diabetes in the mother. BJOG. 2010;117(3):274–281. doi: 10.1111/j.1471-0528.2009.02448.x. [DOI] [PubMed] [Google Scholar]
- 46.Catov JM, Wu CS, Olsen J, et al. Early or recurrent preterm birth and maternal cardiovascular disease risk. Ann Epidemiol. 2010;20(8):604–609. doi: 10.1016/j.annepidem.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nardi O, Zureik M, Courbon D, et al. Preterm delivery of a first child and subsequent mothers’ risk of ischaemic heart disease: a nested case-control study. Eur J Cardiovasc Prev Rehabil. 2006;13(2):281–283. doi: 10.1097/01.hjr.0000183917.35978.a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pell JP, Smith GC, Walsh D. Pregnancy complications and subsequent maternal cerebrovascular events: a retrospective cohort study of 119,668 births. Am J Epidemiol. 2004;159(4):336–342. doi: 10.1093/aje/kwh064. [DOI] [PubMed] [Google Scholar]
- 49.Rich-Edwards JW, Klongsoyr K, Wilcox A, et al. Duration of first pregnancy predicts maternal cardiovascular death, whether delivery was medically indicated or spontaneous [abstract] Am J Epidemiol. 2012;175(suppl 11):S64. [Google Scholar]
- 50.Hastie CE, Smith GC, MacKay DF, et al. Maternal risk of ischaemic heart disease following elective and spontaneous pre-term delivery: retrospective cohort study of 750 350 singleton pregnancies. Int J Epidemiol. 2011;40(4):914–919. doi: 10.1093/ije/dyq270. [DOI] [PubMed] [Google Scholar]
- 51.Wilcox AJ, Skjaerven R, Lie RT. Familial patterns of preterm delivery: maternal and fetal contributions. Am J Epidemiol. 2008;167(4):474–479. doi: 10.1093/aje/kwm319. [DOI] [PubMed] [Google Scholar]
- 52.Bellamy L, Casas JP, Hingorani AD, et al. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373(9677):1773–1779. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 53.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25(10):1862–1868. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 54.Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shah BR, Retnakaran R, Booth GL. Increased risk of cardiovascular disease in young women following gestational diabetes mellitus. Diabetes Care. 2008;31(8):1668–1669. doi: 10.2337/dc08-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carr DB, Utzschneider KM, Hull RL, et al. Gestational diabetes mellitus increases the risk of cardiovascular disease in women with a family history of type 2 diabetes. Diabetes Care. 2006;29(9):2078–2083. doi: 10.2337/dc05-2482. [DOI] [PubMed] [Google Scholar]
- 57.Retnakaran R, Shah BR. Abnormal screening glucose challenge test in pregnancy and future risk of diabetes in young women. Diabet Med. 2009;26(5):474–477. doi: 10.1111/j.1464-5491.2009.02712.x. [DOI] [PubMed] [Google Scholar]
- 58.Retnakaran R, Shah BR. Mild glucose intolerance in pregnancy and risk of cardiovascular disease: a population-based cohort study. CMAJ. 2009;181(6-7):371–376. doi: 10.1503/cmaj.090569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wallis AB, Saftlas AF, Hsia J, et al. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am J Hypertens. 2008;21(5):521–526. doi: 10.1038/ajh.2008.20. [DOI] [PubMed] [Google Scholar]
- 60.Roberts CL, Algert CS, Morris JM, et al. Hypertensive disorders in pregnancy: a population-based study. Med J Aust. 2005;182(7):332–335. doi: 10.5694/j.1326-5377.2005.tb06730.x. [DOI] [PubMed] [Google Scholar]
- 61.McDonald SD, Malinowski A, Zhou Q, et al. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156(5):918–930. doi: 10.1016/j.ahj.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 62.Lin Y-S, Tang C-H, Yang C-YC, et al. Effect of pre-eclampsia–eclampsia on major cardiovascular events among peripartum women in Taiwan. Am J Cardiol. 2011;107(2):325–330. doi: 10.1016/j.amjcard.2010.08.073. [DOI] [PubMed] [Google Scholar]
- 63.Lykke JA, Langhoff-Roos J, Sibai BM, et al. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension. 2009;53(6):944–951. doi: 10.1161/HYPERTENSIONAHA.109.130765. [DOI] [PubMed] [Google Scholar]
- 64.Wilson BJ, Watson MS, Prescott GJ, et al. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ. 2003;326(7394):845. doi: 10.1136/bmj.326.7394.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Funai EF, Friedlander Y, Paltiel O, et al. Long-term mortality after preeclampsia. Epidemiology. 2005;16(2):206–215. doi: 10.1097/01.ede.0000152912.02042.cd. [DOI] [PubMed] [Google Scholar]
- 66.Hannaford P, Ferry S, Hirsch S. Cardiovascular sequelae of toxaemia of pregnancy. Heart. 1997;77(2):154–158. doi: 10.1136/hrt.77.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jónsdóttir L, Arngrimsson R, Geirsson RT, et al. Death rates from ischemic heart disease in women with a history of hypertension in pregnancy. Acta Obstet Gynecol Scand. 1995;74(10):772–776. doi: 10.3109/00016349509021195. [DOI] [PubMed] [Google Scholar]
- 68.Skjaerven R, Wilcox AJ, Klungsoyr K, et al. Cardiovascular mortality after pre-eclampsia in one child mothers: prospective, population based cohort study. BMJ. 2012;345:e7677. doi: 10.1136/bmj.e7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Retnakaran R, Austin PC, Shah BR. Effect of subsequent pregnancies on the risk of developing diabetes following a first pregnancy complicated by gestational diabetes: a population-based study. Diabet Med. 2011;28(3):287–292. doi: 10.1111/j.1464-5491.2010.03179.x. [DOI] [PubMed] [Google Scholar]
- 70.Magnussen EB, Vatten LJ, Lund-Nilsen TI, et al. Prepregnancy cardiovascular risk factors as predictors of pre-eclampsia: population based cohort study. BMJ. 2007;335:978. doi: 10.1136/bmj.39366.416817.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Catov JM, Nohr EA, Olsen J, et al. Chronic hypertension related to risk for preterm and term small-for-gestational-age births. Obstet Gynecol. 2008;112(2 Pt 1):290–296. doi: 10.1097/AOG.0b013e31817f589b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Magnussen EB, Vatten LJ, Myklestad K, et al. Cardiovascular risk factors prior to conception and the length of pregnancy: population-based cohort study. Am J Obstet Gynecol. 2011;204(6):526.e521–526.e528. doi: 10.1016/j.ajog.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 73.Catov JM, Ness RB, Wellons MF, et al. Prepregnancy lipids related to preterm birth risk: the Coronary Artery Risk Development in Young Adults Study. J Clin Endocrinol Metab. 2010;95(8):2009–2028. doi: 10.1210/jc.2009-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Romundstad PR, Davey Smith G, Nilsen TI, et al. Associations of prepregnancy cardiovascular risk factors with the offspring's birth weight. Am J Epidemiol. 2007;166(12):1359–1364. doi: 10.1093/aje/kwm272. [DOI] [PubMed] [Google Scholar]
- 75.Solomon CG, Willett WC, Carey VJ, et al. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA. 1997;278(13):1078–1083. [PubMed] [Google Scholar]
- 76.Riskin-Mashiah S, Damti A, Younes G, et al. First trimester fasting hyperglycemia as a predictor for the development of gestational diabetes mellitus. Eur J Obstet Gynecol Reprod Biol. 2010;152(2):163–167. doi: 10.1016/j.ejogrb.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 77.Sacks DA, Chen W, Wolde-Tsadik G, et al. Fasting plasma glucose test at the first prenatal visit as a screen for gestational diabetes. Obstet Gynecol. 2003;101(6):1197–1203. doi: 10.1016/s0029-7844(03)00049-8. [DOI] [PubMed] [Google Scholar]
- 78.Nanda S, Savvidou M, Syngelaki A, et al. Prediction of gestational diabetes mellitus by maternal factors and biomarkers at 11 to 13 weeks. Prenat Diagn. 2011;31(2):135–141. doi: 10.1002/pd.2636. [DOI] [PubMed] [Google Scholar]
- 79.Williams MA, Qiu C, Muy-Rivera M, et al. Plasma adiponectin concentrations in early pregnancy and subsequent risk of gestational diabetes mellitus. J Clin Endocrinol Metab. 2004;89(5):2306–2311. doi: 10.1210/jc.2003-031201. [DOI] [PubMed] [Google Scholar]
- 80.Lain KY, Daftary AR, Ness RB, et al. First trimester adipocytokine concentrations and risk of developing gestational diabetes later in pregnancy. Clin Endocrinol (Oxf) 2008;69(3):407–411. doi: 10.1111/j.1365-2265.2008.03198.x. [DOI] [PubMed] [Google Scholar]
- 81.Qiu C, Williams MA, Leisenring WM, et al. Family history of hypertension and type 2 diabetes in relation to preeclampsia risk. Hypertension. 2003;41(3):408–413. doi: 10.1161/01.HYP.0000056996.25503.F5. [DOI] [PubMed] [Google Scholar]
- 82.O'Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology. 2003;14(3):368–374. doi: 10.1097/00001648-200305000-00020. [DOI] [PubMed] [Google Scholar]
- 83.Romero R, Espinoza J, Gonçalves LF, et al. The role of inflammation and infection in preterm birth [abstract] Semin Reprod Med. 2007;25(1):21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Monga M, Creasy R. Cardiovascular and renal adaptation to pregnancy. In: Creasy R, Resnik R, Iams JD, et al., editors. Maternal-Fetal Medicine: Principles and Practice. Philadelphia, PA: WB Saunders; 1994. pp. 758–767. [Google Scholar]
- 85.Butte NF. Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus. Am J Clin Nutr. 2000;71(5 suppl):1256S–1261S. doi: 10.1093/ajcn/71.5.1256s. [DOI] [PubMed] [Google Scholar]
- 86.Potter J, Nestel P. The hyperlipidemia of pregnancy in normal and complicated pregnancies. Am J Obstet Gynecol. 1979;133(2):165–170. doi: 10.1016/0002-9378(79)90469-1. [DOI] [PubMed] [Google Scholar]
- 87.Hubel C, Shakir Y, Gallaher M, et al. Low-density lipoprotein particle size decreases during normal pregnancy in association with triglyceride increases. J Soc Gynecol Investig. 1998;5(5):244–250. doi: 10.1016/s1071-5576(98)00022-7. [DOI] [PubMed] [Google Scholar]
- 88.Herrera E. Metabolic adaptations in pregnancy and their implications for the availability of substrates to the fetus. Eur J Clin Nutr. 2000;54(suppl 1):S47–S51. doi: 10.1038/sj.ejcn.1600984. [DOI] [PubMed] [Google Scholar]
- 89.Ducey J, Schulman H, Farmakides G, et al. A classification of hypertension in pregnancy based on Doppler velocimetry. Am J Obstet Gynecol. 1987;157(3):680–685. doi: 10.1016/s0002-9378(87)80028-5. [DOI] [PubMed] [Google Scholar]
- 90.Campbell S, Griffin DR, Pearce JM, et al. New Doppler technique for assessing uteroplacental blood flow. Lancet. 1983;321(8326):675–677. doi: 10.1016/s0140-6736(83)91970-0. [DOI] [PubMed] [Google Scholar]
- 91.Savvidou MD, Hingorani AD, Tsikas D, et al. Endothelial dysfunction and raised plasma concentrations of asymmetric dimethylarginine in pregnant women who subsequently develop pre-eclampsia. Lancet. 2003;361(9368):1511–1517. doi: 10.1016/S0140-6736(03)13177-7. [DOI] [PubMed] [Google Scholar]
- 92.Savvidou MD, Kaihura C, Anderson JM, et al. Maternal arterial stiffness in women who subsequently develop pre-eclampsia. PLoS ONE. 2011;6(5):e18703. doi: 10.1371/journal.pone.0018703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kelly R, Holzman C, Senagore P, et al. Placental vascular pathology findings and pathways to preterm delivery. Am J Epidemiol. 2009;170(2):148–158. doi: 10.1093/aje/kwp131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Clausen T, Djurovic S, Henriksen T. Dyslipidemia in early second trimester is mainly a feature of women with early onset pre-eclampsia. BJOG. 2001;108(10):1081–1087. doi: 10.1111/j.1471-0528.2001.00247.x. [DOI] [PubMed] [Google Scholar]
- 95.Hubel CA, McLaughlin MK, Evans RW, et al. Fasting serum triglycerides, free fatty acids, and malondialdehyde are increased in preeclampsia, are positively correlated, and decrease within 48 hours post partum. Am J Obstet Gynecol. 1996;174(3):975–982. doi: 10.1016/s0002-9378(96)70336-8. [DOI] [PubMed] [Google Scholar]
- 96.Hubel CA, Lyall F, Weissfeld L, et al. Small low-density lipoproteins and vascular cell adhesion molecule-1 are increased in association with hyperlipidemia in preeclampsia. Metabolism. 1998;47(10):1281–1288. doi: 10.1016/s0026-0495(98)90337-7. [DOI] [PubMed] [Google Scholar]
- 97.Sattar N, Bendomir A, Berry C, et al. Lipoprotein subfraction concentrations in preeclampsia: pathogenic parallels to atherosclerosis. Obstet Gynecol. 1997;89(3):403–408. doi: 10.1016/S0029-7844(96)00514-5. [DOI] [PubMed] [Google Scholar]
- 98.Edison RJ, Berg K, Remaley A, et al. Adverse birth outcomes among mothers with low serum cholesterol. Pediatrics. 2007;120(4):723–733. doi: 10.1542/peds.2006-1939. [DOI] [PubMed] [Google Scholar]
- 99.Catov JM, Bodnar LM, Kip KE, et al. Early pregnancy lipid concentrations and spontaneous preterm birth. Am J Obstet Gynecol. 2007;197(6):610.e611–610.e617. doi: 10.1016/j.ajog.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 100.Enquobahrie DA, Williams MA, Qiu C, et al. Early pregnancy lipid concentrations and the risk of gestational diabetes mellitus. Diabetes Res Clin Pract. 2005;70(2):134–142. doi: 10.1016/j.diabres.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 101.Sattar N, Greer I, Galloway P, et al. Lipid and lipoprotein concentrations in pregnancies complicated by intrauterine growth restriction. J Clin Endocrinol Metab. 1999;84(1):128–130. doi: 10.1210/jcem.84.1.5419. [DOI] [PubMed] [Google Scholar]
- 102.Wadsack C, Tabano S, Maier A, et al. Intrauterine growth restriction is associated with alterations in placental lipoprotein receptors and maternal lipoprotein composition. Am J Physiol Endocrinol Metab. 2007;292(2):476–484. doi: 10.1152/ajpendo.00547.2005. [DOI] [PubMed] [Google Scholar]
- 103.Stepan H, Faber R, Walther T. Expression of low density lipoprotein receptor messenger ribonucleic acid in placentas from pregnancies with intrauterine growth retardation. Br J Obstet Gynaecol. 1999;106(11):1221–1222. doi: 10.1111/j.1471-0528.1999.tb08153.x. [DOI] [PubMed] [Google Scholar]
- 104.Ostlund I, Haglund B, Hanson U. Gestational diabetes and preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2004;113(1):12–16. doi: 10.1016/j.ejogrb.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 105.Hedderson MM, Ferrara A, Sacks DA. Gestational diabetes mellitus and lesser degrees of pregnancy hyperglycemia: association with increased risk of spontaneous preterm birth. Obstet Gynecol. 2003;102(4):850–856. doi: 10.1016/s0029-7844(03)00661-6. [DOI] [PubMed] [Google Scholar]
- 106.Lawlor DA, Relton C, Sattar N, et al. Maternal adiposity—a determinant of perinatal and offspring outcomes? Nat Rev Endocrinol. 2012;8(11):679–688. doi: 10.1038/nrendo.2012.176. [DOI] [PubMed] [Google Scholar]
- 107.Smith GCS, Shah I, Pell JP, et al. Maternal obesity in early pregnancy and risk of spontaneous and elective preterm deliveries: a retrospective cohort study. Am J Public Health. 2007;97(1):157–162. doi: 10.2105/AJPH.2005.074294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ernst GDS, de Jonge LL, Hofman A, et al. C-reactive protein levels in early pregnancy, fetal growth patterns, and the risk for neonatal complications: the Generation R Study. Am J Obstet Gynecol. 2011;205(2):132.e1–132.e12. doi: 10.1016/j.ajog.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 109.Pitiphat W, Gillman MW, Joshipura KJ, et al. Plasma C-reactive protein in early pregnancy and preterm delivery. Am J Epidemiol. 2005;162(11):1108–1113. doi: 10.1093/aje/kwi323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Catov JM, Bodnar LM, Ness RB, et al. Inflammation and dyslipidemia related to risk of spontaneous preterm birth. Am J Epidemiol. 2007;166(11):1312–1319. doi: 10.1093/aje/kwm273. [DOI] [PubMed] [Google Scholar]
- 111.Wolf M, Kettyle E, Sandler L, et al. Obesity and preeclampsia: the potential role of inflammation. Obstet Gynecol. 2001;98(5):757–762. doi: 10.1016/s0029-7844(01)01551-4. [DOI] [PubMed] [Google Scholar]
- 112.Freeman DJ, McManus F, Brown EA, et al. Short- and long-term changes in plasma inflammatory markers associated with preeclampsia. Hypertension. 2004;44(5):708–714. doi: 10.1161/01.HYP.0000143849.67254.ca. [DOI] [PubMed] [Google Scholar]
- 113.Curry A, Vogel I, Drews C, et al. Mid-pregnancy maternal plasma levels of interleukin 2, 6, and 12, tumor necrosis factor-alpha, interferon-gamma, and granulocyte-macrophage colony-stimulating factor and spontaneous preterm delivery. Acta Obstet Gynecol Scand. 2007;86(9):1103–1110. doi: 10.1080/00016340701515423. [DOI] [PubMed] [Google Scholar]
- 114.Gammill HS, Powers RW, Clifton RG, et al. Does C-reactive protein predict recurrent preeclampsia? Hypertens Pregnancy. 2010;29(4):399–409. doi: 10.3109/10641950903214633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Heilmann L, Rath W, Pollow K. Hemostatic abnormalities in patients with severe preeclampsia. Clin Appl Thromb Hemost. 2007;13(3):285–291. doi: 10.1177/1076029607299986. [DOI] [PubMed] [Google Scholar]
- 116.Catov J, Bodnar L, Hackney D, et al. Activation of the fibrinolytic cascade early in pregnancy among women with spontaneous preterm birth. Obstet Gynecol. 2008;112(5):1116–1122. doi: 10.1097/AOG.0b013e31818aa5b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hackney DN, Catov JM, Simhan HN. Low concentrations of thrombin-inhibitor complexes and the risk of preterm delivery. Am J Obstet Gynecol. 2010;206(2):184.e181–184.e186. doi: 10.1016/j.ajog.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 118.Girardi G. Role of tissue factor in pregnancy complications: crosstalk between coagulation and inflammation. Thromb Res. 2011;127(suppl 3):S43–S46. doi: 10.1016/S0049-3848(11)70012-3. [DOI] [PubMed] [Google Scholar]
- 119.Gunderson EP, Lewis CE, Murtaugh MA, et al. Long-term plasma lipid changes associated with a first birth: the Coronary Artery Risk Development in Young Adults Study. Am J Epidemiol. 2004;159(11):1028–1039. doi: 10.1093/aje/kwh146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gore SA, Brown DM, West DS. The role of postpartum weight retention in obesity among women: a review of the evidence. Ann Behav Med. 2003;26(2):149–159. doi: 10.1207/S15324796ABM2602_07. [DOI] [PubMed] [Google Scholar]
- 121.Gunderson E, Murtaugh M, Lewis C, et al. Excess gains in weight and waist circumference associated with childbearing: the Coronary Artery Risk Development in Young Adults Study (CARDIA) Int J Obes. 2004;28(4):525–535. doi: 10.1038/sj.ijo.0802551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schwarz EB, Ray RM, Stuebe AM, et al. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet Gynecol. 2009;113(5):974–982. doi: 10.1097/01.AOG.0000346884.67796.ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gunderson EP, Sternfeld B, Wellons MF, et al. Childbearing may increase visceral adipose tissue independent of overall increase in body fat. Obesity. 2008;16(5):1078–1084. doi: 10.1038/oby.2008.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stuebe AM, Rich-Edwards JW. The reset hypothesis: lactation and maternal metabolism. Am J Perinatol. 2009;26(1):81–88. doi: 10.1055/s-0028-1103034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lykke JA, Paidas MJ, Langhoff-Roos J. Recurring complications in second pregnancy. Obstet Gynecol. 2009;113(6):1217–1224. doi: 10.1097/AOG.0b013e3181a66f2d. [DOI] [PubMed] [Google Scholar]
- 126.Ananth CV, Getahun D, Peltier MR, et al. Recurrence of spontaneous versus medically indicated preterm birth. Am J Obstet Gynecol. 2006;195(3):643–650. doi: 10.1016/j.ajog.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 127.Bloom SL, Yost NP, McIntire DD, et al. Recurrence of preterm birth in singleton and twin pregnancies. Obstet Gynecol. 2001;98(3):379–385. doi: 10.1016/s0029-7844(01)01466-1. [DOI] [PubMed] [Google Scholar]
- 128.Agatisa PK, Ness RB, Roberts JM, et al. Impairment of endothelial function in women with a history of preeclampsia: an indicator of cardiovascular risk. Am J Physiol Heart Circ Physiol. 2004;286(4):H1389–H1393. doi: 10.1152/ajpheart.00298.2003. [DOI] [PubMed] [Google Scholar]
- 129.Lawlor D, Davey Smith G, Ebrahim S. Birth weight of offspring and insulin resistance in late adulthood: cross sectional survey. BMJ. 2002;325(7360):359–362. doi: 10.1136/bmj.325.7360.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Berends AL, de Groot CJ, Sijbrands EJ, et al. Shared constitutional risks for maternal vascular-related pregnancy complications and future cardiovascular disease. Hypertension. 2008;51(4):1034–1041. doi: 10.1161/HYPERTENSIONAHA.107.101873. [DOI] [PubMed] [Google Scholar]
- 131.Catov JM, Dodge R, Yamal JM, et al. Prior preterm or small-for-gestational-age birth related to maternal metabolic syndrome. Obstet Gynecol. 2011;117(2 Pt 1):225–232. doi: 10.1097/AOG.0b013e3182075626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Macdonald-Wallis C, Lawlor DA, Fraser A, et al. Blood pressure change in normotensive, gestational hypertensive, preeclamptic, and essential hypertensive pregnancies. Hypertension. 2012;59(6):1241–1248. doi: 10.1161/HYPERTENSIONAHA.111.187039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lauenborg J, Mathiesen E, Hansen T, et al. The prevalence of the metabolic syndrome in a Danish population of women with previous gestational diabetes mellitus is three-fold higher than in the general population. J Clin Endocrinol Metab. 2005;90(7):4004–4010. doi: 10.1210/jc.2004-1713. [DOI] [PubMed] [Google Scholar]
- 134.Meyers-Seifer CH, Vohr BR. Lipid levels in former gestational diabetic mothers. Diabetes Care. 1996;19(12):1351–1356. doi: 10.2337/diacare.19.12.1351. [DOI] [PubMed] [Google Scholar]
- 135.Verma A, Boney CM, Tucker R, et al. Insulin resistance syndrome in women with prior history of gestational diabetes mellitus. J Clin Endocrinol Metab. 2002;87(7):3227–3235. doi: 10.1210/jcem.87.7.8684. [DOI] [PubMed] [Google Scholar]
- 136.Heitritter SM, Solomon CG, Mitchell GF, et al. Subclinical inflammation and vascular dysfunction in women with previous gestational diabetes mellitus. J Clin Endocrinol Metab. 2005;90(7):3983–3988. doi: 10.1210/jc.2004-2494. [DOI] [PubMed] [Google Scholar]
- 137.Anastasiou E, Lekakis JP, Alevizaki M, et al. Impaired endothelium-dependent vasodilatation in women with previous gestational diabetes. Diabetes Care. 1998;21(12):2111–2115. doi: 10.2337/diacare.21.12.2111. [DOI] [PubMed] [Google Scholar]
- 138.Tarim E, Yigit F, Kilicdag E, et al. Early onset of subclinical atherosclerosis in women with gestational diabetes mellitus. Ultrasound Obstet Gynecol. 2006;27(2):177–182. doi: 10.1002/uog.2687. [DOI] [PubMed] [Google Scholar]
- 139.Manten GT, Sikkema MJ, Voorbij HA, et al. Risk factors for cardiovascular disease in women with a history of pregnancy complicated by preeclampsia or intrauterine growth restriction. Hypertens Pregnancy. 2007;26(1):39–50. doi: 10.1080/10641950601146574. [DOI] [PubMed] [Google Scholar]
- 140.Magnussen EB, Vatten LJ, Smith GD, et al. Hypertensive disorders in pregnancy and subsequently measured cardiovascular risk factors. Obstet Gynecol. 2009;114(5):961–970. doi: 10.1097/AOG.0b013e3181bb0dfc. [DOI] [PubMed] [Google Scholar]
- 141.Sattar N, Ramsay J, Crawford L, et al. Classic and novel risk factor parameters in women with a history of preeclampsia. Hypertension. 2003;42(1):39–42. doi: 10.1161/01.HYP.0000074428.11168.EE. [DOI] [PubMed] [Google Scholar]
- 142.Laivuori H, Tikkanen MJ, Ylikorkala O. Hyperinsulinemia 17 years after preeclamptic first pregnancy. J Clin Endocrinol Metab. 1996;81(8):2908–2911. doi: 10.1210/jcem.81.8.8768850. [DOI] [PubMed] [Google Scholar]
- 143.Hubel CA, Powers RW, Snaedal S, et al. C-reactive protein is elevated 30 years after eclamptic pregnancy. Hypertension. 2008;51(6):1499–1505. doi: 10.1161/HYPERTENSIONAHA.108.109934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Romundstad PR, Magnussen EB, Smith GD, et al. Hypertension in pregnancy and later cardiovascular risk: common antecedents? Circulation. 2010;122(6):579–584. doi: 10.1161/CIRCULATIONAHA.110.943407. [DOI] [PubMed] [Google Scholar]
- 145.Smith GN, Walker MC, Liu A, et al. A history of preeclampsia identifies women who have underlying cardiovascular risk factors. Am J Obstet Gynecol. 2009;200(1):e51–e58. doi: 10.1016/j.ajog.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 146.Di Cianni G, Lencioni C, Volpe L, et al. C-reactive protein and metabolic syndrome in women with previous gestational diabetes. Diabetes Metab Res Rev. 2007;23(2):135–140. doi: 10.1002/dmrr.661. [DOI] [PubMed] [Google Scholar]
- 147.Kanagalingam MG, Nelson SM, Freeman DJ, et al. Vascular dysfunction and alteration of novel and classic cardiovascular risk factors in mothers of growth restricted offspring. Atherosclerosis. 2009;205(1):244–250. doi: 10.1016/j.atherosclerosis.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 148.Wolf M, Hubel CA, Lam C, et al. Preeclampsia and future cardiovascular disease: potential role of altered angiogenesis and insulin resistance. J Clin Endocrinol Metab. 2004;89(12):6239–6243. doi: 10.1210/jc.2004-0548. [DOI] [PubMed] [Google Scholar]
- 149.James-Todd TM, Karumanchi SA, Hibert EL, et al. Gestational age, birth weight and subsequent risk of type 2 diabetes in mothers. Preventing Chronic Dis. doi: 10.5888/pcd10.120336. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hastie CE, Smith GCS, Mackay DF, et al. Association between preterm delivery and subsequent C-reactive protein: a retrospective cohort study. Am J Obstet Gynecol. 2011;205(6):556.e551–556.e554. doi: 10.1016/j.ajog.2011.06.080. [DOI] [PubMed] [Google Scholar]
- 151.Hubel CA, Powers R, Snaedal S, et al. C-reactive protein is increased 30 years after eclamptic pregnancy. J Soc Gynecol Invest. 2006;13(suppl 2):292A. [Google Scholar]
- 152.Winzer C, Wagner O, Festa A, et al. Plasma adiponectin, insulin sensitivity, and subclinical inflammation in women with prior gestational diabetes mellitus. Diabetes Care. 2004;27(7):1721–1727. doi: 10.2337/diacare.27.7.1721. [DOI] [PubMed] [Google Scholar]
- 153.Portelinha A, Cerdeira AS, Belo L, et al. Haemostatic factors in women with history of preeclampsia. Thromb Res. 2009;124(1):52–56. doi: 10.1016/j.thromres.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 154.Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2010;122(25):e584–e636. doi: 10.1161/CIR.0b013e3182051b4c. [DOI] [PubMed] [Google Scholar]
- 155.Shaat N, Lernmark A, Karlsson E, et al. A variant in the transcription factor 7-like 2 (TCF7L2) gene is associated with an increased risk of gestational diabetes mellitus. Diabetologia. 2007;50(5):972–979. doi: 10.1007/s00125-007-0623-2. [DOI] [PubMed] [Google Scholar]
- 156.Lauenborg J, Grarup N, Damm P, et al. Common type 2 diabetes risk gene variants associate with gestational diabetes. J Clin Endocrinol Metab. 2009;94(1):145–150. doi: 10.1210/jc.2008-1336. [DOI] [PubMed] [Google Scholar]
- 157.Cho YM, Kim TH, Lim S, et al. Type 2 diabetes-associated genetic variants discovered in the recent genome-wide association studies are related to gestational diabetes mellitus in the Korean population. Diabetologia. 2009;52(2):253–261. doi: 10.1007/s00125-008-1196-4. [DOI] [PubMed] [Google Scholar]
- 158.Kwak SH, Kim SH, Cho YM, et al. A genome-wide association study of gestational diabetes mellitus in Korean women. Diabetes. 2012;61(2):531–541. doi: 10.2337/db11-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bytautiene E, Lu F, Tamayo EH, et al. Long-term maternal cardiovascular function in a mouse model of sFlt-1-induced preeclampsia. Am J Physiol Heart Circ Physiol. 2010;298(1):H189–H193. doi: 10.1152/ajpheart.00792.2009. [DOI] [PubMed] [Google Scholar]
- 160.Regitz-Zagrosek V, Lundqvist CB, Borghi C, et al. ESC guidelines on the management of cardiovascular diseases during pregnancy. The Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC) Eur Heart J. 2011;32(24):3147–3197. doi: 10.1093/eurheartj/ehr218. [DOI] [PubMed] [Google Scholar]
- 161.Rich-Edwards JW, McElrath TF, Karumanchi SA, et al. Breathing life into the lifecourse approach: pregnancy history and cardiovascular disease in women. Hypertension. 2010;56(3):331–334. doi: 10.1161/HYPERTENSIONAHA.110.156810. [DOI] [PMC free article] [PubMed] [Google Scholar]