Abstract
The relationship of postmenopausal hormone therapy with all-cause dementia and Alzheimer's disease dementia has been controversial. Given continued interest in the role of hormone therapy in chronic disease prevention and the emergence of more prospective studies, we conducted a systematic review to identify all epidemiologic studies meeting prespecified criteria reporting on postmenopausal hormone therapy use and risk of Alzheimer's disease or dementia. A systematic search of Medline and Embase through December 31, 2012, returned 15 articles meeting our criteria. Our meta-analysis of any versus never use did not support the hypothesis that hormone therapy reduces risk of Alzheimer's disease (summary estimate = 0.88, 95% confidence interval: 0.66, 1.16). Exclusion of trial findings did not change this estimate. There were not enough all-cause dementia results for a separate meta-analysis, but when we combined all-cause dementia results (n = 3) with Alzheimer's disease results (n = 7), the summary estimate remained null (summary estimate = 0.94, 95% confidence interval: 0.71, 1.26). The limited explorations of timing of use—both duration and early initiation—did not yield consistent findings. Our findings support current recommendations that hormone therapy should not be used for dementia prevention. We discuss trends in hormone therapy research that could explain our novel findings and highlight areas where additional data are needed.
Keywords: Alzheimer disease, clinical trial, cognition, cohort studies, dementia, estrogen replacement therapy, hormone replacement therapy, systematic review
INTRODUCTION
For many years, the relationship between postmenopausal hormone therapy and dementia has been controversial. Although there was no clear consensus, beliefs about the neurocognitive benefits of estrogen were part of a broader view that supplemental estrogen could prevent chronic disease—even aging itself—in postmenopausal women (1). Early observational studies, many of them retrospective, suggested protective associations between hormone therapy and risk of all-cause dementia and Alzheimer's disease dementia (2). These findings were buttressed by a substantial body of animal and in vitro research that supported the idea that supplemental estrogen might stave off dementia-related neurodegeneration by promotion of cholinergic activity (3), by protection from toxic insult (4), by stimulation of neuron formation (5), or by reduced formation and enhanced clearance of β-amyloid (6), the main constituent of the characteristic amyloid plaques of Alzheimer's disease. On the other hand, it was also known that hormone therapy increases inflammatory markers (7) and risk of stroke (8), both of which are associated with increased dementia risk.
Over the last decade, findings from the Women's Health Initiative (WHI) cast doubt on the use of hormone therapy to prevent chronic disease (9), including dementia. The WHI, conducted in approximately 27,000 women aged 50–79 years at enrollment, consisted of 2 parallel, randomized, controlled, double-blind trials. One trial compared the effects of conjugated equine estrogen (CEE) and medroxyprogesterone acetate (MPA) combined (CEE+MPA) with placebo, and the other compared CEE alone with placebo. The WHI was halted early because of increased risk of heart disease, stroke, pulmonary embolism, and breast cancer in the CEE+MPA arm and excess stroke risk and absence of benefit for heart disease in the CEE-alone branch of the trial. The Women's Health Initiative Memory Study (WHIMS) also found a significant increase in dementia risk with CEE+MPA (10) and a nonsignificant increase in dementia risk with CEE alone (11). Analyses of global cognitive function found harmful effects of CEE alone (12) and no benefit of CEE+MPA (13). In the wake of these disappointing findings, the pendulum has generally swung away from prescribing long-term hormone use for chronic disease prevention (14).
One interpretation of the apparent discrepancy between the early observational studies and the WHIMS clinical trial findings is that the observational studies were confounded by factors that influenced use; for example, hormone therapy use was associated with higher socioeconomic status, access to and use of health care, and a variety of health-promoting behaviors (15, 16). Another difference concerns the timing of hormone therapy initiation: Many hormone therapy users in the observational studies began their therapy at the time of menopause, while randomization in the WHI occurred for most participants well beyond menopause. The “critical window hypothesis,” as well as the hypothesized “healthy cell bias” of estrogen action (17), suggests that the findings from the WHI and from observational investigations could both be correct, positing potential cognitive benefits with earlier initiation and potential risks with later initiation.
Although new studies are beginning to address the question of timing, it is unclear whether dementia risk varies with other dimensions of hormone therapy use, such as duration, dose, and mode of delivery. These questions are important, because hormone therapy remains the most effective treatment for vasomotor symptoms that commonly emerge at menopause. Thus, there are still important insights to be gained from further study of hormone therapy use and dementia risk, especially given the large number of women currently in midlife who are expected to develop dementia in the next few decades (18, 19). There have been many commentaries and nonsystematic reviews of hormone therapy and dementia but few systematic reviews (20, 21). Many of the published meta-analyses focused on cognitive decline or impairment rather than dementia (22–24). Moreover, previous meta-analyses of dementia were completed over a decade ago when few prospective studies had been conducted (25–27). In particular, most of the included studies were retrospective case-control studies, many drawing cases that were diagnosed in health-care settings rather than systematically evaluating participants; some relied on self- or proxy report of hormone therapy use, and many did not adjust for major potential sources of confounding. As we describe further (refer to Discussion), these and other limitations present in most of the studies included in these meta-analyses may have contributed to spuriously beneficial summary associations.
Therefore, we conducted a systematic review to more clearly delineate the scope of and gaps in previous findings on hormone therapy use and dementia and to integrate these findings with newly emerging data. Our aim in this review is to improve on the published literature by providing an updated, comprehensive, systematic review of the scientific data linking hormone therapy to risk of all-cause dementia and Alzheimer's disease, limited to data from studies of dementia that were prospectively conducted, including both observational studies and randomized trials. Where appropriate, we conducted meta-analyses of studies relating postmenopausal hormone therapy to dementia/Alzheimer's disease risk.
MATERIALS AND METHODS
Literature search
We conducted our systematic literature search in the Medline (via PubMed and Ovid) and Embase databases through December 31, 2012. We report our methods in accordance with the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines (28).
Two investigators (J. O. and J. W. J.) independently performed the literature search. We used the Medline Medical Subject Headings (MeSH) database and the Embase EMTREE thesaurus, as well as relevant article abstracts and metadata, to compile lists of controlled vocabulary and free text terms that were incorporated into our search strategy. Our search terms included keywords for the exposure (e.g., “postmenopausal” or “hormone” or “estrogen”), the outcome (e.g., “dementia” or “Alzheimer”), and study design (e.g., “cohort” or “case-control”). The full Medline and Embase search strategies are provided in Web Table 1 available at http://aje.oxfordjournals.org/.
The selection process involved 3 stages. First, we removed all duplicate citations from the combined results from the Medline and Embase searches, using EndNote software (Thomson Reuters, New York, New York). Second, we reviewed all citation titles and abstracts for their relevance and selected studies for full text review if the title and/or abstract suggested that the study was at minimum a prospective study of the relationship between hormone therapy and dementia. In the final stage, we reviewed the full text of the articles selected in the second stage.
Inclusion criteria
Studies were eligible for inclusion if they met the following 6 criteria: 1) the study was published in a peer-reviewed journal; 2) the cohort/study population was well defined and followed longitudinally, with exposure (hormone therapy) data collected prospectively with respect to dementia assessment; 3) the study presented original epidemiologic data on the association between incident dementia or Alzheimer's disease and use of postmenopausal hormone therapy; 4) the investigators clearly defined use of postmenopausal hormone therapy (and its variants) and provided details on how the hormone use data were obtained; 5) the investigators appropriately adjusted analyses at a minimum for age, using regression, stratification, matching, or restriction; and 6) the authors provided an estimate of association and at least 1 corresponding measure of statistical uncertainty such as a P value, standard error, or confidence interval. These criteria were designed to identify high-quality studies and to ensure that they provided adequate data for meta-analyses. We communicated with study authors when their report indicated that hormone therapy had been assessed but did not present an estimate and/or measure of uncertainty.
Data extraction
For each eligible study, the following data were extracted (by J. O.): year of publication; cohort; number of participants; number of dementia and Alzheimer's disease cases; follow-up time (including duration of case ascertainment, time between use of hormone therapy and start of dementia/Alzheimer's disease assessment, and time between use of hormone therapy and dementia/Alzheimer's disease diagnosis); participants' ages (including when therapy was taken, when follow-up for dementia outcome began, and when cases were diagnosed); method for collecting data on hormone use (e.g., questionnaire, medical record review, prescription database); hormone therapy use classifications and frequencies (e.g., timing of use, duration of use, route of administration, formulation, or any available information); dementia and Alzheimer's disease diagnostic criteria; and process, effect measure, effect estimates, standard errors or information to compute standard errors, and model covariates or other covariates accounted for via stratification. In instances where multiple models were reported, we extracted data from the model with the maximum covariates.
Data analysis
We grouped study findings on the basis of how hormone therapy was categorized (e.g., any vs. minimal or never use, or current vs. former vs. never use), included effect estimates, and selected study characteristics in the tables.
When studies from 4 or more independent samples reported findings with comparable outcomes (Alzheimer's disease or all-cause dementia) and exposure groupings (binary exposure data or categorical data with sufficiently similar categories), we calculated summary estimates of association and 95% confidence intervals. These summary measures were calculated by using random-effects models (29) that use a weighting scheme that incorporates both within- and between-study variance. We also evaluated the presence of heterogeneity across these associations using the I2 measure (30). To assess whether publication bias may have contributed to the pattern of findings, we used both a quantitative (via the Egger regression asymmetry test) (31) and a qualitative (visual inspection of the Begg funnel plot) approach (31). The power of these tests in our data is limited, however, because of the relatively small number of studies available for each meta-analysis.
We conducted several sensitivity analyses. From the “any use versus never use” findings, we computed summary estimates excluding studies that relied on database records for diagnostic information rather than systematically evaluating their participants. Separately, we computed a summary estimate excluding the randomized trial result. Finally, because Alzheimer's disease pathology underlies the majority of dementia cases, yet individuals with a dementia diagnosis frequently have mixed pathology, we computed a summary estimate that incorporated all results on Alzheimer's disease dementia plus a result on all-cause dementia from an additional study that did not report results on Alzheimer's disease. Only 3 studies reported results on all-cause dementia, too few to conduct a formal meta-analysis, so—using the same reasoning—we computed a summary estimate that combined these all-cause dementia findings with the findings on Alzheimer's disease dementia from 7 additional studies that did not report results on all-cause dementia.
All analyses were conducted by using Stata, version 11, statistical software (StataCorp LP, College Station, Texas).
RESULTS
Literature search results
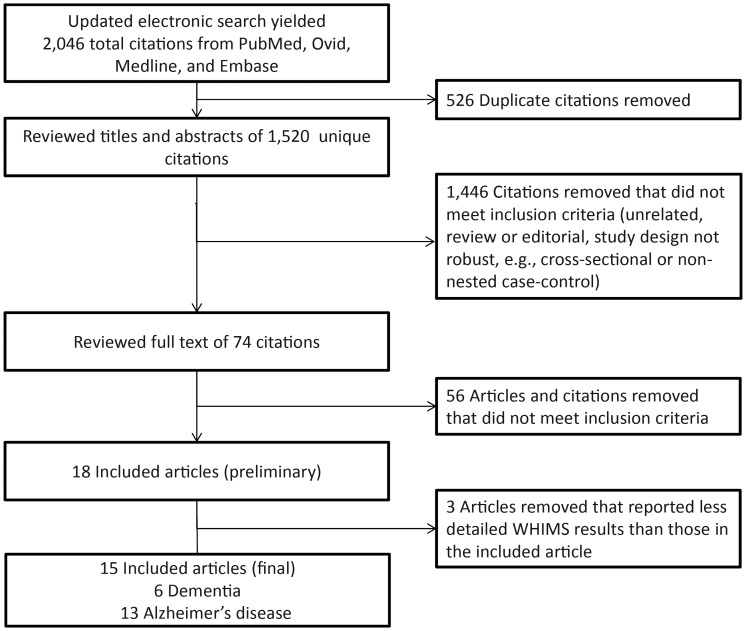
Our systematic, electronic search strategy returned 2,046 citations from PubMed, Ovid Medline, and Embase; 526 duplicates were removed. We excluded 1,446 citations that did not meet our inclusion criteria (e.g., topic not appropriate, review or editorial, cross-sectional or nonnested case-control study design), leaving 74 articles for full-text review. We then excluded 56 additional articles that did not meet the inclusion criteria (and 3 articles that discussed WHIMS results published elsewhere in greater detail), leaving 15 eligible peer-reviewed publications (Figure 1) (11, 32–45).
Figure 1.
Flowchart describing the approach used to identify eligible studies. We conducted a systematic search in the Medline (via PubMed and Ovid) and Embase databases through December 31, 2012. WHIMS, Women's Health Initiative Memory Study.
Description of the included studies
This review includes 13 unique study populations. Population sizes ranged from 227 to 7,479; several studies had small numbers of cases (i.e., <5) in specific exposure categories (35, 40, 42). Two of the cohorts were represented in articles reporting research conducted in separate time periods (Cache County Study (41, 45), Rochester Epidemiology Project (37, 43)). Where there was overlap in at-risk person time, we included only the most updated report in our meta-analyses.
Of the 15 articles included in this review, 9 provided data from a prospective cohort design, 5 from a nested case-control design, and 1 from a randomized clinical trial (Table 1). All studies collected information on use of hormone therapy either by self-report (e.g., interview or questionnaire) at the start of the study or by electronic prescription database. Most of the prospective cohort studies collected information on the use of hormone therapy via questionnaire, either self-administered (35) or administered by a trained interviewer in person (32, 34, 38, 39, 42, 45) or by telephone (41). In the nested case-control studies we reviewed, use of hormone therapy was determined from electronic prescription databases, which were usually maintained by health insurance groups (33, 36, 44) or were part of long-term, regional patient registries (37, 40, 43). The frequency of hormone therapy use varied widely, ranging from 6% to 62% of study participants.
Table 1.
Characteristics of Studies Included in Review
| First Author, Year (Reference No.) | Cohort | Study Designa | No. | Age, years, at Start of Cognitive Follow-upb | Hormone Therapy Ascertainment | When Hormone Therapy Use Assessed | Interval Over Which Dementia Assessed | Diagnostic Criteria | Effect Measure |
|---|---|---|---|---|---|---|---|---|---|
| Barnes, 2003 (32) | Religious Orders Study | Prospective cohort | 577 | 76.1 | Asked at baseline evaluation | At baseline cognitive evaluation, which occurred between 1994 and 2002 | 1994–2003 (mean follow-up = 4.8 years) | NINCDS-ADRDA | HR |
| Brenner, 1994 (33) | University of Washington Alzheimer Disease Patient Registry–Group Health Cooperative | Nested case-control | 227 | 77.6c | From prescription database | 1977 onward | April 1987–February 1992 | DSM-III-R, NINCDS-ADRDA | OR |
| Kawas, 1997 (34) | Baltimore Longitudinal Study of Aging | Prospective cohort | 472 | 61.5 | Asked at biennial evaluations | Beginning in 1978 and every 2 years thereafter | Began in 1978 (maximum follow-up = 16 years) | DSM-III-R, NINCDS-ADRDA | HR |
| Lindsay, 2002 (35) | Canadian Study of Health and Aging | Prospective cohort | 2,079 | 73.3d | From questionnaire | At baseline evaluation in 1991 | 1991–1996 | DSM-IV, NINCDS-ADRDA | OR |
| Petitti, 2008 (36) | Southern California Kaiser Permanente | Nested case-control | 2,906 | 78.7 | From prescription database and self-report | 1992 onward; 6 years before start of cognitive follow-up | 1999–2003 | TICSm (screening), TDQ, medical records | HR |
| Roberts, 2006 (37) | Rochester Epidemiology Project | Nested case-control | 490 | 84e | From prescription database | From perimenopausal period or the onset of menopause to index year for cases and controls | January 1985–December 1989 | DSM-IV | OR |
| Ryan, 2009 (38) | Three-City Study | Prospective cohort | 3,130 | 74 | From questionnaire | At baseline evaluation, beginning in 1999, and at each 2-year follow-up | Began in 1999; results from first 4 years of study | DSM-IV | HR |
| Ryan, 2009 (39) | Enquête de Santé Psychologique-Risques Incidence et Traitement Study | Prospective cohort | 996 | 73 | From questionnaire | At baseline evaluation, which occurred between 1999 and 2001 | Began in 1999; results from first 4 years of study | DSM-IV | HR |
| Seshadri, 2001 (40) | The General Practice Research Database | Nested case-control | 280 | 65.5f | From prescription database | 1990–1998 | January 1992–October 1998 (mean follow-up = 5.3 years) | NINCDS-ADRDA | OR |
| Shao, 2012 (41) | Cache County Study | Prospective cohort | 1,768 | 74.6g | From Women's Health Questionnaire, via telephone | Between first and second waves of follow-up (1995–1999) | 1995–2006 (mean follow-up ≈ 7 years) | NINCDS-ADRDA | HR |
| Shumaker, 2004 (11) | Women's Health Initiative Memory Study | Randomized placebo-controlled trial | 7,479 | 70.6h | Assigned at baseline | Assigned in 1995 | 1995–2004 (mean follow-up = 4.4 yearsi) | DSM-IV | HR |
| Tang, 1996 (42) | Washington Heights–Inwood Columbia Aging Project | Prospective cohort | 1,124 | 74.2 | From questionnaire | At baseline interview | Range, 1–5 years after first interview | DSM-III-R, NINCDS-ADRDA | HR |
| Waring, 1999 (43) | Rochester Epidemiology Project | Nested case-control | 444 | 82j | From prescription database | From menopause onset until Alzheimer's disease onset, or onset of Alzheimer's disease in the matched case patient (for controls) | 1980–1984 | DSM-III-R, NINCDS-ADRDA | OR |
| Whitmer, 2011 (44) | Kaiser Permanente of Northern California | Prospective cohort (using a health-care database) | 5,504 | 80.4k | Midlife use from survey; late-life use from prescription database | Midlife use from 1964 survey; late-life use from 1994 to 1998 | January 1999–June 2008 | ICD-9 | HR |
| Zandi, 2002 (45) | Cache County Study | Prospective cohort | 1,866 | 74.4l | From interview | At baseline evaluation, which occurred between 1995 and 1997 | 1998–2000 (mean follow-up ≈ 3 years) | NINCDS-ADRDA | HR |
Abbreviations: DSM-III-R, Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; HR, hazard ratio; ICD-9, International Classification of Diseases, Ninth Revision; NINCDS-ADRDA, National Institute of Neurological and Communicative Diseases and Stroke-Alzheimer's Disease and Related Disorders Association; OR, odds ratio; TDQ, Telephone Dementia Questionnaire; TICSm, Telephone Interview of Cognitive Status-Modified.
a Here, a prospective cohort design refers to a type of study that evaluates cases of dementia (or Alzheimer's disease) that develop among a clearly defined population of people who do not initially have the condition, over a given observation period, with attention to the timing of diagnosis. A nested case-control design refers to a type of study that evaluates cases of dementia (or Alzheimer's disease) that develop over the course of a defined period among a clearly defined population of people who do not initially have the condition, where cases are matched on certain characteristics (e.g., age, location of residence) to controls who do not develop the disease over this time period.
b The age provided is a mean, unless otherwise noted.
c Mean age at index year, for cases and controls.
d Mean age for cases and controls at the start of follow-up, which includes both men and women.
e Median age at index year for cases and controls.
f Mean age at index year, calculated as the weighted average of cases and controls.
g Mean age at baseline, calculated as weighted average of hormone therapy users and nonusers at baseline.
h Calculated age using a weighted average of the median of reported age categories.
I Calculated as weighted average from data provided in the paper.
j Median age at onset of Alzheimer's disease for cases; controls were matched to case patients within 3 years.
k Median.
l Calculated as the weighted average from data provided in the paper.
Most of the studies we reviewed evaluated Alzheimer's disease as the primary outcome; 9 reported results for Alzheimer's disease only (32–35, 37, 40, 42, 43, 45), 2 reported results for all-cause dementia only (39, 44), and 4 reported results for both Alzheimer's disease and all-cause dementia (11, 36, 38, 41). In 2 instances, we contacted the study authors to obtain additional effect estimates for Alzheimer's disease (32) or dementia (39) when these estimates were mentioned but not reported in the papers.
Participants in the prospective cohort studies underwent regular evaluations for dementia as part of follow-up procedures, which is important because many people with Alzheimer's disease are not formally diagnosed by a health professional (46). Some studies, however, relied solely on medical records for diagnostic information (37, 40, 43, 44) or on a combination of records and cognitive evaluations (33, 36). Investigators for studies that used the medical record approach maintained that this method would capture most cases within the catchment area; for example, the database covered a clearly defined geographical area with detailed electronic medical records for all patients and controls. Furthermore, all studies included in this review except one (36) reported using standard criteria to diagnose Alzheimer's disease and dementia (e.g., National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA); Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised (DSM-III-R); Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV); or International Classification of Diseases, Ninth Revision (ICD-9)). All studies excluded at baseline those with possible dementia or selected controls who were dementia free.
Most of the studies compared Alzheimer's disease or dementia risk among women who ever used hormone therapy (i.e., any history of use) with risk among those who never used hormone therapy. Some studies compared lifetime hormone therapy use with “minimal” use (37, 40, 43), meaning that some women in the reference group used hormone therapy for an interval (e.g., <6 months) that was not considered long enough by investigators to meaningfully affect Alzheimer's disease or dementia risk. Use of estrogen creams was generally not classified as use of hormone therapy because of a lack of evidence that this form of therapy affects the central nervous system (34, 37, 44).
The length of time over which dementia was assessed ranged from 1 to 16 years. The time between hormone therapy use and dementia assessment was not always available, although some studies tracked use in databases dating back many years (33, 37, 43), while others asked only about history of use prior to the start of cognitive follow-up (32, 35, 41, 42, 45).
Meta-analysis and summary of study findings
In this section, we report results for all-cause dementia and Alzheimer's disease dementia together. Articles in this review that reported results for both Alzheimer's disease and all-cause dementia found similar results for each outcome (11, 36, 41).
Any versus never use
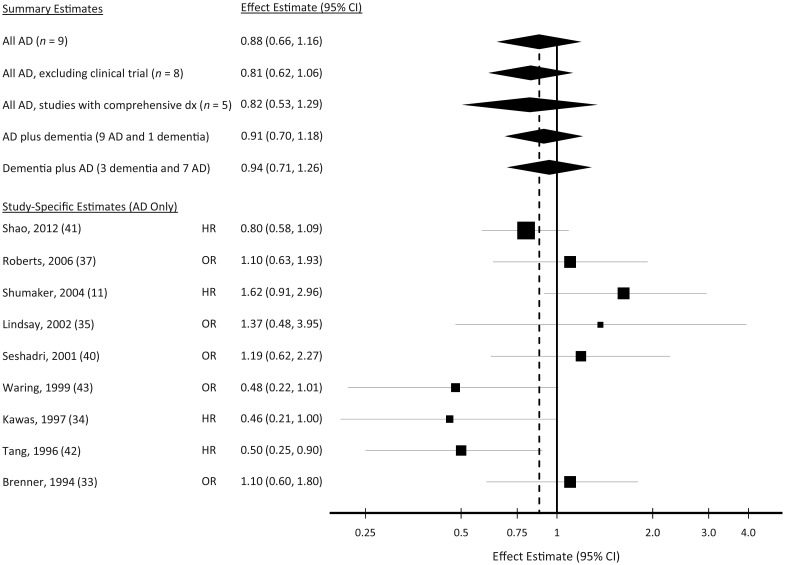
Ten of the articles included in this review, including the WHIMS trial, reported an estimated association between ever-use of hormone therapy and Alzheimer's disease (Table 2). From the articles included in this meta-analysis, there were a total of 14,363 participants and 1,176 cases of Alzheimer's disease (251 cases with ever-hormone therapy use). Four articles reported a significant (42, 45) or marginally significant (P = 0.05) (34, 43) inverse association between any use of hormone therapy and Alzheimer's disease risk. Five reports found a relative risk greater than 1 (11, 33, 35, 37, 40), but confidence intervals were wide and included the null value (relative risk (RR) = 1). Our summary estimate was 0.88 (95% confidence interval (CI): 0.66, 1.16) (Figure 2). This summary estimate did not change meaningfully when we restricted these analyses to studies that comprehensively assessed all of their participants for dementia. Moreover, results were similar when we added the 1 paper that reported only on all-cause dementia to the pool of 9 Alzheimer's disease results (summary RR = 0.91, 95% CI: 0.70, 1.18) and when we combined the all-cause dementia results from papers reporting unique results for all-cause dementia (n = 3) with Alzheimer's disease dementia results from papers reporting only on this outcome (n = 7) (summary RR = 0.94, 95% CI: 0.71, 1.26). The I2 measurement corresponding to the analysis of all Alzheimer's disease results was 49% (95% CI: 0, 73), indicating moderate heterogeneity in the individual study estimates. This heterogeneity estimate was similar for all of the alternative scenarios above.
Table 2.
Summary of Results—Any Use of Hormone Therapy and Alzheimer's Disease/Dementia Risk
| Author, Year (Reference No.) | Study | Study Design | No. of Persons | Covariates | Exposure Distribution |
Alzheimer's Disease |
Dementia |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Description | % | No. of Cases | RRa | 95% CI | P Value | No. of Cases | RR | 95% CI | P Value | |||||
| Brenner, 1994 (33) | Alzheimer Disease Patient Registry-Group Health Cooperative | Nested case-control | 227 | Age, history of hysterectomyb | No hormone therapy | 52 | 55 | 1.00 | Referent | |||||
| Hormone therapy | 48 | 52 | 1.10 | 0.60, 1.80 | 0.73 | |||||||||
| Kawas, 1997 (34) | Baltimore Longitudinal Study of Aging | Prospective cohort | 472 | Age, educationc | No hormone therapy | 51 | 25 | 1.00 | Referent | |||||
| Hormone therapy | 49 | 9 | 0.46 | 0.21, 1.00 | 0.05 | |||||||||
| Lindsay, 2002 (35) | Canadian Study of Health and Aging | Prospective cohort | 2,079 | Age, education | No hormone therapy | 94 | 106 | 1.00 | Referent | |||||
| Hormone therapy | 6 | 4 | 1.37 | 0.48, 3.95 | 0.56 | |||||||||
| Roberts, 2006 (37) | Rochester Epidemiology Project | Nested case-control | 486 | Type of menopause, matched on age | <6 months, never | 89 | 216 | 1.00 | Referent | |||||
| ≥6 months of hormone therapy | 11 | 28 | 1.10 | 0.63, 1.93 | 0.73 | |||||||||
| Ryan, 2009 (39) | Enquête de Santé Psychologique-Risques Incidence et Traitement Study | Prospective cohort | 996 | Age, education | No hormone therapy | 66 | 19 | 1.00 | Referent | |||||
| Hormone therapy | 34d | 8 | 1.41 | 0.59, 3.34 | 0.44 | |||||||||
| Seshadri, 2001 (40) | General Practice Research Database | Nested case-control | 283 | Body mass index, smoking. Matched on age, physician's practice, case index date, date of first prescription in database | <1 year, never | 25 | 44 | 1.00 | Referent | |||||
| Hormone therapy | 75 | 18 | 1.19 | 0.62, 2.27 | 0.60 | |||||||||
| Shao, 2012 (41) | Cache County Study | Prospective cohort | 1,768 | Age, APOE status, education, propensity score for any hormone therapy use | No hormone therapy | 38 | 89 | 1.00 | Referent | NR | 1.00 | Referent | ||
| Hormone therapy | 62 | 87 | 0.80 | 0.58, 1.09 | 0.17 | NR | 0.84 | 0.65, 1.09 | 0.19 | |||||
| Shumaker, 2004 (11) | Women's Health Initiative Memory Study | Randomized placebo-controlled trial | 7,479 | None | Placebo | 51 | 21 | 1.00 | Referent | 40 | 1.00 | Referent | ||
| E or E+P | 49 | 33 | 1.62e | 0.91, 2.96 | 0.08 | 68 | 1.76 | 1.19, 2.60 | 0.005 | |||||
| Tang, 1996 (42) | Washington Heights–Inwood Columbia Aging Project | Prospective cohort | 1,124 | Ethnicity, education, participation group (senior center vs. Medicare sample) | No hormone therapy | 86 | 158 | 1.00 | Referent | |||||
| Hormone therapy | 14 | 9 | 0.50 | 0.25, 0.90 | 0.02 | |||||||||
| Waring, 1999 (43) | Rochester Epidemiology Project | Nested case-control | 444 | Controls matched by age and length of time in record linkage systemf | <6 months, never | 93 | 211 | 1.00 | Referent | |||||
| ≥6 months | 7 | 11 | 0.48 | 0.22, 1.01 | 0.05 | |||||||||
| Zandi, 2002 (45)g | Cache County Study | Prospective case-control | 1,866 | Age, education, APOEh | No hormone therapy | 43 | 58 | 1.00 | Referent | |||||
| Hormone therapy | 57 | 26 | 0.59 | 0.36, 0.96 | 0.03 | |||||||||
Abbreviations: APOE, apolipoprotein E; CI, confidence interval; E, estrogen; E+P, estrogen and progestin combined; NR, not reported; RR, relative risk.
a RR, or the relative risk, can be an odds ratio or a hazard ratio depending on the study design and sampling scheme; refer to Table 1 for the specific effect measure reported in each study.
b The investigators did not include the following variables in their final model because they determined that further adjustment for them did not appreciably change their findings: education, marital status, ethnicity, and history of either smoking or progestin use.
c The investigators examined other variables that did not affect the results of this study, including age at menopause, age at menarche, years of natural cyclic estrogen exposure, menopause duration, and surgical menopause.
d In contrast to many of the studies based in the United States, in this study women used predominantly transdermal estradiol (with or without progesterone), with less than 20% taking oral estradiol. None of the women took conjugated equine estrogens.
e Hazard ratios for Alzheimer's disease were not provided in the paper. We derived incidence rate ratios by using available data on Alzheimer's disease (number of cases, number of noncases, person-time).
f It is unclear which covariates (if any) were included in the final models, but authors reported that the odds ratio did not change noticeably after adjusting for education, age at menopause, and parity, and it was not different in the stratum of women who had undergone natural menopause or in the stratum of women who used estrogen for more than 1 year.
g This study was not included in our meta-analyses of any versus minimal or no hormone therapy use, as the Shao paper (41) from the same cohort provided updated results from the same study population.
h Authors reported that results did not change appreciably when terms were added separately for diabetes mellitus, cardiovascular disease, depression, or use of nonsteroidal antiinflammatory drugs.
Figure 2.
Individual and pooled estimates of the association between hormone therapy use (any vs. minimal or no use) and Alzheimer's disease (AD) risk. “All AD, excluding clinical trial” excludes findings from Shumaker et al. (11). “All AD, studies with comprehensive dx” includes findings only from studies that evaluated all participants for dementia rather than relying on medical records. “AD plus dementia” adds the all-cause dementia findings from Ryan et al. (39) to all AD-specific findings. “Dementia plus AD” uses all all-cause dementia findings, substituting AD-specific findings from 7 studies that did not report results for all-cause dementia. For the study-specific estimates, the reference number is given in parentheses. CI, confidence interval; dx, diagnosis; HR, hazard ratio; OR, odds ratio. “All AD” includes all study-specific estimates shown below.
When we excluded the findings from the clinical trial (WHIMS), the summary estimate for hormone therapy use and Alzheimer's disease risk changed slightly but was not statistically significant (summary RR = 0.81, 95% CI: 0.62, 1.06). This exclusion also resulted in a modest decline in the I2 of 34% (95% CI: 0, 70). However, none of the Egger tests from these meta-analyses, including those that incorporated the WHIMS finding, was consistent with publication bias, and all of the corresponding Begg funnel plots were reasonably symmetrical. Nonetheless, over time, reported estimates have progressed from suggesting a decreased risk of Alzheimer's disease to suggesting an increased risk, with relative risk estimates increasing, on average, by 15% every 5 years (Web Figure 1). Overall, these findings are not consistent with the hypothesis that hormone therapy use, at least classified as an ever-never phenomenon, prevents Alzheimer's disease.
Duration of use
Eight articles involving 7 unique study populations evaluated duration of hormone therapy (Table 3). We were unable to conduct a meta-analysis of these results because studies defined duration of use differently. For instance, 1 study tracked duration by the number of prescriptions filled (33), while the remaining studies used time in years, and these studies used unique cutpoints to define duration categories. Three studies found significantly lower risks of Alzheimer's disease among women with the longest duration of use (>1 year (42), ≥6 months (43), >10 years (45)). In 2 articles women had relative risks of less than 1 in each duration category, although there was no clear trend, and results did not reach significance (33, 34); 3 other reports also did not observe any trend of lower risk with longer use (32, 37, 40).
Table 3.
Summary of Results—Duration of Hormone Therapy Use and Alzheimer's Disease Riska
| Author, Year (Reference No.) | Study | Study Design | No. of Persons | Covariates | Exposure Distribution |
Alzheimer's Disease |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Description | % | No. of Cases | RRb | 95% CI | P Value | |||||
| Barnes, 2003 (32) | Religious Orders Study | Prospective cohort | 577 | Age, education | No hormone therapy | 64 | NR | 1.00 | Referent | |
| <10 years | NRc | NR | 0.57 | 0.21, 1.60 | 0.28 | |||||
| ≥10 years | NR | NR | 1.38 | 0.81, 2.36 | 0.24 | |||||
| Brenner, 1994 (33) | Alzheimer Disease Patient Registry–Group Health Cooperative | Nested case-control | 227 | Age, history of hysterectomyd | No hormone therapy | 52 | 55 | 1.00 | Referent | |
| 1–2 prescriptions | 17 | 22 | 1.50 | 0.70, 3.00 | 0.27 | |||||
| 3–10 prescriptions | 15 | 15 | 0.90 | 0.40, 2.10 | 0.80 | |||||
| 11–50 prescriptions | 16 | 15 | 0.80 | 0.30, 1.80 | 0.63 | |||||
| Kawas, 1997 (34) | Baltimore Longitudinal Study of Aging | Prospective cohort | 472 | Age, educatione | No hormone therapy | NR | NR | 1.00 | Referent | |
| >0–5 years | NR | NR | 0.44 | 0.13, 1.51 | 0.19 | |||||
| >5–10 years | NR | NR | 0.34 | 0.05, 2.52 | 0.29 | |||||
| >10 years | NR | NR | 0.50 | 0.17, 1.47 | 0.21 | |||||
| 34f | ||||||||||
| Roberts, 2006 (37) | Rochester Epidemiology Project | Nested case-control | 486 | Type of menopause, matched on age | <0.5 years, no hormone therapy | 89 | 216 | 1.00 | Referent | |
| 0.5–3 years | 5 | 14 | 1.22 | 0.55, 2.69 | 0.63 | |||||
| >3 years | 6 | 14 | 1.01 | 0.47, 2.20 | 0.97 | |||||
| Seshadri, 2001 (40) | General Practice Research Database | Nested case-control | 280 | Body mass index, smoking. Matched on age, physician's practice, case index date, date of first prescription in database | No hormone therapy | 75 | 44 | 1.00 | Referent | |
| 12–35 months | 7 | 6 | 1.68 | 0.60, 4.69 | 0.32 | |||||
| 36–59 months | 9 | 5 | 0.89 | 0.29, 2.69 | 0.84 | |||||
| ≥60 months | 9 | 4 | 1.05 | 0.32, 3.44 | 0.94 | |||||
| Tang, 1996 (42) | Washington Heights–Inwood Columbia Aging Project | Prospective cohort | 1,124 | Ageg | No hormone therapy | 86 | 158 | 1.00 | Referent | |
| Unknown | 3 | 3 | 1.30 | 0.40, 4.20 | 0.66 | |||||
| ≤1 year | 6 | 5 | 0.47 | 0.20, 1.10 | 0.06 | |||||
| >1 year | 5 | 1 | 0.13 | 0.02, 0.92 | 0.01 | |||||
| Waring, 1999 (43) | Rochester Epidemiology Project | Nested case-control | 444 | Controls matched by age, length of time in record linkage systemh | No hormone therapy | 82 | 189 | 1.00 | Referent | |
| <6 months | 11 | 22 | 0.85 | 0.44, 1.62 | 0.62 | |||||
| ≥6 months | 7 | 11 | 0.42 | 0.18, 0.96 | 0.04 | |||||
| Zandi, 2002 (45) | Cache County Study | Prospective cohort | 1,856 | Age, education, APOEi | No hormone therapy | 43 | 58 | 1.00 | Referent | |
| <3 years | 17 | 10 | 0.82 | 0.38, 1.57 | 0.58 | |||||
| 3–10 years | 17 | 8 | 0.60 | 0.26, 1.22 | 0.2 | |||||
| >10 years | 23 | 7 | 0.41 | 0.17, 0.86 | 0.03 | |||||
Abbreviations: APOE, apolipoprotein A; CI, confidence interval; NR, not reported; RR, relative risk.
a No studies that characterized hormone therapy by duration of use reported results for all-cause dementia.
b RR, or the relative risk, can be an odds ratio or a hazard ratio depending on the study design and sampling scheme; refer to Table 1 for the specific effect measure reported in each study.
c The exposure distribution by category of duration was not reported; 36% reported ever use of hormone therapy, and 64% reported never use of hormone therapy.
d The investigators did not include the following variables in their final model because they determined they were not confounders: education, marital status, ethnicity, and history of either smoking or progestin use.
e Other variables that were examined did not affect the results of this study, including age at menopause, age at menarche, years of natural cyclic estrogen exposure, menopause duration, and surgical menopause.
f Total Alzheimer's disease cases.
g The investigators considered the following variables: ethnicity, education, and participation group (senior center vs. Medicare sample). However, it is unclear if results from the models of duration of use adjusted for these variables.
h It is unclear which covariates (if any) were included in the final models, but the authors report that the odds ratio did not change noticeably after controlling for the effects of education, age at menopause, and parity and when conducting stratified analyses in women who had undergone natural menopause or who used estrogen for more than 1 year.
i The authors reported that results did not change appreciably when terms were added separately for diabetes mellitus, cardiovascular disease, depression, or use of nonsteroidal antiinflammatory drugs.
Timing of use
Reported findings on the timing of hormone therapy use (Table 4) were not consistent across studies. One common but crude approach to explore timing is to categorize women who ever used hormone therapy as current or past users. Four articles reported results for current use of hormone therapy and risk of Alzheimer's disease, and the summary estimate was 0.97 (95% CI: 0.68, 1.38) (I2 = 0%, 95% CI: 0, 68). Three articles provided results for former use of hormone therapy and Alzheimer's disease risk, and results were variable, with only 1 study reporting a significant reduction in risk among former users (45). For all-cause dementia, Ryan et al. (38) reported reduced, but not significant, risk for current and former use of hormone therapy compared with never use. We obtained additional results on dementia incidence by request from investigators of the Enquête de Santé Psychologique-Risques Incidence et Traitement (ESPRIT) study (39), who found elevated, but not significant, risk for current, past, and ever use of hormone therapy.
Table 4.
Summary of Results—Timing of Hormone Therapy Use and Alzheimer's Disease/Dementia Risk
| Author, Year (Reference No.) | Study | Study Design | No. of Persons | Covariates | Exposure Distribution |
Alzheimer's Disease |
Dementia |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Description | % | No. of Cases | RRa | 95% CI | P Value | No. of Cases | RR | 95% CI | P Value | |||||
| Current/Former/Never | ||||||||||||||
| Brenner, 1994 (33) | Alzheimer Disease Patient Registry–Group Health Cooperative | Nested case-control | 227 | Age, history of hysterectomyb | No hormone therapy | 52 | 55 | 1.00 | Referent | |||||
| Former | 24 | 33 | 1.70 | 0.90, 3.20 | 0.10 | |||||||||
| Current | 23 | 19 | 0.60 | 0.30, 1.20 | 0.15 | |||||||||
| Ryan, 2009 (38) | Three-City Study | Prospective cohort | 3,130 | Age, education, study center, high caffeine consumption, depressive symptoms, physical incapacities, comorbidity, marital status, anticholinergic drugs, body mass index, age at menopause | No hormone therapy | 69 | NR | 1.00 | Referent | NR | 1.00 | Referent | ||
| Former | 16 | NR | 0.39 | 0.39, 2.23 | 0.86 | NR | 0.74 | 0.35, 1.55 | 0.42 | |||||
| Current | 15 | NR 53c |
1.36 | 0.44, 4.20 | 0.59 | NR 79d |
0.83 | 0.32, 2.17 | 0.70 | |||||
| Ryan, 2009 (39) | Enquête de Santé Psychologique-Risques Incidence et Traitement Study | Prospective cohort | 996 | Age, education | No use at baseline | 85 | 25 | 1.00 | Referent | |||||
| Current | 15 | 2 | 1.08 | 0.24. 4.92 | 0.93 | |||||||||
| Ryan, 2009 (39) | Enquête de Santé Psychologique-Risques Incidence et Traitement Study | Prospective cohort | 996 | Age, education | No past usee | 81 | 21 | 1.00 | Referent | |||||
| Past use | 19 | 6 | 1.55 | 0.60, 3.97 | 0.36 | |||||||||
| Seshadri, 2001 (40) | General Practice Research Database | Nested case-control | 280 | Body mass index, smoking. Matched on age, physician's practice, case index date, date of first prescription in database | No hormone therapy | 76 | 44 | 1.00 | Referent | |||||
| Current | 24 | 15 | 1.18 | 0.59, 2.37 | 0.64 | |||||||||
| Zandi, 2002 (45) | Cache County Study | Prospective cohort | 1,866 | Age, education, APOEf | No hormone therapy | 43 | 58 | 1.00 | Referent | |||||
| Former | 26 | 9 | 0.33 | 0.15, 0.65 | 0.003 | |||||||||
| Current | 31 | 17 | 1.08 | 0.59, 1.91 | 0.8 | |||||||||
| Time Between Menopause and Hormone Therapy Initiation | ||||||||||||||
| Roberts, 2006 (37) | Rochester Epidemiology Project | Nested case-control | 485 | Type of menopause, matched on age | No hormone therapy | 89 | 216 | 1.00 | Referent | |||||
| ≤2 years | 7 | 17 | 1.24 | 0.61, 2.53 | 0.55 | |||||||||
| >2 years | 5 | 11 | 1.02 | 0.41, 2.54 | 0.97 | |||||||||
| Shao, 2012 (41) | Cache County Study | Prospective cohort | 1,768 | Age, APOE status, education, propensity score for any hormone therapy use | No hormone therapy | 38 | 89 | 1.00 | Referent | NR | 1.00 | Referent | ||
| ≤5 years | 41 | 52 | 0.70 | 0.49, 0.99 | 0.05 | NR | 0.74 | 0.55, 1.00 | 0.05 | |||||
| >5 years | 21 | 35 | 1.03 | 0.68, 1.55 | 0.89 | NR | 1.06 | 0.76, 1.49 | 0.73 | |||||
| Age at Initiation of Hormone Therapy | ||||||||||||||
| Roberts, 2006 (37) | Rochester Epidemiology Project | Nested case-control | 486 | Type of menopause, matched on age | No hormone therapy | 89 | 216 | 1.00 | Referent | |||||
| ≤49.5 years | 6 | 17 | 1.81 | 0.81, 4.05 | 0.15 | |||||||||
| >49.5 years | 6 | 11 | 0.67 | 0.30, 1.51 | 0.34 | |||||||||
| Whitmer, 2011 (44) | Kaiser Permanente of Northern California | Nested case-control | 5,504 | Age, education, race, midlife body mass index, diabetes, hypertension, hyperlipidemia, stroke, and hysterectomy | No hormone therapy | 45 | 699 | 1.00 | Referent | |||||
| Midlife | 25 | 376 | 0.74 | 0.58, 0.94 | 0.01 | |||||||||
| Late life | 12 | 188 | 1.48 | 1.10, 1.98 | 0.01 | |||||||||
| Both | 18 | 261 | 1.02 | 0.78, 1.34 | 0.89 | |||||||||
Abbreviations: APOE, apolipoprotein E; CI, confidence interval; NR, not reported; RR, relative risk.
a RR, or the relative risk, can be an odds ratio or a hazard ratio depending on the study design and sampling scheme; refer to Table 1 for the specific effect measure reported in each study.
b The investigators did not include the following variables in their final model because they determined that further adjustment for them did not appreciably change their findings: education, marital status, ethnicity, and history of either smoking or progestin use.
c Total Alzheimer's disease cases.
d Total all-cause dementia cases.
e Past use and current use were evaluated in separate models.
f The authors reported that results did not change appreciably when terms were added separately for diabetes mellitus, cardiovascular disease, depression, or use of nonsteroidal antiinflammatory drugs.
Other reports contained more detailed information on timing of hormone therapy use. Two articles considered age at initiation. Investigators from the Rochester Epidemiology Project found increased risk among earlier initiators (≤49.5 years) and decreased risk among later initiators (>49.5 years), although neither of these results reached significance (37), and findings from the Northern California Kaiser Permanente cohort observed that midlife (ages 40–55 years) use was associated with a significant 26% decreased all-cause dementia risk, while late-life (ages ≈70–85 years) use was associated with 48% increased risk (44). Two articles considered time between menopause and initiation of therapy. Roberts et al. (37) reported an adverse but nonsignificant association among women who started therapy within 2 years of menopause, while new findings from the Cache County Study included a significant and inverse association corresponding to hormone therapy use when initiated within 5 years of menopause onset (RR = 0.70, 95% CI: 0.49, 0.99) (41), although the upper bound of the confidence interval was close to the null value (RR = 1.0).
Additional Alzheimer's disease results
Relative Alzheimer's disease risks corresponding to other exposure categorizations—related to dose, formulation of hormone therapy, and route of administration of hormone therapy—are displayed together in Table 5. There were too few studies of any exposure categorization to combine in meta-analyses. Observational studies that reported results according to whether therapy included progestin were inconsistent; some found higher Alzheimer's disease risk for hormone therapy with progestin than for hormone therapy without progestin, although formulation-specific relative risks were not significant (36, 40). The recent Cache County Study found an inverse association of unopposed estrogen use with Alzheimer's disease risk (RR = 0.70, 95% CI: 0.49, 1.01) (41). In contrast, the WHIMS randomized trial found increased risk of Alzheimer's disease for both unopposed estrogen use and estrogen plus progestin use (from our extracted Alzheimer's disease results for CEE alone: RR = 1.47, 95% CI: 0.58, 3.91; for CEE+MPA: RR = 1.74, 95% CI: 0.71, 3.91). However, none of these findings from the Cache County Study and the WHIMS was statistically significant.
Table 5.
Summary of Results—Other Hormone Therapy Exposure Categorizations and Alzheimer's Disease/Dementia Risk
| Author, Year (Reference No.) | Study | Study Design | No. of Persons | Covariates | Exposure Distribution |
Alzheimer's Disease |
Dementia |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Description | % | No. of Cases | RRa | 95% CI | P Value | No. of Cases | RR | 95% CI | P Value | |||||
| Cumulative Dose | ||||||||||||||
| Roberts, 2006 (37) | Rochester Epidemiology Project | Nested case-control | 475 | Type of menopause, matched on age | No hormone therapy | 91 | 216 | 1.00 | Referent | |||||
| ≤756 mg | 5 | 13 | 1.52 | 0.64, 3.61 | 0.34 | |||||||||
| >756 mg | 4 | 9 | 0.77 | 0.32, 1.84 | 0.56 | |||||||||
| Waring, 1999 (43) | Rochester Epidemiology Project | Nested case-control | 444 | Controls matched by age, length of time in record linkage systemb | 0 mg | 89 | 189 | 1.00 | Referent | |||||
| ≤20 mg | 3 | 7 | 1.34 | 0.42, 4.28 | 0.63 | |||||||||
| 21–500 mg | 4 | 7 | 0.77 | 0.26, 2.25 | 0.63 | |||||||||
| >500 mg | 4 | 7 | 0.60 | 0.28, 2.06 | 0.6 | |||||||||
| Formulation | ||||||||||||||
| Petitti, 2008 (36) | Southern California Kaiser Permanente | Nested case-control | 2,906 | Age, education, myocardial infarction, stroke, Parkinson's disease, diabetes mellitus, hypertension | No hormone therapy | 48 | NR | 1.00 | Referent | 121 | 1.00 | Referent | ||
| E with P | 15 | NR | 1.41 | 0.94, 2.12 | 0.10 | 50 | 1.34 | 0.95, 1.89 | 0.10 | |||||
| E without P | 37 | NR 191c |
1.22 | 0.89, 1.68 | 0.22 | 112 | 1.23 | 0.94, 1.59 | 0.12 | |||||
| Seshadri, 2001 (40) | General Practice Research Database | Nested case-control | 280 | Body mass index, smoking. Matched on age, physician's practice, case index date, date of first prescription in database | No hormone therapy | 75 | 44 | 1.00 | Referent | |||||
| Oral E with P | 13 | 9 | 1.45 | 0.60, 3.49 | 0.41 | |||||||||
| Oral E without P | 7 | 4 | 0.89 | 0.35, 2.30 | 0.81 | |||||||||
| Transdermal | 4 | 2 | 0.73 | 0.15, 3.57 | 0.7 | |||||||||
| Shao, 2012 (41) | Cache County Study | Prospective cohort | 1,732 | Age, APOE status, education, propensity score for any hormone therapy use | No hormone therapy | 38 | 89 | 1.00 | Referent | NR | 1.00 | Referent | ||
| E with P | 24 | 32 | 0.93 | 0.60, 1.43 | 0.74 | NR | 0.93 | 0.64, 1.35 | 0.70 | |||||
| E without P | 38 | 49 | 0.70 | 0.49, 1.01 | 0.05 | NR | 0.79 | 0.59, 1.07 | 0.12 | |||||
| Shumaker, 2004 (11) | Women's Health Initiative Memory Study (estrogen with/without progestin) | Randomized placebo-controlled trial | 4,531 | None | Placebo | 51 | 12 | 1.00 | Referent | 21 | 1.00 | Referent | ||
| E with P | 49 | 20 | 1.74d | 0.71, 3.91 | 0.20 | 40 | 2.05 | 1.21, 3.48 | 0.01 | |||||
| Shumaker, 2004 (11) | Women's Health Initiative Memory Study (estrogen with/without progestin) | Randomized placebo-controlled trial | 2,947 | None | Placebo | 50 | 9 | 1.00 | Referent | 19 | 1.00 | Referent | ||
| E without P | 50 | 13 | 1.47d | 0.58, 3.91 | 0.44 | 28 | 1.49 | 0.83, 2.66 | 0.18 | |||||
| Route of Administration | ||||||||||||||
| Brenner, 1994 (33) | Alzheimer Disease Patient Registry-Group Health Cooperative | Nested case-control | 252 | Age, history of hysterectomye | No hormone therapy | 46 | 55 | 1.00 | Referent | |||||
| Oral | 23 | 25 | 0.70 | 0.40, 1.50 | 0.29 | |||||||||
| Vaginal | 31 | 38 | 1.30 | 0.70, 2.30 | 0.39 | |||||||||
Abbreviations: APOE, apolipoprotein E; CI, confidence interval; E, estrogen; NR, not reported; P, progestin; RR, relative risk.
a RR, or the relative risk, can be an odds ratio or a hazard ratio depending on the study design and sampling scheme; refer to Table 1 for the specific effect measure reported in each study.
b It is unclear which covariates (if any) were included in the final models, but the authors report that the odds ratio did not change noticeably after controlling for the effects of education, age at menopause, and parity and when conducting stratified analyses in women who had undergone natural menopause or who used estrogen for more than 1 year.
c Total Alzheimer's disease cases.
d Hazard ratios for Alzheimer's disease were not provided in the paper. We derived incidence rate ratios by using available data on Alzheimer's disease (number of cases, number of noncases, person-time).
e The investigators did not include the following variables in their final model because they determined that adjusting for them did not appreciably change the findings: education, marital status, ethnicity, and history of either smoking or progestin use.
One study differentiated use by route of administration (33) (oral vs. vaginal) and found a nonsignificant decreased risk for use of oral estrogens but a nonsignificant increased risk for use of vaginal estrogens. Finally, the 2 studies from the Rochester Epidemiology Project, which had detailed records of prescriptions in a local population, considered how cumulative dose of hormone therapy may influence Alzheimer's disease risk, and, while women with the highest cumulative doses had lower Alzheimer's disease risk, results did not reach significance (37, 43).
DISCUSSION
In this review, we present results from a series of prospective studies on postmenopausal hormone therapy (including estrogen alone or in combination with progestin) in relation to dementia and Alzheimer's disease risk that suggest no impact on risk for Alzheimer's disease or all-cause dementia. Our summary estimates for ever versus never users were null whether or not we included the clinical trial result that hormone therapy actually increases dementia risk and were robust to a variety of alternative outcome definitions.
These null findings are noteworthy in light of prior meta-analyses of observational studies, conducted over a decade ago, that found inverse associations between hormone therapy use and dementia risk. These earlier meta-analyses were interpreted with caution because the included studies often suffered from methodological limitations (25–27). By contrast, our meta-analysis included only prospective studies meeting prespecified inclusion criteria. In addition, an examination of the studies included in our review shows that the reported associations between hormone therapy use and dementia tend to shift over the 18-year span from suggesting protection to suggesting no benefit or harm. We discuss here potential reasons for this shift in findings over time, both from previous reviews to the present one and within our own review over time. We also explore possible ways that our null results could mask potential nuances in the relation of hormone therapy use to dementia risk, whether beneficial or harmful (e.g., aspects of hormone therapy such as formulation, timing, duration, and so on), and identify areas where further research is needed.
Prior findings and time trends
The differences between our findings and those of earlier meta-analyses are partly accounted for by our inclusion criteria. Many of the observational studies included in prior reviews did not meet our inclusion criteria; for example, of the 10 studies included in 1 previous meta-analysis (25), only 2 were in our meta-analysis. We did not include case-control studies unless they were conducted within well-defined study populations, and we required that all included studies follow participants longitudinally, collecting hormone therapy data prospectively with respect to dementia assessment. In addition, our analysis includes many studies published after previous meta-analyses were conducted, and, as noted earlier, there was a time trend, with studies from the 1990s more often showing protection to those from 2000 forward more often showing no association or harm. This pattern may reflect publication bias prior to the release of the first—deleterious—WHI findings in 2002. It could also reflect improvements in how the studies were conducted or changes in the predictors of hormone therapy use over time.
Measurement error and study design
All studies included in our review evaluated hormone therapy use prospectively in relation to dementia risk, and most systematically evaluated their study populations for dementia. By contrast, when previous systematic reviews were conducted, many of the available studies were either cross-sectional (47) or had collected data on hormone therapy use retrospectively (e.g., after dementia diagnosis occurred). In addition, many previous studies drew cases and controls from populations that were not well defined or comprehensively evaluated.
Determining history of hormone therapy use is a concern in studies that relied on self-report, but it is especially challenging in retrospective settings. People with prodromal memory symptoms—undetected by screening—may be less likely to recall past use of hormone therapy, which could lead to spurious protective effects. This is especially problematic in studies that collected exposure information from older women, who would have been trying to recall hormone therapy use over a longer interval and would have been more likely to have early cognitive changes. Worse still, in retrospective studies, some women may have ceased using hormone therapy because they had developed dementia symptoms.
Misclassification of the outcome is also possible, and some older studies were especially susceptible to this problem by defining cases according to cause of death (48). Although our review did not include studies that relied solely on hospital records or death certificate data, we did include several studies that used medical databases for case detection (33, 36, 37, 40, 43, 44). Even though this approach can also incompletely capture all dementia cases, most of these databases included entries for outpatient visits in addition to hospitalizations and therefore were more comprehensive in detecting cases than older studies that relied on death certificate or hospitalization data alone. Moreover, excluding the medical database studies did not appreciably change our summary estimate.
Finally, many studies included in previous reviews followed a case-control design in which cases and controls may have been sampled in ways that were related to hormone therapy use or its measurement. For example, some studies selected cases from hospital rosters, which may represent the most severe cases and also those with the least reliable data on hormone therapy use.
Confounding
One of the most noted problems in observational studies of hormone therapy is that users differ from nonusers in fundamental ways, including being more educated, being more affluent, having better access to health care, and (at least until the publication of the WHI results) being more health conscious. Consistent with this, users tended to have fewer cardiovascular risk factors (16) and to have access to more and higher quality health services. Many studies included in previous systematic reviews did not adjust for education or other indices of socioeconomic position. Although many of the studies in our review adjusted for more potential sources of confounding than in older studies, very few considered a wide constellation of socioeconomic confounders, even though hormone therapy use appears to vary independently by several dimensions of socioeconomic position (49–51). These issues could have led to spurious protective effects or to a masking of detrimental effects, especially in studies that did not account for other dementia risk factors beyond age and education (52). In addition, socioeconomic predictors of hormone therapy use are likely to have shifted over time in complex ways, with use initially and gradually growing more widespread followed by changes in use in response to the publicity around the WHI results (53, 54). Overall, complex changes in patterns of confounding could explain some of the differences between earlier reviews and our own, as well as the time trends within our own review.
Publication bias
Publication bias could certainly account for the differences between our review and previous meta-analyses, as nonnull findings—especially those supporting the benefits suggested in animal data—would be more interesting early on, and null findings more so over time, particularly after the release of the first deleterious findings from the WHI in 2002. While Egger's tests and Begg's funnel plots did not reveal any blatant publication bias in our study, these tests are underpowered when fewer than 20 studies are assessed.
Questions regarding key dimensions of hormone therapy use
The effects of hormone therapy on dementia risk may depend on a variety of factors, including formulation (especially whether progestin is present), the dosing schedule (i.e., formulas where both estrogen and progestin are taken daily vs. formulas that include progestin for only part of the month), the route of administration (e.g., oral, transdermal), and the timing of use in the life span (timing relative to menopause as well as duration of use). Few studies were able to capture all of the differences in hormone therapy usage that may bear on dementia risk, because either the investigators lacked the necessary information or sample size did not allow for separate or subgroup analyses. These factors may have contributed to some of the changes in findings over time. In addition, there might be undetected differences in the impact of hormone therapy on dementia risk across these dimensions, so further research is needed in all of these areas.
Formulation and dosing schedule
During the time the studies were conducted, the majority of participants in the United States likely took unopposed, oral conjugated estrogen (e.g., Premarin; Pfizer, Inc. (formerly Wyeth-Ayerst Pharmaceuticals), New York, New York). Many studies reviewed here either did not provide information about formulation (34, 35), or they analyzed opposed and unopposed estrogen therapies together typically because they had insufficient sample size for separate analyses (38, 42, 43, 45). Studies reporting results for specific formulations of hormone therapy (36, 40, 41) were inconsistent. In the WHIMS, the largest data set providing information on formulation, women assigned to estrogen plus progestin had a significantly elevated all-cause dementia risk, but women assigned to estrogen alone had elevated all-cause dementia risk that was not statistically significant. Risk of Alzheimer's disease was elevated for both treatment groups but did not achieve statistical significance (11).
The formulation of hormone therapy could be important for dementia risk; in some tissues (e.g., the uterine lining), progestin prevents certain actions of estrogen (55), and the specific effects on the nervous system are known to differ depending on whether the hormone preparation includes progestin (56, 57). If opposed and unopposed formulations have different effects on Alzheimer's disease risk, then combining different hormone therapy types into one exposure group could mask formulation-specific associations with dementia risk. Furthermore, the dosing schedule (i.e., whether estrogen and progestin are taken daily in continuous therapy or whether daily estrogen is accompanied by progestin for 10–14 days of the month to simulate hormone levels in a menstrual cycle in sequential therapy) could affect dementia risk. The WHIMS trial used a continuous dosing schedule, while most women in the observational studies (who used combined hormone therapy) used sequential preparations (58). To our knowledge, no study has directly compared the effects of sequential versus continuous combined hormone therapy on dementia risk.
Route of administration
The observational studies reported here rarely evaluated different routes of administration, but, during the period when most of these studies were conducted, hormone therapy was typically taken orally in the United States, so these findings cannot be generalized to hormones administered by transdermal patches, gels and creams, vaginal rings, and injections, which are in increasingly common use in recent years (59). For example, orally—but not transdermally—administered estrogen induces hepatic effects such as increased production of C-reactive protein, an acute-phase reactant, via first-pass metabolism in the liver (60). Even though the relation of peripheral C-reactive protein to Alzheimer's disease is unclear, a higher level of C-reactive protein does appear to be associated with increased dementia risk (61). Other delivery-based differences in metabolism (62) may also alter any impact of estrogen on Alzheimer's disease risk. That said, a 2-year trial of ultra-low-dose transdermal estrogen found no significant difference in cognitive outcomes between those using the patch and those on placebo (63). Yet there is a lack of evidence from large, randomized clinical trials that examine the efficacy and safety of alternative hormone therapy formulations (e.g., low-dose estrogen, transdermal estrogens, vaginal estrogens).
Timing and duration of use
Most of the studies in this review did not distinguish at what age or at what time relative to menopause the participating women used hormone therapy. However, hormone use in the observational studies was more likely to begin in midlife, near the time of menopause, while in the WHIMS women were assigned hormone therapy long after menopause onset. To the extent that timing of initiation has been analyzed separately, the current literature provides no indication that early initiation of hormone therapy use is related to reduced dementia risk. Two recent observational studies found significant reductions in risks for Alzheimer's disease (41) and all-cause dementia (41, 44) associated with initiation near the time of menopause. However, not all studies in this review found inverse associations for earlier initiation of hormone therapy; in one study, risk was actually higher in those who used hormone therapy earlier, although the findings were not significant (37), and another study found no benefit of starting hormone therapy within 10 years of menopause (36). Findings from the WHIMS, where hormone therapy was assigned long after menopause onset, support an increased dementia risk (17, 64, 65).
The duration of hormone therapy use is closely tied to time of initiation (i.e., longer duration of use implies earlier initiation), and several of the studies reviewed here categorized exposure by duration of use (32–34, 37, 40, 42, 43, 45). However, definitions of “long” duration varied widely, and even though some studies found significantly reduced risks of Alzheimer's disease in longer duration categories (42, 43, 45), there were very few cases in the longest duration categories, and taken together these findings do not provide evidence that duration of use plays a role in dementia risk. There is an urgent need for further research on the role of the timing and duration of hormone therapy use on cognitive outcomes, and randomized trials addressing this issue are underway (e.g., the Kronos Early Estrogen Prevention Study (KEEPS) (66) and Early versus Late Intervention Trial with Estradiol (ELITE)). Earlier initiation may also correspond to hormone therapy use in the presence of minimal existing disease burden, and further research should clarify the risk-benefit profile of hormone therapy use for varying degrees of preexisting cardiovascular and neurological disease.
Strengths and limitations
This systematic review and meta-analysis has several strengths, including our comprehensive and systematic search strategy, methodology, and stringent criteria to ensure that only high-quality studies were included. Furthermore, the review provides information from many additional studies that could not be included in a meta-analysis, as well as qualitative information on all of the studies that help to interpret the body of research. The display of findings not only shows the full scope of prospective research conducted on hormone therapy and dementia but also highlights areas where data are lacking, sometimes severely (e.g., data on Alzheimer's disease and dementia risk by timing of hormone therapy use, duration of use, formulation, and route of administration).
Our work also has several limitations. Our conclusions about hormone therapy use and all-cause dementia risk are limited because of the small number of studies that evaluated this outcome. However, consistent with findings for Alzheimer's disease or dementia outcome, a brief review of analyses of studies examining cognitive decline, which in many cases represents the prodromal phase of Alzheimer's disease or other dementias (67), also revealed inconsistent findings in prospective observational studies (20, 68–70), in the WHIMS (12, 13, 71, 72), and in other clinical trials over the short or long (up to 5 years) term (22). Studies also varied in the disparate ways that they categorized the exposure. Additionally, analyses of hormone therapy typically have smaller sample sizes than those of other risk factors because only women can be included, which may limit statistical power. Smaller sample sizes and, in particular, the smaller case numbers that result also limit the number of covariates that can be included in the analytical models and, therefore, the ability to adjust for confounding. In addition, the wide variety of formulations, broad age range, and other factors described above call for stratified analyses, but sample sizes limit these approaches.
Conclusion and recommendations
For many years, postmenopausal hormone therapy was viewed as a promising treatment for the prevention of age-related diseases in women. However, in our systematic review of the literature, there does not appear to be a benefit for dementia. The widely publicized results of the Women's Health Initiative Study and associated Women's Health Initiative Memory Study, published in 2003 (10) and 2004 (11), led to markedly decreased use of hormone therapy (73). Prescribing patterns continue to change since the publication of these results. There have been an overall decrease in hormone therapy use and, specifically, a decrease in use of oral high-dose estrogen formulations and relative increase in use of low-dose estrogen oral formulations and transdermal and vaginal formulations (74, 75). However, there is little information about how these other forms of hormone therapy affect dementia or Alzheimer's disease risk.
Although ongoing studies address issues about formulation and timing, hormone therapy is not recommended for prevention of dementia or other chronic diseases (14). However, estrogen remains one of the most effective treatments for relief from the vasomotor symptoms of menopause. The North American Menopause Society stresses the importance of considering individual risk profiles, because temporary use of hormone therapy may be appropriate to treat low-risk women with severe menopausal symptoms (76). In terms of dementia, there is a lack of available data on key questions, particularly related to the timing, form, and dosage of hormone therapy use, and we look forward to the results of future research addressing these questions as more women face decisions about hormone therapy as they enter menopause.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts (Jacqueline O'Brien, John W. Jackson, Francine Grodstein, Deborah Blacker); Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital, Boston, Massachusetts (Jacqueline O'Brien, Francine Grodstein); Division of Aging, Department of Medicine, Brigham and Women's Hospital, Boston, Massachusetts (Jacqueline O'Brien); Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women's Hospital, Boston, Massachusetts (John W. Jackson); Department of Psychiatry, Massachusetts General Hospital/Harvard Medical School, Boston, Massachusetts (Deborah Blacker); Rush Institute for Healthy Aging, Rush University Medical Center, Chicago, Illinois (Jennifer Weuve); and Department of Environmental Health, Harvard School of Public Health, Boston, Massachusetts (Jennifer Weuve).
Deborah Blacker and Jennifer Weuve contributed equally to this work.
This work was supported by the National Institutes of Health/National Institute of Environmental Health Sciences (T32-AG000158 (J. O.), T32-MH017119 (J. W. J.), R21ES020404 (J. W.)); the Alzheimer's Association (NIRG 242395 (J. W.)); the Horace W. Goldsmith Fellowship at Harvard University (J. W. J.); and a grant from an anonymous foundation (D. B.).
We thank Gautam Sajeev for his assistance with the systematic literature search, Dr. Matthew McQueen for advice regarding meta-analysis methods, Dr. Melinda Power for statistical and programming guidance, and Dr. Kristine Yaffe for review of an earlier version of this work.
REFERENCES
- 1.Krieger N, Lowy I, Aronowitz R, et al. Hormone replacement therapy, cancer, controversies, and women's health: historical, epidemiological, biological, clinical, and advocacy perspectives. J Epidemiol Community Health. 2005;59(9):740–748. doi: 10.1136/jech.2005.033316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henderson VW. Action of estrogens in the aging brain: dementia and cognitive aging. Biochim Biophys Acta. 2010;1800(10):1077–1083. doi: 10.1016/j.bbagen.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Gibbs RB. Estrogen therapy and cognition: a review of the cholinergic hypothesis. Endocr Rev. 2010;31(2):224–253. doi: 10.1210/er.2009-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brinton RD, Chen S, Montoya M, et al. The estrogen replacement therapy of the Women's Health Initiative promotes the cellular mechanisms of memory and neuronal survival in neurons vulnerable to Alzheimer's disease. Maturitas. 2000;34(2):S35–S52. doi: 10.1016/s0378-5122(00)00107-9. [DOI] [PubMed] [Google Scholar]
- 5.Tanapat P, Hastings NB, Reeves AJ, et al. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19(14):5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pike CJ, Carroll JC, Rosario ER, et al. Protective actions of sex steroid hormones in Alzheimer's disease. Front Neuroendocrinol. 2009;30(2):239–258. doi: 10.1016/j.yfrne.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Georgiadou P, Sbarouni E. Effect of hormone replacement therapy on inflammatory biomarkers. Adv Clin Chem. 2009;47:59–93. doi: 10.1016/s0065-2423(09)47003-3. [DOI] [PubMed] [Google Scholar]
- 8.Nelson HD, Walker M, Zakher B, et al. Menopausal hormone therapy for the primary prevention of chronic conditions: a systematic review to update the U.S. Preventive Services Task Force recommendations. Ann Intern Med. 2012;157(2):104–113. doi: 10.7326/0003-4819-157-2-201207170-00466. [DOI] [PubMed] [Google Scholar]
- 9.Coker LH, Espeland MA, Rapp SR, et al. Postmenopausal hormone therapy and cognitive outcomes: the Women's Health Initiative Memory Study (WHIMS) J Steroid Biochem Mol Biol. 2010;118(4-5):304–310. doi: 10.1016/j.jsbmb.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shumaker SA, Legault C, Rapp SR, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289(20):2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 11.Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291(24):2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 12.Espeland MA, Rapp SR, Shumaker SA, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291(24):2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- 13.Rapp SR, Espeland MA, Shumaker SA, et al. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289(20):2663–2672. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- 14.Moyer VA on behalf of the USPSTF. Menopausal hormone therapy for the primary prevention of chronic conditions: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;158(1):47–54. doi: 10.7326/0003-4819-158-1-201301010-00553. [DOI] [PubMed] [Google Scholar]
- 15.Gleason CE, Dowling NM, Friedman E, et al. Using predictors of hormone therapy use to model the healthy user bias: how does healthy user status influence cognitive effects of hormone therapy? Menopause. 2012;19(5):524–533. doi: 10.1097/gme.0b013e318238ff2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matthews KA, Kuller LH, Wing RR, et al. Prior to use of estrogen replacement therapy, are users healthier than nonusers? Am J Epidemiol. 1996;143(10):971–978. doi: 10.1093/oxfordjournals.aje.a008678. [DOI] [PubMed] [Google Scholar]
- 17.Brinton RD. The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends Neurosci. 2008;31(10):529–537. doi: 10.1016/j.tins.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seshadri S, Wolf PA, Beiser A, et al. Lifetime risk of dementia and Alzheimer's disease. The impact of mortality on risk estimates in the Framingham Study. Neurology. 1997;49(6):1498–1504. doi: 10.1212/wnl.49.6.1498. [DOI] [PubMed] [Google Scholar]
- 19.Hebert LE, Weuve J, Scherr PA, et al. Alzheimer disease in the United States (2010–2050) estimated using the 2010 Census. Neurology. 2013;80(19):1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrett-Connor E, Laughlin GA. Endogenous and exogenous estrogen, cognitive function, and dementia in postmenopausal women: evidence from epidemiologic studies and clinical trials. Semin Reprod Med. 2009;27(3):275–282. doi: 10.1055/s-0029-1216280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dye RV, Miller KJ, Singer EJ, et al. Hormone replacement therapy and risk for neurodegenerative diseases. Int J Alzheimer Dis. 2012;2012:258454. doi: 10.1155/2012/258454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lethaby A, Hogervorst E, Richards M, et al. Hormone replacement therapy for cognitive function in postmenopausal women. Cochrane Database Syst Rev. 2008;(1):CD003122. doi: 10.1002/14651858.CD003122.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maki PM. A systematic review of clinical trials of hormone therapy on cognitive function: effects of age at initiation and progestin use. Ann N Y Acad Sci. 2005;1052:182–197. doi: 10.1196/annals.1347.012. [DOI] [PubMed] [Google Scholar]
- 24.Marjoribanks J, Farquhar C, Roberts H, et al. Long term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2012;7:CD004143. doi: 10.1002/14651858.CD004143.pub4. [DOI] [PubMed] [Google Scholar]
- 25.Yaffe K, Sawaya G, Lieberburg I, et al. Estrogen therapy in postmenopausal women: effects on cognitive function and dementia. JAMA. 1998;279(9):688–695. doi: 10.1001/jama.279.9.688. [DOI] [PubMed] [Google Scholar]
- 26.LeBlanc ES, Janowsky J, Chan BK, et al. Hormone replacement therapy and cognition: systematic review and meta-analysis. JAMA. 2001;285(11):1489–1499. doi: 10.1001/jama.285.11.1489. [DOI] [PubMed] [Google Scholar]
- 27.Hogervorst E, Williams J, Budge M, et al. The nature of the effect of female gonadal hormone replacement therapy on cognitive function in post-menopausal women: a meta-analysis. Neuroscience. 2000;101(3):485–512. doi: 10.1016/s0306-4522(00)00410-3. [DOI] [PubMed] [Google Scholar]
- 28.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 29.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 30.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 31.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barnes LL, Wilson RS, Schneider JA, et al. Gender, cognitive decline, and risk of AD in older persons. Neurology. 2003;60(11):1777–1781. doi: 10.1212/01.wnl.0000065892.67099.2a. [DOI] [PubMed] [Google Scholar]
- 33.Brenner DE, Kukull WA, Stergachis A, et al. Postmenopausal estrogen replacement therapy and the risk of Alzheimer's disease: a population-based case-control study. Am J Epidemiol. 1994;140(3):262–267. doi: 10.1093/oxfordjournals.aje.a117245. [DOI] [PubMed] [Google Scholar]
- 34.Kawas C, Resnick S, Morrison A, et al. A prospective study of estrogen replacement therapy and the risk of developing Alzheimer's disease: the Baltimore Longitudinal Study of Aging. Neurology. 1997;48(6):1517–1521. doi: 10.1212/wnl.48.6.1517. [DOI] [PubMed] [Google Scholar]
- 35.Lindsay J, Laurin D, Verreault R, et al. Risk factors for Alzheimer's disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;156(5):445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- 36.Petitti DB, Crooks VC, Chiu V, et al. Incidence of dementia in long-term hormone users. Am J Epidemiol. 2008;167(6):692–700. doi: 10.1093/aje/kwm362. [DOI] [PubMed] [Google Scholar]
- 37.Roberts RO, Cha RH, Knopman DS, et al. Postmenopausal estrogen therapy and Alzheimer disease: overall negative findings. Alzheimer Dis Assoc Disord. 2006;20(3):141–146. doi: 10.1097/00002093-200607000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Ryan J, Carriere I, Scali J, et al. Characteristics of hormone therapy, cognitive function, and dementia: the prospective 3C Study. Neurology. 2009;73(21):1729–1737. doi: 10.1212/WNL.0b013e3181c34b0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ryan J, Carriere I, Scali J, et al. Life-time estrogen exposure and cognitive functioning in later life. Psychoneuroendocrinology. 2009;34(2):287–298. doi: 10.1016/j.psyneuen.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 40.Seshadri S, Zornberg GL, Derby LE, et al. Postmenopausal estrogen replacement therapy and the risk of Alzheimer disease. Arch Neurol. 2001;58(3):435–440. doi: 10.1001/archneur.58.3.435. [DOI] [PubMed] [Google Scholar]
- 41.Shao H, Breitner JC, Whitmer RA, et al. Hormone therapy and Alzheimer disease dementia: new findings from the Cache County Study. Neurology. 2012;79(18):1846–1852. doi: 10.1212/WNL.0b013e318271f823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang MX, Jacobs D, Stern Y, et al. Effect of oestrogen during menopause on risk and age at onset of Alzheimer's disease. Lancet. 1996;348(9025):429–432. doi: 10.1016/S0140-6736(96)03356-9. [DOI] [PubMed] [Google Scholar]
- 43.Waring SC, Rocca WA, Petersen RC, et al. Postmenopausal estrogen replacement therapy and risk of AD: a population-based study. Neurology. 1999;52(5):965–970. doi: 10.1212/wnl.52.5.965. [DOI] [PubMed] [Google Scholar]
- 44.Whitmer RA, Quesenberry CP, Zhou J, et al. Timing of hormone therapy and dementia: the critical window theory revisited. Ann Neurol. 2011;69(1):163–169. doi: 10.1002/ana.22239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zandi PP, Carlson MC, Plassman BL, et al. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. JAMA. 288(17):2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- 46.Boustani M, Peterson B, Hanson L, et al. Screening for dementia in primary care: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2003;138(11):927–937. doi: 10.7326/0003-4819-138-11-200306030-00015. [DOI] [PubMed] [Google Scholar]
- 47.Baldereschi M, Di Carlo A, Lepore V, et al. Estrogen-replacement therapy and Alzheimer's disease in the Italian Longitudinal Study on Aging. Neurology. 1998;50(4):996–1002. doi: 10.1212/wnl.50.4.996. [DOI] [PubMed] [Google Scholar]
- 48.Olichney JM, Hofstetter CR, Galasko D, et al. Death certificate reporting of dementia and mortality in an Alzheimer's disease research center cohort. J Am Geriatr Soc. 1995;43(8):890–893. doi: 10.1111/j.1532-5415.1995.tb05532.x. [DOI] [PubMed] [Google Scholar]
- 49.Friedman-Koss D, Crespo CJ, Bellantoni MF, et al. The relationship of race/ethnicity and social class to hormone replacement therapy: results from the Third National Health and Nutrition Examination Survey 1988–1994. Menopause. 2002;9(4):264–272. doi: 10.1097/00042192-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 50.Lawlor DA, Davey Smith G, Ebrahim S. Socioeconomic position and hormone replacement therapy use: explaining the discrepancy in evidence from observational and randomized controlled trials. Am J Public Health. 2004;94(12):2149–2154. doi: 10.2105/ajph.94.12.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Persson I, Bergkvist L, Lindgren C, et al. Hormone replacement therapy and major risk factors for reproductive cancers, osteoporosis, and cardiovascular diseases: evidence of confounding by exposure characteristics. J Clin Epidemiol. 1997;50(5):611–618. doi: 10.1016/s0895-4356(97)00004-8. [DOI] [PubMed] [Google Scholar]
- 52.Grodstein F, Clarkson TB, Manson JE. Understanding the divergent data on postmenopausal hormone therapy. N Engl J Med. 2003;348(7):645–650. doi: 10.1056/NEJMsb022365. [DOI] [PubMed] [Google Scholar]
- 53.Haskell SG, Bean-Mayberry B, Goulet JL, et al. Determinants of hormone therapy discontinuation among female veterans nationally. Mil Med. 2008;173(1):91–96. doi: 10.7205/milmed.173.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei F, Miglioretti DL, Connelly MT, et al. Changes in women's use of hormones after the Women's Health Initiative estrogen and progestin trial by race, education, and income. J Natl Cancer Inst Monogr. 2005;(35):106–112. doi: 10.1093/jncimonographs/lgi047. [DOI] [PubMed] [Google Scholar]
- 55.Nilsen J, Brinton RD. Divergent impact of progesterone and medroxyprogesterone acetate (Provera) on nuclear mitogen-activated protein kinase signaling. Proc Natl Acad Sci U S A. 2003;100(18):10506–10511. doi: 10.1073/pnas.1334098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hogervorst E, Yaffe K, Richards M, et al. Hormone replacement therapy to maintain cognitive function in women with dementia. Cochrane Database Syst Rev. 2009;(1):CD003799. doi: 10.1002/14651858.CD003799.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Henderson VW, Brinton RD. Menopause and mitochondria: windows into estrogen effects on Alzheimer's disease risk and therapy. Prog Brain Res. 2010;182:77–96. doi: 10.1016/S0079-6123(10)82003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Henderson VW. The neurology of menopause. Neurologist. 2006;12(3):149–159. doi: 10.1097/01.nrl.0000215750.52786.b1. [DOI] [PubMed] [Google Scholar]
- 59.Lakey SL, Reed SD, LaCroix AZ, et al. Self-reported changes in providers’ hormone therapy prescribing and counseling practices after the Women's Health Initiative. J Womens Health. 2010;19(12):2175–2181. doi: 10.1089/jwh.2010.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Modena MG, Bursi F, Fantini G, et al. Effects of hormone replacement therapy on C-reactive protein levels in healthy postmenopausal women: comparison between oral and transdermal administration of estrogen. Am J Med. 2002;113(4):331–334. doi: 10.1016/s0002-9343(02)01209-3. [DOI] [PubMed] [Google Scholar]
- 61.Koyama A, O'Brien J, Weuve J, et al. The role of peripheral inflammatory markers in dementia and Alzheimer's disease: a meta-analysis. J Geront Ser A Biol Sci Med Sci. 2013;68(4):433–440. doi: 10.1093/gerona/gls187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Steingold KA, Matt DW, DeZiegler D, et al. Comparison of transdermal to oral estradiol administration on hormonal and hepatic parameters in women with premature ovarian failure. J Clin Endocrinol Metab. 1991;73(2):275–280. doi: 10.1210/jcem-73-2-275. [DOI] [PubMed] [Google Scholar]
- 63.Yaffe K, Vittinghoff E, Ensrud KE, et al. Effects of ultra-low-dose transdermal estradiol on cognition and health-related quality of life. Arch Neurol. 2006;63(7):945–950. doi: 10.1001/archneur.63.7.945. [DOI] [PubMed] [Google Scholar]
- 64.Mikkola TS, Clarkson TB. Estrogen replacement therapy, atherosclerosis, and vascular function. Cardiovasc Res. 2002;53(3):605–619. doi: 10.1016/s0008-6363(01)00466-7. [DOI] [PubMed] [Google Scholar]
- 65.Brinton RD. Investigative models for determining hormone therapy-induced outcomes in brain: evidence in support of a healthy cell bias of estrogen action. Ann N Y Acad Sci. 2005;1052:57–74. doi: 10.1196/annals.1347.005. [DOI] [PubMed] [Google Scholar]
- 66.Tsagkas V, Turner S. Phoenix, AZ: Kronos Longevity Research Institute; 2012. Hormone therapy has many favorable effects in newly menopausal women: initial findings of the Kronos Early Estrogen Prevention Study (KEEPS) http://www.keepstudy.org/news/pr_100312_a.cfm. Accessed February 7, 2013. [Google Scholar]
- 67.Wilson RS, Leurgans SE, Boyle PA, et al. Cognitive decline in prodromal Alzheimer disease and mild cognitive impairment. Arch Neurol. 2011;68(3):351–356. doi: 10.1001/archneurol.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Low LF, Anstey KJ. Hormone replacement therapy and cognitive performance in postmenopausal women—a review by cognitive domain. Neurosci Biobehav Rev. 2006;30(1):66–84. doi: 10.1016/j.neubiorev.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 69.Kang JH, Grodstein F. Postmenopausal hormone therapy, timing of initiation, APOE and cognitive decline. Neurobiol Aging. 2012;33(7):1129–1137. doi: 10.1016/j.neurobiolaging.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carlson MC, Zandi PP, Plassman BL, et al. Hormone replacement therapy and reduced cognitive decline in older women: the Cache County Study. Neurology. 2001;57(12):2210–2216. doi: 10.1212/wnl.57.12.2210. [DOI] [PubMed] [Google Scholar]
- 71.Resnick SM, Maki PM, Rapp SR, et al. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. J Clin Endocrinol Metab. 2006;91(5):1802–1810. doi: 10.1210/jc.2005-2097. [DOI] [PubMed] [Google Scholar]
- 72.Resnick SM, Espeland MA, An Y, et al. Effects of conjugated equine estrogens on cognition and affect in postmenopausal women with prior hysterectomy. J Clin Endocrinol Metab. 2009;94(11):4152–4161. doi: 10.1210/jc.2009-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guay MP, Dragomir A, Pilon D, et al. Changes in pattern of use, clinical characteristics and persistence rate of hormone replacement therapy among postmenopausal women after the WHI publication. Pharmacoepidemiol Drug Safety. 2007;16(1):17–27. doi: 10.1002/pds.1273. [DOI] [PubMed] [Google Scholar]
- 74.Tsai SA, Stefanick ML, Stafford RS. Trends in menopausal hormone therapy use of US office-based physicians, 2000–2009. Menopause. 2011;18(4):385–392. doi: 10.1097/gme.0b013e3181f43404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Steinkellner AR, Denison SE, Eldridge SL, et al. A decade of postmenopausal hormone therapy prescribing in the United States: long-term effects of the Women's Health Initiative. Menopause. 2012;19(6):616–621. doi: 10.1097/gme.0b013e31824bb039. [DOI] [PubMed] [Google Scholar]
- 76.North American Menopause Society. The 2012 hormone therapy position statement of: the North American Menopause Society. Menopause. 2012;19(3):257–271. doi: 10.1097/gme.0b013e31824b970a. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.