Abstract
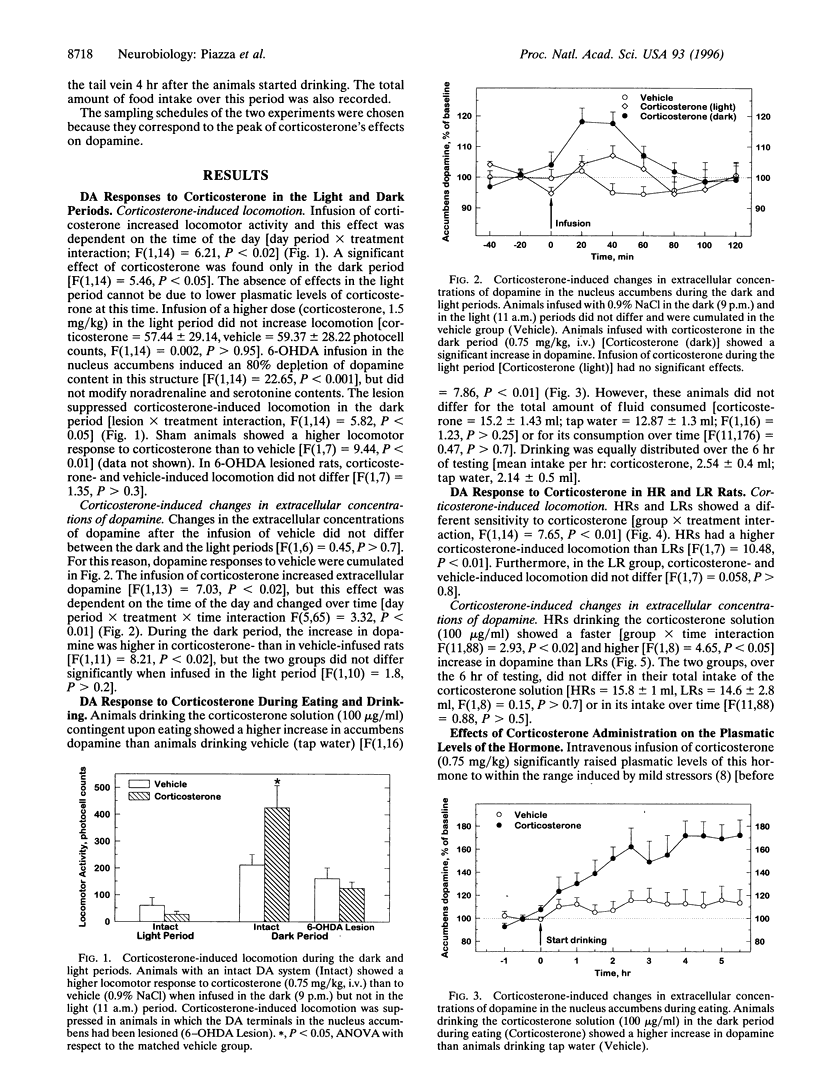
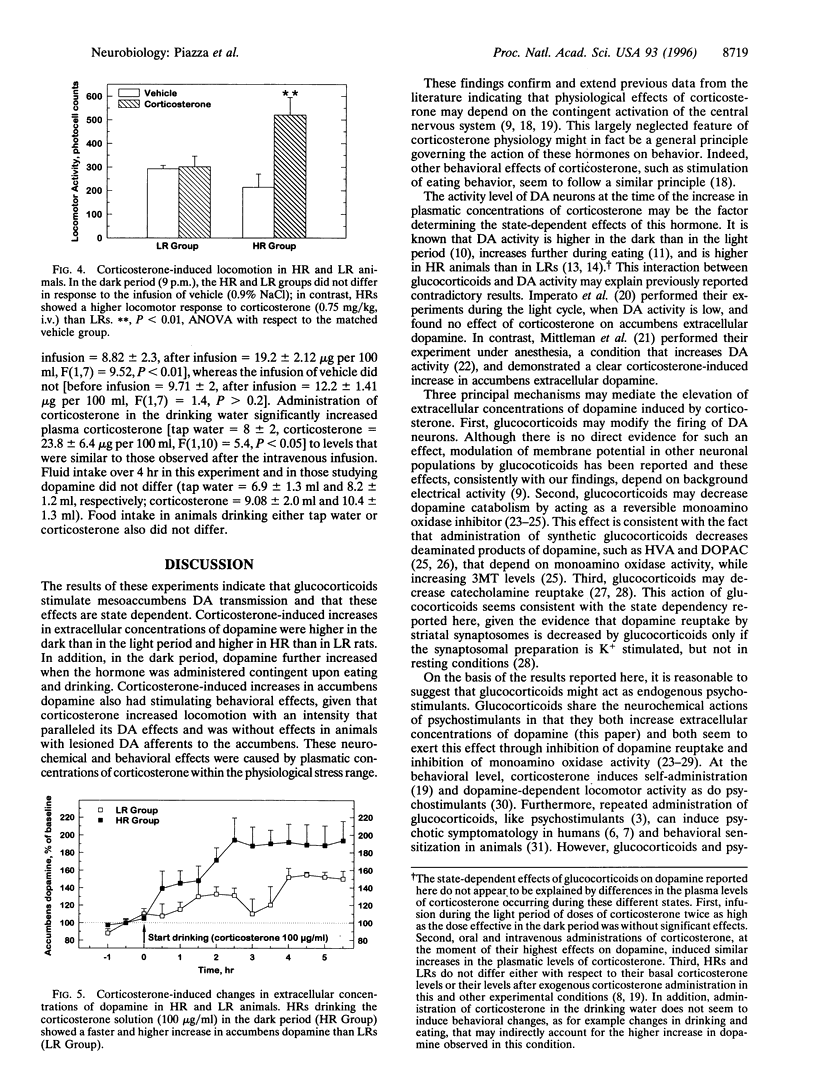
An increase in the activity of mesencephalic dopaminergic neurons has been implicated in the appearance of pathological behaviors such as psychosis and drug abuse. Several observations suggest that glucocorticoids might contribute to such an increase in dopaminergic activity. The present experiments therefore analyzed the effects of corticosterone, the major glucocorticoid in the rat, both on dopamine release in the nucleus accumbens of freely moving animals by means of microdialysis, and on locomotor activity, a behavior dependent on accumbens dopamine. Given that glucocorticoids have certain state-dependent neuronal effects, their action on dopamine was studied in situations differing in dopaminergic tonus, including during the light and dark phases of the circadian cycle, during eating, and in groups of animals differing in their locomotor reactivity to novelty. Dopaminergic activity is increased in the dark period, further increased during food-intake, and is higher in rats defined as high responders to novelty than in low responders. Corticosterone, peripherally administered in a dose that approximates stress-induced plasma concentrations, increased extracellular concentrations of dopamine, and this increase was augmented in the dark phase, during eating, and in high responder rats. Corticosterone had little or no effects in the light phase and in low responder rats. Corticosterone also stimulated locomotor activity, an effect that paralleled the release of dopamine and was abolished by neurochemical (6-hydroxydopamine) depletion of accumbens dopamine. In conclusion, glucocorticoids have state-dependent stimulant effects on mesencephalic dopaminergic transmission, and an interaction between these two factors might be involved in the appearance of behavioral disturbances.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badiani A., Stewart J. The kappa-opioid U-50,488H suppresses the initiation of nocturnal spontaneous drinking in normally hydrated rats. Psychopharmacology (Berl) 1992;106(4):463–473. doi: 10.1007/BF02244816. [DOI] [PubMed] [Google Scholar]
- Caesar P. M., Collins G. G., Sandler M. Catecholamine metabolism and monoamine oxidase activity in adrenalectomized rats. Biochem Pharmacol. 1970 Mar;19(3):921–926. doi: 10.1016/0006-2952(70)90255-8. [DOI] [PubMed] [Google Scholar]
- Deroche V., Piazza P. V., Maccari S., Le Moal M., Simon H. Repeated corticosterone administration sensitizes the locomotor response to amphetamine. Brain Res. 1992 Jul 3;584(1-2):309–313. doi: 10.1016/0006-8993(92)90911-r. [DOI] [PubMed] [Google Scholar]
- Gilad G. M., Rabey J. M., Gilad V. H. Presynaptic effects of glucocorticoids on dopaminergic and cholinergic synaptosomes. Implications for rapid endocrine-neural interactions in stress. Life Sci. 1987 Jun 22;40(25):2401–2408. doi: 10.1016/0024-3205(87)90754-5. [DOI] [PubMed] [Google Scholar]
- Halbach M., Henning U. Abnormal glucocorticoid dependent increase of spiperone binding sites on lymphocytes from schizophrenics in vitro. Pharmacopsychiatry. 1989 Sep;22(5):169–173. doi: 10.1055/s-2007-1014601. [DOI] [PubMed] [Google Scholar]
- Hall R. C., Popkin M. K., Stickney S. K., Gardner E. R. Presentation of the steroid psychoses. J Nerv Ment Dis. 1979 Apr;167(4):229–236. doi: 10.1097/00005053-197904000-00006. [DOI] [PubMed] [Google Scholar]
- Ho-Van-Hap A., Babineau L. M., Berlinguet L. Hormonal action on monoamine oxidase activity in rats. Can J Biochem. 1967 Mar;45(3):355–362. doi: 10.1139/o67-042. [DOI] [PubMed] [Google Scholar]
- Hoebel B. G., Hernandez L., Schwartz D. H., Mark G. P., Hunter G. A. Microdialysis studies of brain norepinephrine, serotonin, and dopamine release during ingestive behavior. Theoretical and clinical implications. Ann N Y Acad Sci. 1989;575:171–193. doi: 10.1111/j.1749-6632.1989.tb53242.x. [DOI] [PubMed] [Google Scholar]
- Hooks M. S., Colvin A. C., Juncos J. L., Justice J. B., Jr Individual differences in basal and cocaine-stimulated extracellular dopamine in the nucleus accumbens using quantitative microdialysis. Brain Res. 1992 Aug 7;587(2):306–312. doi: 10.1016/0006-8993(92)91012-4. [DOI] [PubMed] [Google Scholar]
- Härfstrand A., Fuxe K., Cintra A., Agnati L. F., Zini I., Wikström A. C., Okret S., Yu Z. Y., Goldstein M., Steinbusch H. Glucocorticoid receptor immunoreactivity in monoaminergic neurons of rat brain. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9779–9783. doi: 10.1073/pnas.83.24.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperato A., Puglisi-Allegra S., Casolini P., Angelucci L. Changes in brain dopamine and acetylcholine release during and following stress are independent of the pituitary-adrenocortical axis. Brain Res. 1991 Jan 4;538(1):111–117. doi: 10.1016/0006-8993(91)90384-8. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Salt P. J. Inhibition of catecholamine Uptake-2 by steroids in the isolated rat heart. Br J Pharmacol. 1970 Nov;40(3):528–530. doi: 10.1111/j.1476-5381.1970.tb10637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M., de Kloet E. R. Control of neuronal excitability by corticosteroid hormones. Trends Neurosci. 1992 Jan;15(1):25–30. doi: 10.1016/0166-2236(92)90345-9. [DOI] [PubMed] [Google Scholar]
- Koob G. F., Bloom F. E. Cellular and molecular mechanisms of drug dependence. Science. 1988 Nov 4;242(4879):715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Kumar B. A., Leibowitz S. F. Impact of acute corticosterone administration on feeding and macronutrient self-selection patterns. Am J Physiol. 1988 Feb;254(2 Pt 2):R222–R228. doi: 10.1152/ajpregu.1988.254.2.R222. [DOI] [PubMed] [Google Scholar]
- Le Moal M., Simon H. Mesocorticolimbic dopaminergic network: functional and regulatory roles. Physiol Rev. 1991 Jan;71(1):155–234. doi: 10.1152/physrev.1991.71.1.155. [DOI] [PubMed] [Google Scholar]
- Ling M. H., Perry P. J., Tsuang M. T. Side effects of corticosteroid therapy. Psychiatric aspects. Arch Gen Psychiatry. 1981 Apr;38(4):471–477. doi: 10.1001/archpsyc.1981.01780290105011. [DOI] [PubMed] [Google Scholar]
- Marinelli M., Piazza P. V., Deroche V., Maccari S., Le Moal M., Simon H. Corticosterone circadian secretion differentially facilitates dopamine-mediated psychomotor effect of cocaine and morphine. J Neurosci. 1994 May;14(5 Pt 1):2724–2731. doi: 10.1523/JNEUROSCI.14-05-02724.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittleman G., Blaha C. D., Phillips A. G. Pituitary-adrenal and dopaminergic modulation of schedule-induced polydipsia: behavioral and neurochemical evidence. Behav Neurosci. 1992 Apr;106(2):408–420. doi: 10.1037//0735-7044.106.2.408. [DOI] [PubMed] [Google Scholar]
- Osborne P. G., O'Connor W. T., Drew K. L., Ungerstedt U. An in vivo microdialysis characterization of extracellular dopamine and GABA in dorsolateral striatum of awake freely moving and halothane anaesthetised rats. J Neurosci Methods. 1990 Sep;34(1-3):99–105. doi: 10.1016/0165-0270(90)90047-j. [DOI] [PubMed] [Google Scholar]
- Paulson P. E., Robinson T. E. Relationship between circadian changes in spontaneous motor activity and dorsal versus ventral striatal dopamine neurotransmission assessed with on-line microdialysis. Behav Neurosci. 1994 Jun;108(3):624–635. doi: 10.1037//0735-7044.108.3.624. [DOI] [PubMed] [Google Scholar]
- Piazza P. V., Deminière J. M., Le Moal M., Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989 Sep 29;245(4925):1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza P. V., Deroche V., Deminière J. M., Maccari S., Le Moal M., Simon H. Corticosterone in the range of stress-induced levels possesses reinforcing properties: implications for sensation-seeking behaviors. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11738–11742. doi: 10.1073/pnas.90.24.11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza P. V., Le Moal M. L. Pathophysiological basis of vulnerability to drug abuse: role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu Rev Pharmacol Toxicol. 1996;36:359–378. doi: 10.1146/annurev.pa.36.040196.002043. [DOI] [PubMed] [Google Scholar]
- Piazza P. V., Maccari S., Deminière J. M., Le Moal M., Mormède P., Simon H. Corticosterone levels determine individual vulnerability to amphetamine self-administration. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2088–2092. doi: 10.1073/pnas.88.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson T. E., Becker J. B. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986 Jun;396(2):157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Rothschild A. J., Langlais P. J., Schatzberg A. F., Miller M. M., Saloman M. S., Lerbinger J. E., Cole J. O., Bird E. D. The effects of a single acute dose of dexamethasone on monoamine and metabolite levels in rat brain. Life Sci. 1985 Jul 1;36(26):2491–2501. doi: 10.1016/0024-3205(85)90145-6. [DOI] [PubMed] [Google Scholar]
- Rougé-Pont F., Piazza P. V., Kharouby M., Le Moal M., Simon H. Higher and longer stress-induced increase in dopamine concentrations in the nucleus accumbens of animals predisposed to amphetamine self-administration. A microdialysis study. Brain Res. 1993 Jan 29;602(1):169–174. doi: 10.1016/0006-8993(93)90260-t. [DOI] [PubMed] [Google Scholar]
- Seiden L. S., Sabol K. E., Ricaurte G. A. Amphetamine: effects on catecholamine systems and behavior. Annu Rev Pharmacol Toxicol. 1993;33:639–677. doi: 10.1146/annurev.pa.33.040193.003231. [DOI] [PubMed] [Google Scholar]
- Veals J. W., Korduba C. A., Symchowicz S. Effect of dexamethasone on monoamine oxidase inhibiton by iproniazid in rat brain. Eur J Pharmacol. 1977 Feb 7;41(3):291–299. doi: 10.1016/0014-2999(77)90322-3. [DOI] [PubMed] [Google Scholar]


