Abstract
Background
Cerebral malaria (CM) is responsible for most of the malaria-related deaths in children in sub-Saharan Africa. Although, not well understood, the pathogenesis of CM involves parasite and host factors which contribute to parasite sequestration through cytoadherence to the vascular endothelium. Cytoadherence to brain microvasculature is believed to involve host endothelial receptor, CD54 or intercellular adhesion molecule (ICAM)-1, while other receptors such as CD36 are generally involved in cytoadherence of parasites in other organs. We therefore investigated the contributions of host ICAM-1 expression and levels of antibodies against ICAM-1 binding variant surface antigen (VSA) on parasites to the development of CM.
Methodology/Principal Findings
Paediatric malaria patients, 0.5 to 13 years were recruited and grouped into CM and uncomplicated malaria (UM) patients, based on well defined criteria. Standardized ELISA protocol was used to measure soluble ICAM-1 (sICAM-1) levels from acute plasma samples. Levels of IgG to CD36- or ICAM-1-binding VSA were measured by flow cytometry during acute and convalescent states. Wilcoxon sign rank-test analysis to compare groups revealed association between sICAM-1 levels and CM (p<0.0037). Median levels of antibodies to CD36-binding VSA were comparable in the two groups at the time of admission and 7 days after treatment was initiated (p>0.05). Median levels of antibodies to CD36-binding VSAs were also comparable between acute and convalescent samples within any patient group. Median levels of antibodies to ICAM-1-binding VSAs were however significantly lower at admission time than during recovery in both groups.
Conclusions/Significance
High levels of sICAM-1 were associated with CM, and the sICAM-1 levels may reflect expression levels of the membrane bound form. Anti-VSA antibody levels to ICAM-binding parasites was more strongly associated with both UM and CM than antibodies to CD36 binding parasites. Thus, increasing host sICAM-1 levels were associated with CM whilst antibodies to parasite expressing non-ICAM-1-binding VSAs were not.
Introduction
Malaria infection in man is caused by five species of Plasmodium; Plasmodium falciparum, P. knowlesi, P malariae, P. ovale and P. vivax [1]–[5] and transmitted by the bites of infected female mosquitoes of more than 30 anopheline species. Globally, an estimated 3.3 billion people were at risk of malaria in 2010 with populations living in sub-Saharan Africa having the highest risk of acquiring malaria. Majority of the cases and deaths occur annually in sub-Saharan Africa with children less than five years of age and pregnant women most severely affected [1].
Depending upon the degree of resistance to malaria by an individual, infection could be asymptomatic or manifest clinically as mild or severe malaria. Approximately 2% of all clinical malaria cases in African children results in severe disease that is attributable to P. falciparum [6]. This severe clinical manifestation of malaria is responsible for most of the malaria associated deaths [1]. Field isolates of P. falciparum that cause severe disease in children tend to express a subset of variant surface antigens (VSAs) with limited recognition by the developing immune system of infants and young children [7], [8]. Plasmodium falciparum erythrocyte membrane protein (PfEMP)-1, a component of VSAs, elicits natural immune responses to disease [9], [10]. PfEMP-1 mediates adhesion of infected red blood cells (iRBC) to endothelial receptors such as CD54 or ICAM-1, and CD36. Unlike CD36, ICAM-1 expression on vascular endothelium in the brain is up-regulated during malaria infection [11]–[13]. The degree of ICAM-1 up-regulation is influenced by the parasite and inflammatory cytokines [5], [14]–[17]. Following proteolytic cleavage, ICAM-1 becomes detectable in the blood [18]–[21] and the plasma levels of soluble ICAM-1 (sICAM-1) may perhaps reflect its membrane expression levels. ICAM-1 mediates adhesion of a smaller number of field parasite isolates to vascular endothelium compared to CD36 [11], [22]. The binding of CD36 and ICAM-1 to their respective PfEMP-1 have been shown to be specific and results in a selection of parasites expressing those PfEMP-1 variants [23]. Cytoadherence and subsequent sequestration of iRBC in the brain micro-vessels has been implicated in the pathophysiology of cerebral malaria. Up-regulation of ICAM-1 during infection and the possibility of its increased interaction with parasites expressing the appropriate corresponding PfEMP-1 phenotype may thus be important in the pathogenesis of cerebral malaria. Additionally, naturally induced antibodies that bind to parasite VSAs and have been associated with protection [8]–[10] may prevent parasite interaction with the host receptors, CD36 and ICAM-1, thereby preventing parasite cytoadherence and sequestration. Thus, the expression levels of ICAM-1 in the endothelium, here reflected by the plasma levels of the soluble form of this receptor, and the physiological levels of antibodies against ICAM-1-binding VSA phenotypes of infecting parasites might modulate clinical disease progression. This hypothesis was investigated by analyzing plasma samples from 69 paediatric malaria patients, of which 37 presented with cerebral malaria (CM) and 32 presented with uncomplicated malaria (UM). The data revealed an association of sICAM-1 levels with CM relative to UM. Levels of antibodies against ICAM-1-binding VSA in CM patients were significantly lower at the time of admission than during recovery (p = 0.0031). Thus an increase in sICAM-1 levels may be predictive of increased ICAM-1 expression in the brain microvasculature, and hence the development of cerebral complications in malaria. Also, increase in antibody levels to ICAM-1-binding VSA in CM patients after recovery does affirm that CM patients were infected with parasites that express ICAM-1-binding VSAs.
Materials and Methods
Malaria patients and sampling
Patients aged 0.5 to13 years who reported to the Department of Child Health, Korle-Bu Teaching Hospital, University of Ghana Medical School, during the 2003–2004 malaria transmission seasons with a diagnosis of P. falciparum malaria were enroled in the study after signed informed consent was obtained from their guardians/parents. All participants were febrile (>37.5 °C) at enrolment and had asexual blood parasitaemia of >2500/ul. Strict criteria were used to categorize patients into cerebral malaria and uncomplicated malaria [24]. Briefly, cerebral malaria patients had coma with a score of <3 on the Blantyre scale [25] and with no other attributable cause of cerebral dysfunction while those with uncomplicated malaria were fully conscious and had haemoglobin level of ≥ 8 g/dl.
Blood samples of 5ml were collected into K3EDTA vacutainers from study participants during the period of acute infection and for a subset of participants, samples were also taken 7 days later after recovery from disease. Plasma was prepared from blood samples by centrifugation and aliquots stored at –20°C until used for the estimation of the levels of the three biomarkers (sICAM-1 and antibodies to CD36- and ICAM-1-selected parasites).
The study received ethical approval from the Institutional Review Board of the Noguchi Memorial Institute for Medical Research and the Ethics Committee of the University of Ghana Medical School. Malaria treatment was carried out in accordance with the existing institutional guidelines at the time. Patients with UM were treated with a daily dose of chloroquine at 25 mg/kg body weight over 3 days. When treatment failure occurred, 10 mg/kg body weight per day of amodiaquine was administered for 3 days. Patients with cerebral malaria were treated with either amodiaquine syrup via a nasogastric tube at the same dosage as stated or intramuscular quinine sulphate (10 mg/kg body weight every 8 hours). The treatment with intramuscular quinine was changed to syrup at the same dosage when patients regained full consciousness or after 72 h (whichever was earlier) to complete a 7-day course.
P. falciparum lines, immunostaining and flow cytometry
Two genetically distinct laboratory established isolates of P. falciparum, generated as described earlier [26], [27] and kindly donated by Professor Lars Hviid of Centre for Medical Parasitology, Copenhagen were used. The FRC54 clone binds to host ICAM-1 while the FRC36 clone binds to CD36 of the host were used. Parasites were maintained in culture with human type O+ RBCs at 4% haematocrit in RPMI 1640 supplemented with Albumax II (GIBCO, USA) and L-glutamine according to previously described standard methods [28]. Cultures were maintained for not more than 14 days in order to minimize parasite mutations and switching to another phenotype. Specific antibodies to ICAM-1 and CD36-selected parasites in the plasma of patients were measured by flow cytometry as described earlier [29], [30]. In brief, RBC infected with late trophozoite and schizont stages of CD36 or ICAM-1-binding parasites were purified (to > 95%) using the magnetic activated cell sorting (MACS) technique. Aliquots of 2×105 purified late stage parasite infected erythrocytes (LSPEs) were labeled with ethidium bromide in a tube to allow for exclusion of uninfected RBCs from the flow cytometric analysis. Labeled LSPEs were sequentially incubated with 5 µl of test plasma followed by 100 µl of 10 µg/ml goat anti-human IgG conjugated to fluorescein isothiocyanate (Vector Laboratories Inc, USA). Samples were washed twice between incubation periods using 2% foetal bovine serum (FBS) in phosphate buffered saline (PBS). A minimum of 5000 LSPEs were acquired on a FACScan flow cytometer (BD Biosciences, San Jose, CA) and the mean fluorescence intensity (MFI) levels of anti-VSA antibodies was analyzed using CellQuest v3.3 (BD Biosciences, San Jose, CA). Pooled hyper-immune Ghanaian and malaria-naïve American plasma samples were used as positive and negative controls respectively. For each parasite line, the test and control plasma samples were assayed on the same day using the same parasite preparation.
Measurement of plasma sICAM-1 levels
Plasma levels of sICAM-1 were measured by an in-house optimized sandwich ELISA protocol. Nunc microtitre plates weire coated at 50 µl/well with 2.5 µg/ml purified monoclonal sheep anti-human ICAM-1 antibody (MAB 720, R and D Systems, USA) in PBS as diluent, incubated overnight at 4°C and blocked with 300 µl/well of 5% BSA, 0.05% Tween 20 in PBS for 2 h. Recombinant human ICAM-1, serially diluted from 4000 to 31.25 pg/ml in 0.5% BSA, 0.05% Tween 20 in PBS, and plasma samples diluted 500 times in the same buffer, were added at 50 µl/well and incubated for 2 h. This was followed by incubation with 50 µl/well of a 0.1 µg/ml biotinylated anti-human ICAM-1 (BAF 720, R and D Systems, USA) for 2 h, and subsequently by incubation with 50 µl/well of 1 µg/ml streptavidin-peroxidase (Insight Biotechnology Limited, UK) for 25 min. Colour development was achieved by the addition of 100 µl/well TMB substrate (KEM-EM-TEC, Denmark) for 30 min and the colour reaction stopped by addition of 50 µl/well of 0.2M H2SO4. Optical densities were subsequently read at 450 nm with a 620 nm reference wavelength. Except for the coating step, all incubations were done on a shaker at room temperature and plates were washed with PBS, 0.05% Tween 20 between incubation periods.
Statistical analysis
The Wilcoxon sign rank-test was used to assess differences between study groups at baseline and for the pair-wise analysis of possible differences in antibody levels between acute and convalescent plasma samples. Antibody levels to CD36- and ICAM-1-binding VSAs were compared in only 15 pairs of acute and convalescent plasma samples since the other patients were lost to follow-up. A logistic regression model was used to find association between the levels of sICAM-1 and anti-VSA antibodies and the development of CM. The R statistical software (version 2.14.0, R development Core Team) was used for all statistical analyses and graphical presentations. P<0.05 was considered statistically significant.
Results
Samples from 37 cerebral malaria patients and 32 uncomplicated malaria patients were analyzed in this study, and a summary of patient characteristics is presented in Table 1. The median ages, haemoglobin levels and parasite densities were not statistically significantly different between the two disease groups.
Table 1. Patient characteristics and laboratory data at baseline.
| Disease category (n) | Age (years) | Haemoglobin levels (g/dl) | Parasite density (×103 perµl blood) | sICAM-1 levels (µg/ml)# | Anti-VSA (CD36-binding) antibodies (MFI)§ | Anti-VSA (CD54-binding) antibodies (MFI) | ||
| UM (32) | 5 (1–13) | 10.20 (8.1–13) | 51.61 (3.15–4340.2) | 1.5 (0.09–5.21) | 60.42 (31.82–104.5) | 16.64 (9.74– 35.41) | ||
| CM (37) | 3.5 (0.5–12) | 7.3 (5.2–10.7) | 117.81 (4.41–296.17) | 2.27 (0.35–6.3) | 76.07 (19.3–132.28) | 15.8 (11.26–22.8) | ||
Values reported as median (minimum-maximum) and comparisons made using Wilcoxon sign rank test. Antibody levels are expressed as mean fluorescence intensity (MFI). #ICAM-1 levels were statistically significantly higher in CM than in UM (p = 0.0037).
Anti-VSA (CD36-binding) levels were statistically significantly higher in CM than in UM (p = 0.048).
The aim of the study was to find possible associations between the membrane expression of ICAM-1 and the plasma levels of antibodies to CD36- and ICAM-1-binding VSAs on one hand, and the development of cerebral complications in malaria patients. Expression levels of ICAM-1 were assessed by measuring the plasma soluble form of ICAM-1 (sICAM-1) using sandwiched ELISA technique on the assumption that the soluble form reflects its membrane expression levels. Antibody levels to CD36- and ICAM-1-binding VSAs were however measured by a flow cytometric assay. Levels of anti-VSA antibodies were also estimated for the subset (15 samples) of study participants whose plasma samples, taken 7 days after treatment was initiated, were available for comparison. Median levels of soluble ICAM-1 during acute infection were statistically significantly higher in CM patients compared to the median level in UM patients (Table 1, p = 0.0037, Wilcoxon sign rank-test). A logistic regression model with the three biomarkers as independent variables confirmed a strong association of sICAM-1 levels with the CM patient group (Table 2). Median levels of antibodies to CD36-selected parasites were also significantly higher in CM patients compared to median levels in UM patients with a borderline p-value (Table 1, p = 0.048), but there was no statistically significant difference in median levels of antibodies to ICAM-1-selected parasites between the two study groups (Table 1).
Table 2. Logistic regression data for association of sICAM-1 and anti-VSA antibodies with CM.
| crude OR (95%CI) | adj. OR (95%CI) | P (Wald's test) | P (LR-test) | |
| Anti-VSA (CD36) | 1.02 (1, 1.04) | 1.02 (0.99, 1.04) | 0.158 | 0.147 |
| Anti-VSA (CD54) | 0.96 (0.84, 1.08) | 0.95 (0.83, 1.09) | 0.439 | 0.428 |
| sICAM-1 | 1.82 (1.18, 2.82) | 1.82 (1.15, 2.87) | 0.01 | 0.005 |
UM is the reference patient category.
Pairwise comparisons of antibody levels to CD36- and ICAM-1-selected parasites were performed using 15 paired acute and convalescent plasma samples from UM and CM patients to assess changes in antibody levels between disease groups and between Day 0 (D0) and Day 7 (D7) plasma samples. While antibody levels to ICAM-1-selected parasites generally increased for most participants on D7 compared to D0 (Figure 1), antibody levels to CD36-selected parasites did not show a general trend of increase in any direction (Figure 2). Thus antibody levels to CD36-selected parasites were not significantly different in study groups, irrespective of whether the pairwise comparison was for the same patient group (D0 versus D7 for UM, D0 versus D7 for CM) or for the same time point sampling (UM versus CM for D0, UM versus CM for D7) (Table 3). Similarly, no significant differences were observed when levels of antibodies to ICAM-1-selected parasites were compared between patient groups for the same time point (UM versus CM for D0, UM versus CM for D7). Pairwise comparison of antibodies to ICAM-1-selected parasites for both UM (p = 0.037) and CM (p = 0.0031) groups however showed statistically significant increases on D7 compared to D0 levels (Table 3). Thus, antibody levels to ICAM-1-selected parasites were lower during acute infection but increased significantly after recovery from both uncomplicated and cerebral malaria.
Figure 1. Acute and convalescent levels of antibodies to ICAM-1 selected parasites.
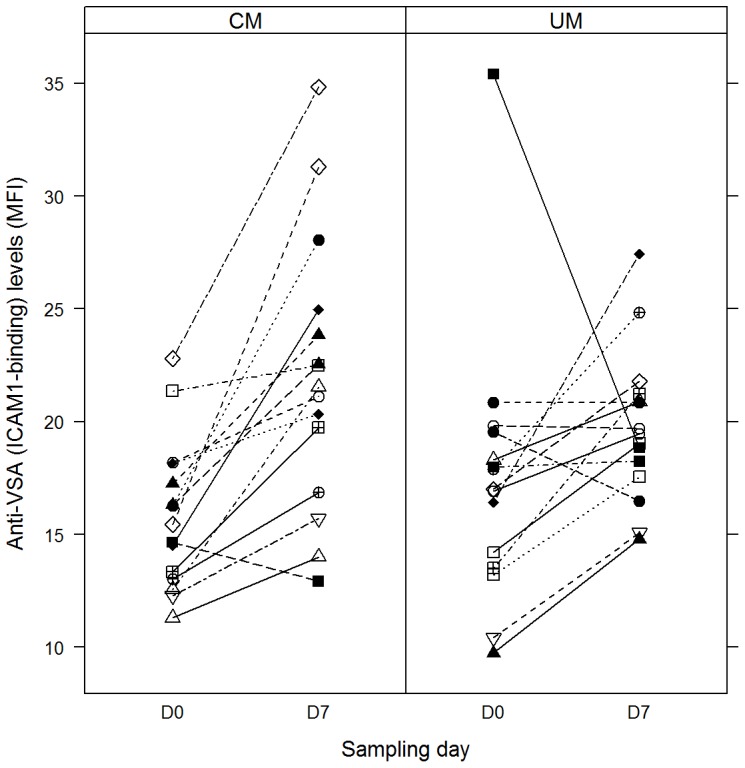
Symbols linked by straight line represent anti-VSA antibody levels on days 0 and 7 for individual participants.
Figure 2. Acute and convalescent levels of antibodies to CD36- selected parasites.
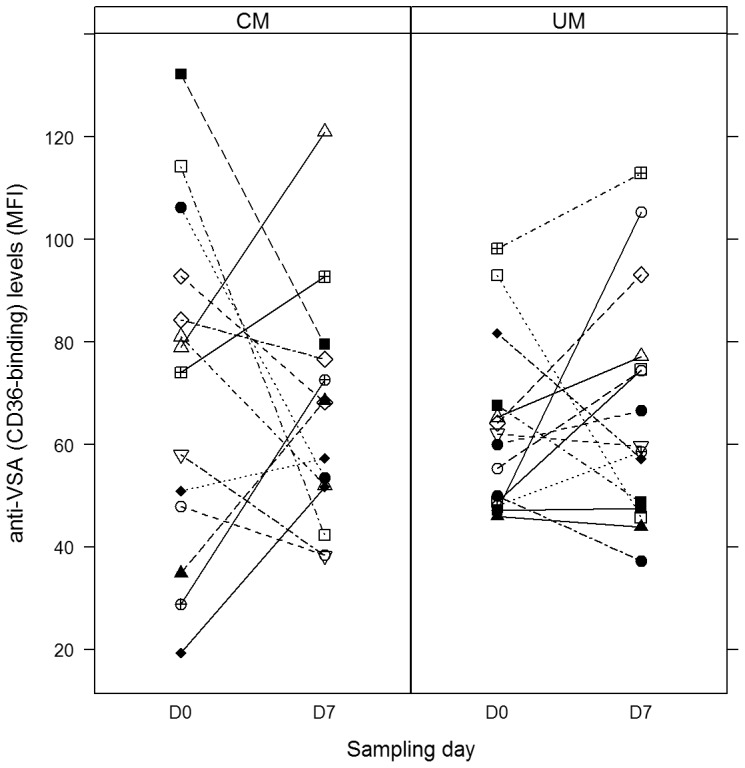
Symbols linked by straight line represent anti-VSA antibody levels on days 0 and 7 for individual participants.
Table 3. Comparison of anti-CD36 and anti-CD54 antibody levels between D0 and D7 samples for CM and UM patients.
| Anti-VSA (CD36-binding) | Anti-VSA (CD54-binding) | |||||
| Patient group (n) | D0 | D7 | P value | D0 | D7 | P value |
| UM (15) | 60.04 (46.02 – 98.22) | 59.68 (37.25 – 112.92) | 0.81 | 17 (9.74 – 35.41) | 19.47 (14.79 – 27.43) | 0.037 |
| CM (15) | 76.5 (19.31 – 132.28) | 62.77 (38.3 – 121) | 0.57 | 14.44 (11.3 – 22.79) | 21.54 (12.92 – 34.83) | 0.0031 |
| P value | 0.45 | 0.88 | 0.41 | 0.2 | ||
All values reported as median (minimum-maximum). Comparisons were made using the Wilcoxon sign rank- test. Antibody levels are expressed as mean fluorescence intensity (MFI).
Discussion
During Plasmodium’s life cycle in humans, it expresses a number of antigens including PfEMP-1, a component of the VSAs that are expressed on the surface of infected RBCs (iRBCs) by late stage parasites [31]. Adhesion of iRBCs to the host endothelium is mediated by specific host receptor molecules such as intercellular adhesion molecule 1 (ICAM-1), which is the predominant receptor in the brain, chondroitin sulfate A (CSA) in the placenta, and CD36 in other organs. The extent of parasite sequestration has been shown to correlate positively with endothelial ICAM-1 expression [11]–[13], [31]–[37]. The level of soluble ICAM-1 (sICAM-1), detected in circulation following proteolytic cleavage of the membrane form, is also believed to increase during infection [12], [38]. ICAM-1 is also believed to play a role in inflammation as it binds to leukocyte function associated antigen (LFA-1) or integrin Mac-1 expressed on leukocytes and potentiates leukocyte stimulation [39]. PfEMP-1 elicits specific antibody which has been shown to be protective against disease [8]–[10]. In this study, levels of anti-VSA (CD36- and ICAM-1-binding parasites) antibodies in plasma samples from CM patients during the active disease phase (D0) and seven days after treatment was commenced (D7) were quantitatively determined by flow cytometry and compared with the levels in UM control patients. Levels of sICAM-1 in the plasma of both patient groups during the active disease phase were also quantitatively measured by ELISA and compared.
Consistent with earlier reports [40]–[42], this study found that CM patients had higher levels of sICAM-1 compared to UM patients. This may indeed be a result of shedding of the receptor after its increased expression on endothelial cells and leukocytes following endothelial cell activation in CM patients. The increased expression of membrane bound ICAM-1 followed by binding of parasites to it could therefore enhance sequestration of the parasites to cause vascular obstruction and ischaemia as postulated by the mechanical hypothesis of cerebral malaria development [43]–[46]. The ability of sICAM-1 to elicit inflammatory responses could also mediate an increased production of pro-inflammatory cytokines such as TNF-α, whose overproduction has been implicated in pathogenesis of CM as postulated by the inflammatory hypothesis of cerebral malaria development [47]–[50].
For the subgroup of patients whose convalescent (D7) plasma samples were available for analysis, median antibody levels to ICAM-1-binding VSAs were significantly lower in D0 samples compared to D7 samples for both UM and CM patients. Median levels of antibodies to CD36-binding VSA were however not statistically significantly different between D0 and D7 samples for both the UM and CM groups (Table 3). Thus the anti-VSA antibody levels measured in this study were neither predictive of the development of cerebral complications, nor are they predictive of the parasite variants (ICAM1-binding or CD36-binding) that caused disease. These collectively suggest that both UM and CM patients have had previous exposure to both ICAM-1-binding and CD36-binding parasites, and the boosting of antibodies to ICAM-1-binding parasites in both study groups would suggest that the disease causing parasites in both groups might possibly express ICAM-1-binding VSA. The difference in disease outcome between the two groups could then be attributable to the degree of ICAM-1 expression in the presence of parasite expressing ICAM-1 binding VSA and/or other factors aside just infection with ICAM-binding parasites. Such other factors may include amongst others pre-existing anti-malarial immunity, and the time lapse between infection and initiation of appropriate chemotherapy. Under these conditions, a delay in seeking medical care early could allow parasites to multiply enough and sequester in the brain microvasculture and result in a quick progression from UM to CM. Differences in disease outcome could also be attributable to possible multiple parasite variant infections, with the CM group having a greater proportion of ICAM-1-binding parasites as it is not uncommon to find individuals who are infected by different clones of parasite that express different VSAs at the same time [51], [52].
Limitations of this study include our inability to measure sICAM-1 levels in matching convalescent plasma samples for comparison with D0 samples as this would have provided some insight on the expression levels/shedding of the receptor in absence of the infecting parasites and also whether the ICAM-1 levels influence the rate of recovery. In addition, findings in this study would have to be confirmed with much larger sample sizes, although with the current trend of decline in severe malaria cases even in areas that were considered to be highly endemic some years ago, larger sample sizes might be difficult to achieve.
In conclusion, the study found a strong association between elevated levels of plasma sICAM-1 and CM. Anti-VSA antibodies against both CD36- and ICAM-1-selected parasites did not show any association with protection from CM in acute samples. However, the levels of anti-VSA antibodies to ICAM-1-selected parasites, but not CD36-selected parasites, were higher in convalescent samples compared to acute samples for both patient groups, suggesting that there might have been a disproportionate multiplication of parasites expressing ICAM-1-binding VSA compared to those expressing CD-36 binding VSA in both UM and CM patients. This presupposes that infection with ICAM-1 binding parasite variants or pre-existing/induced antibodies to this parasite type may not be predictive of the development of CM, and raises the possibility of the contribution of other factors to CM development. Thus, protection from CM may therefore require contribution of other factors including antibodies to other parasite types. These findings are relevant to our current understanding of the development of complications such as cerebral malaria during P. falciparum infection in children
Acknowledgments
We thank the study participants and their parents/guardians for agreeing to partake in the study. We also thank Professor Lars Hviid, Centre for Medical Parasitology, Copenhagen for donating the parasite lines used while the staff in Immunology Department of Noguchi Memorial Institute For Medical Research, University of Ghana, Legon are thanked for their technical and laboratory support.
Funding Statement
The study was funded by Multinational Initiative on Malaria-Training in Tropical Diseases (MIM-TDR grant A11034). However, the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO (2011) World malaria report 2011. WHO.
- 2. Cox-Singh J, Davis TM, Lee KS, Shamsul SS, Matusop A, et al. (2008) Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin Infect Dis 46: 165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee KS, Cox-Singh J, Singh B (2009) Morphological features and differential counts of Plasmodium knowlesi parasites in naturally acquired human infections. Malar J 8: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singh B, Daneshvar C (2013) Human Infections and Detection of Plasmodium knowlesi. Clin Microbiol Rev 26: 165–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ta TT, Salas A, Ali-Tammam M, Martinez MC, Lanza M, et al. (2010) First case of detection of Plasmodium knowlesi in Spain by Real Time PCR in a traveller from Southeast Asia. Malar J 9: 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Greenwood B, Marsh K, Snow R (1991) Why do some African children develop severe malaria? Parasitol Today 7: 277–281. [DOI] [PubMed] [Google Scholar]
- 7. Bull PC, Lowe BS, Kortok M, Marsh K (1999) Antibody recognition of Plasmodium falciparum erythrocyte surface antigens in Kenya: evidence for rare and prevalent variants. Infect Immun 67: 733–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bull PC, Lowe BS, Kortok M, Molyneux CS, Newbold CI, et al. (1998) Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat Med 4: 358–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dodoo D, Staalsoe T, Giha H, Kurtzhals JA, Akanmori BD, et al. (2001) Antibodies to variant antigens on the surfaces of infected erythrocytes are associated with protection from malaria in Ghanaian children. Infect Immun 69: 3713–3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ofori MF, Dodoo D, Staalsoe T, Kurtzhals JA, Koram K, et al. (2002) Malaria-induced acquisition of antibodies to Plasmodium falciparum variant surface antigens. Infect Immun 70: 2982–2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Newbold C, Warn P, Black G, Berendt A, Craig A, et al. (1997) Receptor-specific adhesion and clinical disease in Plasmodium falciparum. Am J Trop Med Hyg 57: 389–398. [DOI] [PubMed] [Google Scholar]
- 12. Silamut K, Phu NH, Whitty C, Turner GD, Louwrier K, et al. (1999) A quantitative analysis of the microvascular sequestration of malaria parasites in the human brain. Am J Pathol 155: 395–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Turner GD, Morrison H, Jones M, Davis TM, Looareesuwan S, et al. (1994) An immunohistochemical study of the pathology of fatal malaria. Evidence for widespread endothelial activation and a potential role for intercellular adhesion molecule-1 in cerebral sequestration. Am J Pathol 145: 1057–1069. [PMC free article] [PubMed] [Google Scholar]
- 14. Armah H, Dodoo AK, Wiredu EK, Stiles JK, Adjei AA, et al. (2005) High-level cerebellar expression of cytokines and adhesion molecules in fatal, paediatric, cerebral malaria. Ann Trop Med Parasitol 99: 629–647. [DOI] [PubMed] [Google Scholar]
- 15. Armah H, Wired EK, Dodoo AK, Adjei AA, Tettey Y, et al. (2005) Cytokines and adhesion molecules expression in the brain in human cerebral malaria. Int J Environ Res Public Health 2: 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vastag M, Skopal J, Voko Z, Csonka E, Nagy Z (1999) Expression of membrane-bound and soluble cell adhesion molecules by human brain microvessel endothelial cells. Microvasc Res 57: 52–60. [DOI] [PubMed] [Google Scholar]
- 17. Wong D, Dorovini-Zis K (1992) Upregulation of intercellular adhesion molecule-1 (ICAM-1) expression in primary cultures of human brain microvessel endothelial cells by cytokines and lipopolysaccharide. J Neuroimmunol 39: 11–21. [DOI] [PubMed] [Google Scholar]
- 18. Budnik A, Grewe M, Gyufko K, Krutmann J (1996) Analysis of the production of soluble ICAM-1 molecules by human cells. Exp Hematol 24: 352–359. [PubMed] [Google Scholar]
- 19. McGuire W, Hill AV, Greenwood BM, Kwiatkowski D (1996) Circulating ICAM-1 levels in falciparum malaria are high but unrelated to disease severity. Trans R Soc Trop Med Hyg 90: 274–276. [DOI] [PubMed] [Google Scholar]
- 20. Tchinda VH, Tadem AD, Tako EA, Tene G, Fogako J, et al. (2007) Severe malaria in Cameroonian children: correlation between plasma levels of three soluble inducible adhesion molecules and TNF-alpha. Acta Trop 102: 20–28. [DOI] [PubMed] [Google Scholar]
- 21. Tsakadze NL, Sithu SD, Sen U, English WR, Murphy G, et al. (2006) Tumor necrosis factor-alpha-converting enzyme (TACE/ADAM-17) mediates the ectodomain cleavage of intercellular adhesion molecule-1 (ICAM-1). J Biol Chem 281: 3157–3164. [DOI] [PubMed] [Google Scholar]
- 22. Gray C, McCormick C, Turner G, Craig A (2003) ICAM-1 can play a major role in mediating P. falciparum adhesion to endothelium under flow. Mol Biochem Parasitol 128: 187–193. [DOI] [PubMed] [Google Scholar]
- 23. Ho M, Hickey MJ, Murray AG, Andonegui G, Kubes P (2000) Visualization of Plasmodium falciparum-endothelium interactions in human microvasculature: mimicry of leukocyte recruitment. J Exp Med 192: 1205–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kurtzhals JA, Adabayeri V, Goka BQ, Akanmori BD, Oliver-Commey JO, et al. (1998) Low plasma concentrations of interleukin 10 in severe malarial anaemia compared with cerebral and uncomplicated malaria. Lancet 351: 1768–1772. [DOI] [PubMed] [Google Scholar]
- 25. Molyneux ME, Taylor TE, Wirima JJ, Borgstein A (1989) Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children. Q J Med 71: 441–459. [PubMed] [Google Scholar]
- 26. Joergensen L, Bengtsson DC, Bengtsson A, Ronander E, Berger SS, et al. (2010) Surface co-expression of two different PfEMP1 antigens on single plasmodium falciparum-infected erythrocytes facilitates binding to ICAM1 and PECAM1. PLoS Pathog 6: e1001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Staalsoe T, Nielsen MA, Vestergaard LS, Jensen AT, Theander TG, et al. (2003) In vitro selection of Plasmodium falciparum 3D7 for expression of variant surface antigens associated with severe malaria in African children. Parasite Immunol 25: 421–427. [DOI] [PubMed] [Google Scholar]
- 28. Trager W, Jensen JB (1976) Human malaria parasites in continuous culture. Science 193: 673–675. [DOI] [PubMed] [Google Scholar]
- 29. Paul F, Roath S, Melville D, Warhurst DC, Osisanya JO (1981) Separation of malaria-infected erythrocytes from whole blood: use of a selective high-gradient magnetic separation technique. Lancet 2: 70–71. [DOI] [PubMed] [Google Scholar]
- 30. Staalsoe T, Giha HA, Dodoo D, Theander TG, Hviid L (1999) Detection of antibodies to variant antigens on Plasmodium falciparum-infected erythrocytes by flow cytometry. Cytometry 35: 329–336. [DOI] [PubMed] [Google Scholar]
- 31. Baruch DI, Gormely JA, Ma C, Howard RJ, Pasloske BL (1996) Plasmodium falciparum erythrocyte membrane protein 1 is a parasitized erythrocyte receptor for adherence to CD36, thrombospondin, and intercellular adhesion molecule 1. Proc Natl Acad Sci U S A 93: 3497–3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cooke BM, Rogerson SJ, Brown GV, Coppel RL (1996) Adhesion of malaria-infected red blood cells to chondroitin sulfate A under flow conditions. Blood 88: 4040–4044. [PubMed] [Google Scholar]
- 33. McGilvray ID, Serghides L, Kapus A, Rotstein OD, Kain KC (2000) Nonopsonic monocyte/macrophage phagocytosis of Plasmodium falciparum-parasitized erythrocytes: a role for CD36 in malarial clearance. Blood 96: 3231–3240. [PubMed] [Google Scholar]
- 34. Ockenhouse CF, Ho M, Tandon NN, Van Seventer GA, Shaw S, et al. (1991) Molecular basis of sequestration in severe and uncomplicated Plasmodium falciparum malaria: differential adhesion of infected erythrocytes to CD36 and ICAM-1. J Infect Dis 164: 163–169. [DOI] [PubMed] [Google Scholar]
- 35. Roberts DD, Sherwood JA, Spitalnik SL, Panton LJ, Howard RJ, et al. (1985) Thrombospondin binds falciparum malaria parasitized erythrocytes and may mediate cytoadherence. Nature 318: 64–66. [DOI] [PubMed] [Google Scholar]
- 36. Treutiger CJ, Heddini A, Fernandez V, Muller WA, Wahlgren M (1997) PECAM-1/CD31, an endothelial receptor for binding Plasmodium falciparum-infected erythrocytes. Nat Med 3: 1405–1408. [DOI] [PubMed] [Google Scholar]
- 37. Turner GD, Ly VC, Nguyen TH, Tran TH, Nguyen HP, et al. (1998) Systemic endothelial activation occurs in both mild and severe malaria. Correlating dermal microvascular endothelial cell phenotype and soluble cell adhesion molecules with disease severity. Am J Pathol 152: 1477–1487. [PMC free article] [PubMed] [Google Scholar]
- 38. Hviid L, Theander TG, Elhassan IM, Jensen JB (1993) Increased plasma levels of soluble ICAM-1 and ELAM-1 (E-selectin) during acute Plasmodium falciparum malaria. Immunol Lett 36: 51–58. [DOI] [PubMed] [Google Scholar]
- 39. Mendez MP, Morris SB, Wilcoxen S, Du M, Monroy YK, et al. (2008) Disparate mechanisms of sICAM-1 production in the peripheral lung: contrast between alveolar epithelial cells and pulmonary microvascular endothelial cells. Am J Physiol Lung Cell Mol Physiol 294: L807–L814. [DOI] [PubMed] [Google Scholar]
- 40. Erdman LK, Dhabangi A, Musoke C, Conroy AL, Hawkes M, et al. (2011) Combinations of host biomarkers predict mortality among Ugandan children with severe malaria: a retrospective case-control study. PLoS One 6: e17440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cserti-Gazdewich CM, Dzik WH, Erdman L, Ssewanyana I, Dhabangi A, et al. (2010) Combined measurement of soluble and cellular ICAM-1 among children with Plasmodium falciparum malaria in Uganda. Malar J 9: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cserti-Gazdewich CM, Dhabangi A, Musoke C, Ssewanyana I, Ddungu H, et al. (2012) Cytoadherence in paediatric malaria: ABO blood group, CD36, and ICAM1 expression and severe Plasmodium falciparum infection. Br J Haematol 159: 223–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kampfl A, Pfausler B, Haring HP, Denchev D, Donnemiller E, et al. (1997) Impaired microcirculation and tissue oxygenation in human cerebral malaria: a single photon emission computed tomography and near-infrared spectroscopy study. Am J Trop Med Hyg 56: 585–587. [DOI] [PubMed] [Google Scholar]
- 44. Kaul DK, Roth EF Jr, Nagel RL, Howard RJ, Handunnetti SM (1991) Rosetting of Plasmodium falciparum-infected red blood cells with uninfected red blood cells enhances microvascular obstruction under flow conditions. Blood 78: 812–819. [PubMed] [Google Scholar]
- 45. Warrell DA (1987) Pathophysiology of severe falciparum malaria in man. Parasitology 94 Suppl: S53–S76 [DOI] [PubMed] [Google Scholar]
- 46. Ponsford MJ, Medana IM, Prapansilp P, Hien TT, Lee SJ, et al. (2012) Sequestration and microvascular congestion are associated with coma in human cerebral malaria. J Infect Dis 205: 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Grau GE, Bieler G, Pointaire P, De KS, Tacchini-Cotier F, et al. (1990) Significance of cytokine production and adhesion molecules in malarial immunopathology. Immunol Lett 25: 189–194. [DOI] [PubMed] [Google Scholar]
- 48. Kern P, Hemmer CJ, Gallati H, Neifer S, Kremsner P, et al. (1992) Soluble tumor necrosis factor receptors correlate with parasitemia and disease severity in human malaria. J Infect Dis 166: 930–934. [DOI] [PubMed] [Google Scholar]
- 49. Kwiatkowski D, Hill AV, Sambou I, Twumasi P, Castracane J, et al. (1990) TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet 336: 1201–1204. [DOI] [PubMed] [Google Scholar]
- 50. Day NP, Hien TT, Schollaardt T, Loc PP, Chuong LV, et al. (1999) The prognostic and pathophysiologic role of pro- and antiinflammatory cytokines in severe malaria. J Infect Dis 180: 1288–1297. [DOI] [PubMed] [Google Scholar]
- 51. Mayengue PI, Ndounga M, Malonga FV, Bitemo M, Ntoumi F (2011) Genetic polymorphism of merozoite surface protein-1 and merozoite surface protein-2 in Plasmodium falciparum isolates from Brazzaville, Republic of Congo. Malar J 10: 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ntoumi F, Contamin H, Rogier C, Bonnefoy S, Trape JF, et al. (1995) Age-dependent carriage of multiple Plasmodium falciparum merozoite surface antigen-2 alleles in asymptomatic malaria infections. Am J Trop Med Hyg 52: 81–88. [DOI] [PubMed] [Google Scholar]


