Abstract
Context:
Articular cartilage has a unique functional architecture capable of providing a lifetime of pain-free joint motion. This tissue, however, undergoes substantial age-related physiologic, mechanical, biochemical, and functional changes that reduce its ability to overcome the effects of mechanical stress and injury. Many factors affect joint function in the maturing athlete—from chondrocyte survival and metabolism to structural composition and genetic/epigenetic factors governing cartilage and synovium. An evaluation of age-related changes for joint homeostasis and risk for osteoarthritis is important to the development of new strategies to rejuvenate aging joints.
Objective:
This review summarizes the current literature on the biochemical, cellular, and physiologic changes occurring in aging articular cartilage.
Data Sources:
PubMed (1969-2013) and published books in sports health, cartilage biology, and aging.
Study Selection:
Keywords included aging, athlete, articular cartilage, epigenetics, and functional performance with age.
Study Design:
Systematic review.
Level of Evidence:
Level 3.
Data Extraction:
To be included, research questions addressed the effect of age-related changes on performance, articular cartilage biology, molecular mechanism, and morphology.
Results:
The mature athlete faces challenges in maintaining cartilage health and joint function due to age-related changes to articular cartilage biology, morphology, and physiology. These changes include chondrocyte loss and a decline in metabolic response, alterations to matrix and synovial tissue composition, and dysregulation of reparative responses.
Conclusion:
Although physical decline has been regarded as a normal part of aging, many individuals maintain overall fitness and enjoy targeted improvement to their athletic capacity throughout life. Healthy articular cartilage and joints are needed to maintain athletic performance and general activities. Genetic and potentially reversible epigenetic factors influence cartilage physiology and its response to mechanical and injurious stimuli. Improved understandings of the physical and molecular changes to articular cartilage with aging are important to develop successful strategies for joint rejuvenation.
Keywords: articular cartilage, aging, athlete, exercise
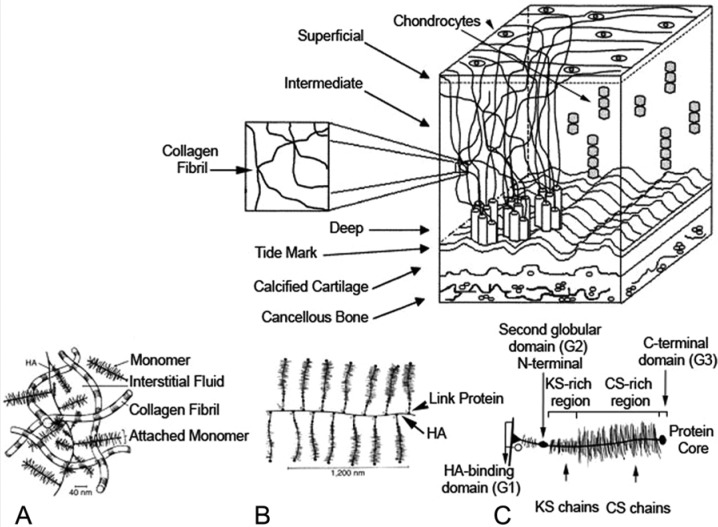
Articular cartilage is the highly specialized connective tissue of diarthrodial joints. Its principal function is to provide a smooth, lubricated surface for articulation and to facilitate the transmission of loads with a low frictional coefficient.126 Articular cartilage is hyaline cartilage and is 2- to 4-mm thick. Unlike most tissues, articular cartilage does not have blood vessels, nerves, or lymphatics. It is composed of a dense extracellular matrix (ECM) with a sparse distribution of highly specialized cells called chondrocytes. The ECM is principally composed of water, collagen, and proteoglycans, with other noncollagenous proteins and glycoproteins present in lesser amounts.20,22 Together, these components help to retain water within the ECM, which is critical to maintain its unique mechanical properties. Along with collagen fiber ultrastructure and ECM, chondrocytes contribute to the secretion, organization, and maintenance of the ECM (Figure 1).
Figure 1.
Organization of articular cartilage. Normal articular cartilage is composed of matrix, chondrocytes, and water. The matrix is principally composed of type II collagen fibers. Sulfated proteoglycans are linked to hyaluronate. (A-C) Structure of aggregating proteoglycans and their association with collagen. (A) Proteoglycans, chondrocytes, and water are trapped in a collagen fibril matrix. (B) Hyaluronic acid (HA) is a large polysaccharide composed of repeat glycosaminoglycans. (C) The gylcosaminoglycans keratin sulfate (KS) and chondroitin sulfate (CS) attach to aggrecan, which in turn binds to HA via its N-terminal globular domain facilitated by link protein.27,99,120,132 Upper image reproduced with permission from Silver et al.122 Image A reproduced with permission from Mow and Hung.99 Image B reproduced with permission from Mankin et al.83 Image C reproduced with permission from Buckwalter et al.21
The chondrocyte is the resident cell type in articular cartilage. Chondrocytes are highly specialized, metabolically active cells that play a unique role in the development, maintenance, and repair of the ECM. Chondrocytes originate from mesenchymal stem cells and constitute about 2% of the total volume of articular cartilage.4 Chondrocytes vary in shape, number, and size, depending on the anatomic regions of the articular cartilage (Figure 1). Each chondrocyte establishes a specialized microenvironment and is responsible for the turnover of the ECM in its immediate vicinity. This microenvironment essentially traps the chondrocyte within its own matrix and prevents ready migration to adjacent areas of cartilage. Chondrocytes are linked to the ECM and respond to a variety of stimuli, including growth factors, mechanical loads, piezoelectric forces, and hydrostatic pressures.20,142 Similarly, chondrocytes possess cell surface receptors for a variety of growth factors and cytokines, and they regulate both anabolic and catabolic activities in response to these agents.132 Unfortunately, chondrocytes encapsulated within the dense ECM have limited room for replication, a factor further limiting the intrinsic healing capacity of articular cartilage.
Another tissue important to chondrocyte function and joint physiology is the synovium. While the cartilage is devoid of blood vessels, lymphatics, and nerves, the synovium is a vascularized connective tissue that contains specialized cells that produce fluid to lubricate and nourish articular cartilage (Figure 1). Changes to the synovium due to aging, inflammation, and disease can adversely affect chondrocyte metabolism and joint homeostasis.
Definition of Age
Aging is defined as “a persistent decline in the age-specific fitness components of an organism due to internal physiological degeneration.”112 Most evolutionary biologists define aging as an age-progressive decline in intrinsic physiologic function, leading to an increase in age-specific mortality rate and a decrease in age-specific reproductive rate.40,93 According to the American Academy of Orthopaedic Surgeons, aging of joints occurs when (1) joint motion becomes more restricted and flexibility decreases with age because of changes in tendons and ligaments and (2) as the cushioning cartilage begins to break down from a lifetime of use, joints become inflamed and arthritic.1
Articular Cartilage
Surprisingly few abnormalities are noted when considering the overall effect of aging on articular cartilage, which can remain intact for a lifetime. With advanced age, the cartilage may take on a yellowish discoloration, the nature of which is obscure. Cartilage appears to be thinner, but accurate measurements have not been systematically carried out to definitively support this concept. Histologically, no alterations are evident on light microscopy, but electron microscopic studies have suggested an increased number of cells showing organelle degeneration and intracytoplasmic fine filaments.101 Electron microscopic study of the collagen fibers shows increased fiber size in aging similar to that seen in osteoarthritis. Occasionally, giant fibers are seen, particularly in the deeper layers.101 A frequently encountered age-associated structural change in articular cartilage is increasing surface fibrillation.24,71 However, this does not necessarily lead to progressive articular cartilage degeneration.87 As well, the frequency of advanced cartilage loss and osteoarthritis substantially increases with age. While the macroscopic changes are subtle in individuals who retain intact articular cartilage throughout life, studies have described a number of cellular and matrix changes associated with aging. These changes contribute to increased vulnerability of aging articular cartilage to injury and stress overload.39,65,117
Chondrocyte Cellular Events and Function
There are fewer chondrocytes in the articular cartilage of older individuals.85 Chondrocytes in aging individuals also show decreased functional activity. With fewer and less healthy chondrocytes to repair and maintain the matrix, previously healthy levels of loading may be greater than what the tissue can now tolerate. As such, there is greater risk for cartilage degeneration not just following injury or overuse but potentially even to normal activities.
Several reasons have been proposed for the age-related reduction in chondrocyte numbers. The ability of chondrocytes to proliferate and hence repair and maintain the cartilage matrix decreases with age.3 Programmed cell death may also play a role. Aging rats have higher levels of apoptotic chondrocytes in the calcified layer of knee cartilage.2 Increased apoptosis in aging chondrocytes and reduced cellularity decrease the ability to repair and restore articular cartilage and result in lower functional capacity.13,74,128
Loss of chondrocyte function with aging also occurs and represents an area where improved understanding of the mechanisms may reveal new opportunities for restorative therapy. There is progressive senescence of articular cartilage chondrocytes, as evidenced by increased expression of the cell senescence markers P16/INK4A and decreased telomere length with age.86,88,90,144 Cytoskeletal networks of old chondrocyte cells were also altered.33 Aged chondrocytes had a different response to mechanical stimulation when compared with young and adult chondrocytes. Their viscoelastic properties are altered because of cell structure changes and cytoskeleton composition.33
Insulin Growth Factor 1
Further evidence for chondrocyte senescence is the altered mitogenic response of aging cells to growth factors.106 Insulin growth factor 1 (IGF-1) is an anabolic factor that can be synthesized locally in articular chondrocytes,32 and it initiates proteoglycan synthesis.49 Furthermore, IGF-1 downregulates proteoglycan degradation induced by interleukin 1α (IL-1α).131 Aging articular chondrocytes show a decline in the responsiveness of the cells to IGF-1 and its downstream signaling pathway.80,89,95
Transforming Growth Factor β
Members of the transforming growth factor β (TGF-β) superfamily, such as TGF-β1, elicit chondrogenic properties and stimulate proteoglycan synthesis by articular chondrocytes.56 Furthermore, TGF-β1 can counteract the suppression of cartilage proteoglycan synthesis induced by IL-1α.108,118 Aging articular chondrocytes show a decline in glycosaminoglycan synthesis and composition as a result of a lack of responsiveness of the cells to TGF-β1 and its downstream signaling pathway.56 Other key regulators that belong to the TGF-β superfamily are bone morphogenetic proteins and osteogenic protein 1 (OP-1). Both are important anabolic factors that enable homeostasis and repair of the cartilage.41,72 A remarkable decline in OP-1 expression level and function was found in individuals aged 35 to 75 years.28
Molecular Changes
Other mediators of chondrocyte senescence that are associated with aging cells include reduced expression of the telomeric proteins that aim to maintain telomere function,143 XRCC5 (x-ray repair complementing defective repair in Chinese hamster cells 5) that repairs DNA double-strand breaks,129,130 and sirtuin 1 that prevents growth arrest and apoptosis via P53 cascade.48 Aging-related loss of the chromatin protein high-mobility group protein B2 (HMGB2) in articular cartilage was linked to reduced cellularity of cartilage.128 Chondrocyte-downstream signaling (eg, protein kinase and caveolin) also is altered as a result of age.82
Whatever the reason may be—telomere erosion, oxidative stress, or both—chondrocyte senescence is an essential component in cartilage physiology and ultimately leads to changes in the matrix.85 Aged chondrocytes characteristically have decreased functional activity. Along with that is the ECM protein synthesis and reduced responsiveness to anabolic growth factors.88 They also produce fewer link proteins and smaller and less uniform aggregan.85
Extracellular Matrix
The major matrix proteins in cartilage are proteoglycans and collagen. Aggrecan is the key proteoglycan responsible for the resiliency of the tissue, while type II collagen provides tensile strength.114 Structural changes due to aging in any of the articular cartilage components contribute to a loss of tensile strength and joint stiffness.139
Collagen
Collagen is the most abundant structural macromolecule in ECM, and it makes up about 60% of the dry weight of cartilage. It is organized in fibrils and fibers intertwined with proteoglycan aggregates. The triple helix structure of the collagen polypeptide chains provides articular cartilage with important shear and tensile properties, which help to stabilize the matrix.92 Type II collagen is the predominant collagen in articular cartilage. Collagen type II is more abundantly expressed in young cartilage rats than in older rats.96 Nevertheless, there is very little turnover of type II collagen network (which has a half-life over 100 years) in healthy cartilage.134 However, over time, an increase in cross-links between adjacent fibrils has been described. An increase in the number of bonds between collagen fibrils78 is associated with the age-related increased stiffness and brittleness of the articular cartilage.2,58 Little evidence regarding the levels of nonenzymatic glycations (Maillard reaction) of collagen over age is documented. However, Bank et al7 reported that the formation of nonenzymatic glycations (like pentosidine) of collagen with age results in stiffer and more brittle collagen fibrils.7
Proteoglycan
All hyaline cartilages are characterized by high content of proteoglycans, which consist of a protein core with one or more linear glycosaminoglycan chains covalently attached. These chains may be composed of more than 100 monosaccharides. They extend from the protein core and remain separated from one another because of charge repulsion (Figure 2). Of the proteoglycans, aggrecan is the largest in size and most abundant by weight. Aggrecan possesses many chondroitin sulfate and keratin sulfate chains that interact with hyaluronan to form large proteoglycan aggregates via link proteins (Figure 2).25,126 Aggrecan occupies the interfibrillar space of the cartilage ECM and provides articular cartilage with its osmotic properties, which are critical to its ability to resist compressive loads.126 Age-related changes in size, structure, and sulfation of aggrecan affect cartilage resilience and hydration.47
Figure 2.
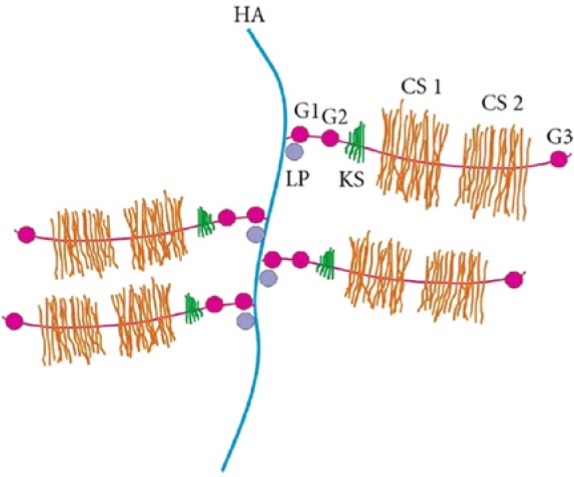
Schematic representation of the aggrecan structure. HA, hyaluronic acid; CS, chondroitin sulfate domain; KS, keratin sulfate; G, globular domain; LP, link protein.63 Image reproduced with permission from Jerosch.63
Size and structure. The size of proteoglycan aggregates within the ECM decreases with age. This may occur as a result of a decrease in the available binding sites of the hyaluronan chain or as a result of proteolytic damage that enables the link between proteins and their glycosaminoglycans chains.8,97,98,126,135 Bolton et al15,16 were able to show that both protein expression and mRNA levels of link proteins decrease with age, and these changes are largely reflected by altered gene expression. Link proteins also undergo different degrees of glycosylation, but it is unclear if this is an implication of function and structure.97 Furthermore, a notable increase in the heterogeneity of proteoglycan monomers was observed with age9,10,26,118 along with experiments showing an irregular aggregate structure in cultures of older chondrocytes.26 Finally, proteoglycan monomers interact with hyaluronic acid via its protein core to form the macromolecular aggregates.50-54 Although the size of the aggregate depends partly on the size of the monomeric proteoglycans, it is determined by principally the length of the hyaluronic acid chain and number of monomers attached to it.51 Holmes et al57 showed that the molecular mass of hyaluronic acid is not constant and that it decreases considerably (approximately 7-fold) during maturation and aging, thus suggesting that 2 factors regulate the size of proteoglycan aggregates in aging articular cartilage. Changes in aggregates are also associated with its hydration content.
Molecular composition of proteoglycans: Ratio of chondroitin sulfate to keratin sulfate. The glycosaminoglycan chains that are covalently bound to the core protein in the proteoglycans are long, unbranched disaccharide units. The 3 most common types of glycosaminoglycans are chondroitin sulfate, keratin sulfate, and dermatan sulfate. The negative charges attributed from the repeating sulfate and/or the carboxyl groups are important for the osmotic pressure and charged repulsive forces that maintain the structural integrity of articular cartilage.23,27,115 In humans, increasing age is accompanied with a decreasing proportion of chondroitin sulfates in the ECM of nonosteoarthritic articular cartilage.12 This change results in a decrease in the ratio of chondroitin sulfate to keratan sulfate. Since the elastic properties of cartilage are determined by the 3-dimensional organization and fixation of the charged groups (ie, mainly the chondroitin sulphate chains), a decrease in chondroitin sulfate will ultimately affect proteoglycan size.59 Finally, Lee et al73 studied aggrecan monomers and their glycosaminoglycan side chains using atomic force microscopy–based imaging and force spectroscopy. They showed that the decrease of chondroitin sulfate chains essentially transformed aggrecan into a linear core protein, with only traces of shorter keratan sulfate chains. These observations confirmed previous data showing that adult aggrecans are significantly weaker in compression based on these molecular changes.73
Water content. Because of the hydrophilic nature of aggrecan’s negatively charged sulfates, articular cartilage has about 70% to 80% water content attributing to its resilient properties.75 Aggrecan has high affinity for water by virtue of its high negative fixed-charge density, and it is trapped in a 3-dimensional network of type II collagen fibrils.7 The hydrodynamic properties of aggregates determine the load-bearing capacity of articular tissue. As the electronegative charges of aggrecan draw water into the tissue, a large osmotic pressure is created that swells and expands the ECM. This pressure produces tension within the interlacing collagen network of the matrix; balance is achieved when tension in the collagen network prevents further entry of water.84
Increasing age is accompanied by a decreasing proportion of chondroitin sulfates in the ECM of nonosteoarthritic articular cartilage.12 Furthermore, the average size of proteoglycans decreases, impairing the ability of proteoglycans to aggregate spontaneously, all of which affect the hydration state of articular cartilage.78
Summary
Age-dependent changes in articular cartilage increase the risk for cartilage degeneration and its ability to repair or regenerate itself. The synthetic activity of chondrocytes in all articular cartilage layers declines with age.68 This decline leads to structural changes in the articular cartilage and its mechanical functions (Table 1). The decline in cellular activity in all articular cartilage layers can be associated with a decrease in the growth factor response and apoptosis of chondrocytes (Figure 3).2,78,113 This also decreases the ability of the cells to repair the tissue, counteract the catabolic mediators, and maintain homeostasis (Figure 3).
Table 1.
Structure changes with age
| Component | Aging Effect | Functional Change |
|---|---|---|
| Chondrocyte | Senescence: Decreased cellular activity Decreased absolute number of cells (apoptosis) |
Reduced synthetic activity and repair |
| Extracellular matrix | Increased passive glycation Increase in the number of bonds between collagen fibrils |
Increased stiffness and brittleness |
| Changes in size, structure, and sulfation of aggrecans: Increase in ratio of proteoglycan keratin sulfate to chondroitin sulfate | Decreased resilience and hydration | |
| Decreased size of proteoglycan aggregates | Decreased hydration and cartilage resiliency | |
| Water | Decrease in water content | Increased compressive stiffness |
Figure 3.
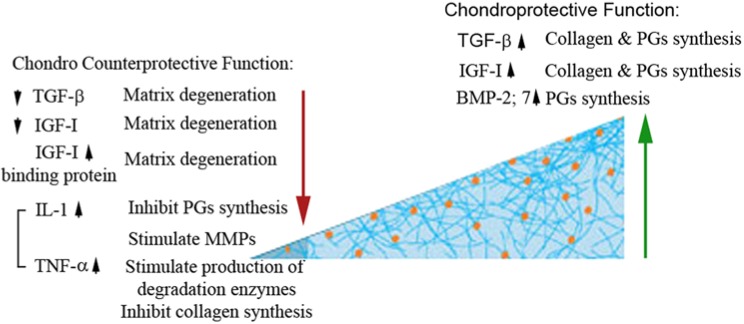
Anabolic versus catabolic changes in chondrocyte homeostasis. Autocrine, paracrine, and endocrine mediators manipulate chondrocyte cellular response in either anabolic or catabolic activity. TGF-β (transforming growth factor β), IGF-1 (insulin growth factor 1), and bone morphogenetic protein have anabolic activity on chondrocyte function and homeostasis. However, IL-1 (interleukin 1) and tumor necrosis factor α (TNFα), which are secreted from the synovial fluids, have the opposite catabolic effect on chondrocytes.132 Imbalance between these mediators can lead to cartilage degeneration and, ultimately, osteoarthritis.
Synovium and Synovial Fluid in Normal and Aged Cartilage
The term synovium refers to the soft tissue lining the spaces of diarthrodial joints, tendon sheaths, and bursae (Figure 4). When healthy, it is a thin layer of tissue that is only a few cells thick. The synovium includes the continuous surface layer of cells (intima) and the underlying tissue (subintima). The intima consists of macrophages and fibroblasts, while the subintima includes blood and lymphatic vessels. Between the intimal surfaces is a small amount of fluid, usually rich in hyaluronan (hyaluronic acid). Together, this structure provides a nonadherent surface between tissue elements.123,124
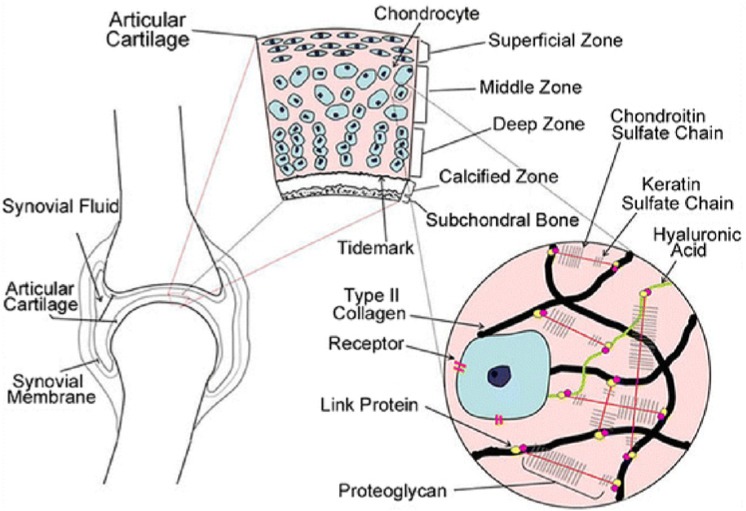
Figure 4.
Schematic representation of articular cartilage, which is an avascular tissue with a heterogeneous composition. Chondrocytes exist in a depth-dependent arrangement based on cell size and shape. Cells in the tangential zone are aligned parallel to the articular surface, cells in the middle zone are spherical and randomly distributed, and cells in the deep zone are aligned perpendicular to the tidemark and calcified zone and integrate with the subchondral bone. The matrix consists of a network of type II collagen fibers, reinforced by cross-links formed among chains of hyaluronic acid, proteoglycans, and other noncollagenous proteins.141 Image reproduced with permission from Wescoe et al.141
Because articular cartilage is avascular, chondrocytes derive both oxygen and nutrition from the synovial fluid by simple diffusion.132 The synovium therefore acts to control the environment within joints. It does this in 2 ways: First, it serves as a membrane to determine what can pass into the joint space and what stays outside. Second, the cells within the synovium produce substances that lubricate the joint (Figure 4). Paracrine factors from the synovium have an important impact on cartilage metabolism.101 Systemic hormones that diffuse into the synovial fluid also influence articular chondrocyte metabolism.101 Thus, the health of the synovium factors significantly into joint and cartilage homeostasis. Changes due to aging can compromise joint performance and its response to loading and injury.
Synoviocyte Cellular Events and Function
In general, 2 types of cells are found: synoviocytes type A and type B. Type A cells are greater in number and contain vacuoles related to phagocytic function. Type B cells have a developed ergastoplasm and are capable of transforming into fibrocytes depending on the inflammatory response to coexistent cytokines. A wide array of cytokines are produced by synovial stimulation, including tumor necrosis factor, IL-1, IL-6, and IL-8; all play a role in the inflammatory process and tissue necrosis.11
Synovium has various purposes, including an immune function, phagocytosis, lubrication, and cartilage nutrition. With regard to phagocytosis, synovium can remove bacteria and envelope small cartilage fragments that may result from joint overload, arthritis, or direct trauma. Elimination of intra-articular debris reduces the deleterious effect of inflammation over time.11 Synovial lubrication is extremely important to the healthy joint by diminishing the joint frictional coefficient, reducing heat and wear.119,125 Hyaluronic acid, a deformable gel that increases elasticity as force is applied, is synthesized by type A synoviocytes. Joint forces promote the secretion of hyaluronic acid. The ability of synovial fluid to lubricate cartilage surfaces is also dependent on the presence of lubricin, a mucinous glycoprotein that is a product of megakaryocyte-stimulating factor gene expression. Loss of synovial lubricating ability has been implicated in the pathogenesis of degenerative joint disease.62
It is difficult to describe age-associated changes in synovium and synovial fluid without describing the most relevant disease related to age. The following description of several changes is linked to common aging processes such as osteoarthritis.
Aging of Synovium and Synovial Membrane
With aging, the surface of the synovial membrane becomes more corrugated and sometimes folded into numerous villi in individuals older than 40 years.104 The synovial villi, which provide versatile deformability during movement, become more common with age and may compensate for the growing inelasticity and increasingly fibrous character of the subintima.14,60 Morphologic studies of the aging synovium revealed an overall loss of synovium lining cells. Synovial intimal fibroblast numbers decrease with advancing age. In contrast, a relative increase in synovial intimal macrophages has been observed with age.104 Moreover, fibrosis and decreased vascularity of the intimal layer are caused by collagen accumulation.64 In older individuals, the number of fibroblasts, mast cells, and macrophages per unit were notably higher in the subintimal layer.104 When microarthroscopy of human knee joints was analyzed for individuals over the age range of 15 to 56 years, there were generally more villi in aged individuals. In addition, the vascular network, cell distribution, and profiles were less regular. Particular attention was paid to synovial lining cells, among which 3 main phenotypes could be recognized: synthetic type (present at all ages and hypertrophied in aged persons), macrophage-like (increasing with age), and fibroblast-like.104 With age, blood vessels do not change in overall number; however, they tend to become more superficial, and their individual wall thickness increases.104
Matrix Metalloproteinase
Aging is associated with elevation of advanced glycation end products in articular cartilage.133 This increase in pentosidine has a strong correlation with a decrease in metalloproteinase-mediated tissue degradation.30 The increased advanced glycation end products resulted in decreased cartilage degradation by metalloproteinases from synovial fluids, indicating that aged cartilage is less sensitive than young cartilage to metalloproteinase-mediated cartilage degradation.30 This study was further confirmed in equine,17 showing that levels of active metalloproteinase 1 were lower in aged animals, unlike its activity in pathologic cases of osteoarthritis.
Prostaglandin E2 Synthesis
In response to acute inflammation induced by lipopolysaccharide, synoviocyte response to lipopolysaccharide can be measured by prostaglandin E2 levels.18 Young horses showed a drastic and significant increase in prostaglandin E2 concentration in synoviocytes, while older horses had less dramatic and lower prostaglandin E2 levels.18
Synovial Fluid
Hyaluronic acid plays an important role in joint lubrication and has beneficial effects on the joint tissues, including an anti-inflammatory effect, an inhibition of cartilage degeneration, and a positive role in cartilage repair.100 Hyaluronic acid concentration gradually decreases in individuals between 40 and 70 years old.6,100 There is a direct relationship between age and the C6S:C4S ratio (ie, chondroitin-6-sulfate to chondroitin-4-sulfate).121 At 70 years, the concentrations of C6S and the C6S:C4S ratio decreased 48.4% and 35.4%, respectively, compared with those at 20 years of age.100
Inflammatory Response
Inflammation is a part of the body’s healing response. This response is stimulated by injury, infection, surgery, or allergic reactions. Normally, inflammatory response removes unhealthy and foreign material from the area. It also begins the repair process in which new blood vessels and tissue-rebuilding cells enter the injury site. Under healthy conditions, a normal inflammatory response (due to either acute traumatic loading or exposure to inflammatory insults) will activate chondrocytes and cells of the synovium and drive biochemical events that lead to the synthesis of proinflammatory mediators known to be destructive to joints (Figure 5).43 IL-1β and tumor necrosis factor α (TNFα) are prominent mediators of cartilage destruction.35,43,81,127 These proinflammatory cytokines are responsible for cartilage destruction and intracellular pathways mediated by NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) and activator protein 1 that are involved in cytokine-mediated tissue destruction.81 Both can activate chondrocytes and synovial cells to produce IL-1β, TNFα, IL-8, IL-18, and IL-6, as well as matrix metalloproteinases, nitric oxide, and prostaglandin E2. Proinflammatory mediators also induce apoptosis and inhibit anabolic pathways (ie, the synthesis of proteoglycans, collagen type II, and tissue inhibitors of metalloproteinases). The induction of proinflammatory genes and the inhibition of matrix synthesis thus set up a self-sustaining inflammatory loop between synoviocytes and chondrocytes that exacerbates cartilage destruction.
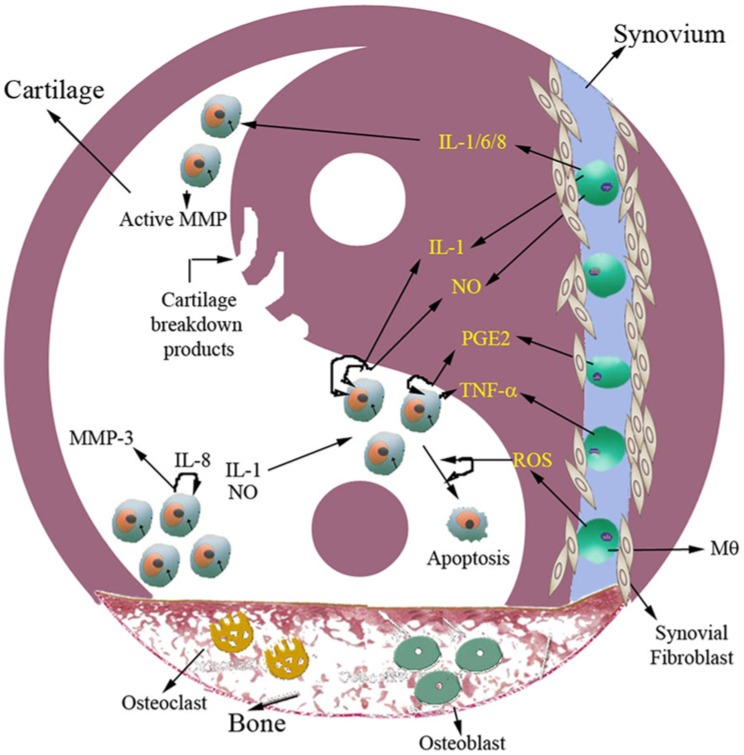
Figure 5.
Crosstalk between chondrocyte and synoviocyte. Activation of signaling pathway either by mechanical injury or subinflammation produces cytokines, chemokines, and proteolytic enzymes that shift the homeostasis into a catabolic state. The expression of several matrix-degrading enzymes (metalloproteinase 1 [MMP-1], MMP-2, MMP-3, MMP-9, MMP-13) is increased and causes cartilage breakdown. Apoptosis seems to be mediated by proinflammatory mediators and reactive oxygen species. Aged chondrocytes have an insufficient response to growth factor and hence fail to restore the cartilage. The degradation pathway stays on, and continuation of matrix degradation leads to the development of osteoarthritis.
Yin and Yang of Chondrocyte and Synoviocyte Function in Aging Joints
A low-grade inflammatory state is an integral part of the aging process and occurs at an earlier age in those who are overweight or obese.37 Adipocytes, damaged organs, and/or tissue may serve as sources of cytokines. Low-grade infections may also result in chronic activation of the immune system, and this may predispose to a variety of chronic disease stages, including arthritis, which is well documented to be an age-related disease.29,37 While data are scant, it is possible that the ability to dampen inflammation following joint injury decreases with age. It is almost impossible to describe age-associated changes in the articular cartilage without mentioning osteoarthritis. Changes within joint tissues affect repair and remodeling processes and predispose the joint to failure and the development of osteoarthritis when other factors are present, such as obesity, joint injury, and altered mechanics.
It appears that age-related changes in the ECM of cartilage result in a tissue that is less able to handle mechanical stress. The ability of the chondrocyte to maintain cartilage homeostasis declines with aging. This appears to be primarily due to decreased anabolic activity, although recent studies have shown an increase in catabolic responsiveness with age.42
The lack of homeostasis in an aged joint leads to the progression of osteoarthritis.67 Chondrocytes release chemoattractants such as IL-8 and monocyte chemoattractant protein 1 that direct the migration of leukocytes. Leukocytes, in turn, are a source of proinflammatory cytokines, such as IL-1 and TNFα, that induce apoptosis in chondrocytes (Figure 5). IL-1 can also induce chondrocytes to produce sufficient nitric oxide to cause cell death.105 These proinflammatory cytokines upregulate the expression of intercellular adhesion molecule 1 on chondrocytes, which allows the attachment of leukocytes and facilitates the accumulation of toxic agents within the chondrocyte cytoplasm.105 In addition, these cytokines stimulate the release of metalloproteinases by injured chondrocytes and leukocytes, and these metalloproteinases promote the degradation of the ECM.44,55,79,105 The resultant activation of signaling pathways, including reactive oxygen species generation, results in increased production of cytokines, chemokines, and proteolytic enzymes and induces chondrocyte apoptosis.46 This catabolic response to injury serves to degrade the damaged matrix. Matrix degradation results in the release of growth factors stored in the matrix that would normally feed back on the cell and shut down the catabolic pathways (Figure 5). However, aged chondrocytes have an insufficient response to growth factor stimulation, resulting in continued matrix destruction from unbalanced catabolic and anabolic activity.76
Maturing Athlete’s Healing Process in Response to Various Types of Injuries
Two major types of injuries should be considered in maturing athletes: prior injuries that accelerated joint degeneration and osteoarthritis and new injuries. Younger athletes experience traumatic injury to their ligaments and tendons.94 The aging athlete’s greatest menace is degenerative tissue. These are wear-and-tear disorders resulting from chronic overuse or trauma experienced over years of athletic stress.94 DeHaven and Lintner31 reviewed the incidence of various athletic injuries with aging and found that inflammatory injuries increase until the age of 70 years. This raises a valid question: whether the aging athlete heals at a slower rate.31 Jackson and Rouse61 concluded that the presence of degenerative joints at the time of injury adversely affects the healing process, suggesting that the early treatment of injuries to prevent the development of degenerative changes at a later age.
Osteoarthritis
Many older athletes have trained from a very young age, making them vulnerable to osteoarthritis. Middle-aged athletes who participate in high-intensity physical loading are 8.5 times more likely to develop osteoarthritis of the hip than are age-matched controls.136 Repetitive, high-impact loading results in cartilage microtrauma and degeneration of the weightbearing joints.66 This effect may be exacerbated by previous injury or surgery, such as prior meniscectomy in the knee, which diminishes the ability of the joint to dissipate loads.
Epigenetics and Cartilage Physiology
Modifications of chromatin can change and affect transcription and regulation of genes. Methylation and/or histone modifications (acetylation, methylation, or phosphorylation) are described as epigenetic modifications that are heritable changes that affect transcription.34 The methylation of sequences in or near regulatory elements can suppress gene expression through effects on DNA binding proteins and chromatin structure. Both increases and decreases in methylation occur with aging,34,109 depending on the tissue and the gene. These changes can have pathologic consequences, contributing to the development of malignancies and autoimmunity with aging and possibly to other disorders as well. Thus, while aging can affect DNA methylation, the changes in DNA methylation can affect aging.109
In normal articular chondrocytes, most proteases are not expressed, probably because of silencing transcription via DNA methylation.110 However, osteoarthritic chondrocytes express a new set of genes involved in cartilage catabolism (metalloproteinases and a disintegrin and metalloproteinase with thrombospondin motifs).102,111 These enzymatic changes result in a loss of DNA methylation in the relevant promoter region. Furthermore, DNA methylation in chondrocytes are not the only age-related changes in DNA methylation, as inflammatory mediators such as ILβ and TNFα produced by osteoarthritic chondrocytes can also affect DNA methylation.36,38,45,82,110 Age-related methylation of the OP-1 promoter may contribute to a decrease in OP-1 production in cartilage and a decrease in expression of OP-1-responsive genes, such as IGF-1, the IGF-1 receptor.77 Adult mice develop osteoarthritis-like disease when nuclear factor of activated T cells transcription is changed. Nuclear factor of activated T cells specifically regulates the function of adult articular chondrocytes through its age-dependent expression, which is mediated by dynamic histone modifications.140 Yet, expression levels of aggrecan in aged cartilage are not associated with DNA methylation.107
Conclusion
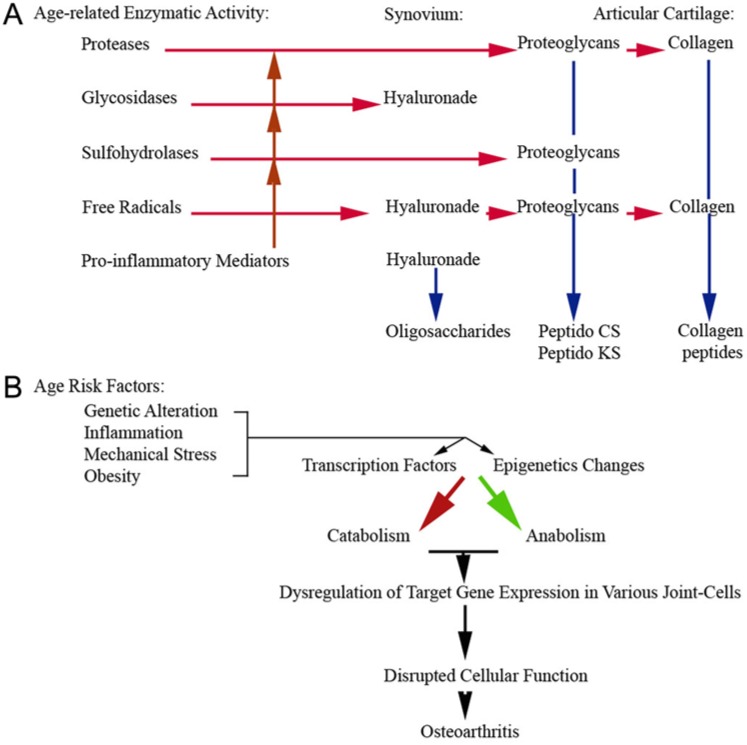
Maturing athletes are at risk for compromised joint function due to degenerative changes accruing from past injuries as well as reduced ability to recover and repair from new injuries either due to stress overload or a catastrophic event.94 This increased vulnerability is due to age-related changes (Figure 6) involving the individual as well as the joint—most notably, the articular cartilage, the synovium, and the interplay between these tissues.39,65,117
Figure 6.
Summary of (A) age-related changes in gene expression and enzymatic activity and (B) the age risk factors that lead into these changes.
The greatest threat to the health of the aging athlete is not the aging process itself but rather inactivity. Motion is critical to articular cartilage health, repair, and homeostasis. The application of constant compressive loading is important to maintain the normal structure of articular cartilage.19,69,91 Regular to moderate physical activity leads to improvements in the biomechanical and biological properties of articular cartilage70 by acting as a chondroprotective agent,103 increasing the synthesis and concentrations of proteoglycans and glycosaminoglycans70 and the other components of cartilage matrix.137,138 As stated by Astrand,5 “there is less risk in activity than in continuous inactivity.” Maintaining joint health is critical to independent living and to maintaining health.
Acknowledgments
This work was supported by National Institutes of Health grants AR052784 and AR051963 (Dr Chu).
Footnotes
The authors report no potential conflicts of interest in the development and publication of this manuscript.
References
- 1. AAOS Effects of aging. http://orthoinfo.aaos.org/topic.cfm?topic=A00191 Accessed November 6, 2013
- 2. Adams CS, Horton WE., Jr Chondrocyte apoptosis increases with age in the articular cartilage of adult animals. Anat Rec. 1998;250:418-425 [DOI] [PubMed] [Google Scholar]
- 3. Ahmed MS, Matsumura B, Cristian A. Age-related changes in muscles and joints. Phys Med Rehabil Clin N Am. 2005;16:19-39 [DOI] [PubMed] [Google Scholar]
- 4. Alford JW, Cole BJ. Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33:295-306 [DOI] [PubMed] [Google Scholar]
- 5. Astrand PO. Exercise physiology of the mature athlete. In: Sutton A, Jr, Brock RM, eds. Sport Medicine for the Mature Athlete. Indianapolis, IN: Benchmark Press; 1986:3-13 [Google Scholar]
- 6. Balazs EA. The physical properties of synovial fluid and the special role of hyaluronic acid. In: Helfet A, ed. Disorders of the Knee. Philadelphia, PA: JB Lippincott; 1982:63-75 [Google Scholar]
- 7. Bank RA, Bayliss MT, Lafeber FP, Maroudas A, Tekoppele JM. Ageing and zonal variation in post-translational modification of collagen in normal human articular cartilage: the age-related increase in non-enzymatic glycation affects biomechanical properties of cartilage. Biochem J. 1998;330(pt 1):345-351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bayliss MT. Proteoglycan structure and metabolism during maturation and ageing of human articular cartilage. Biochem Soc Trans. 1990;18:799-802 [DOI] [PubMed] [Google Scholar]
- 9. Bayliss MT, Ali SY. Age-related changes in the composition and structure of human articular cartilage proteoglycans. Biochem J. 1978;176:683-693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bayliss MT, Ali SY. Age-related changes in human articular cartilage proteoglycans. Semin Arthritis Rheu. 1981;11:20-21 [Google Scholar]
- 11. Berumen-Nafarrate E, Leal-Berumen I, Luevano E, Solis FJ, Munoz-Esteves E. Synovial tissue and synovial fluid. J Knee Surg. 2002;15:46-48 [PubMed] [Google Scholar]
- 12. Bihari-Varga M, Biro T. Thermoanalytical investigations on the age-related changes in articular cartilage, meniscus and tendon. Gerontologia. 1971;17:2-15 [DOI] [PubMed] [Google Scholar]
- 13. Bobacz K, Erlacher L, Smolen J, Soleiman A, Graninger WB. Chondrocyte number and proteoglycan synthesis in the aging and osteoarthritic human articular cartilage. Ann Rheum Dis. 2004;63:1618-1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bobacz K, Sunk IG. Age and joints. In: Conn PM, ed. Handbook of Models for Human Aging. Waltham, MA: Elsevier Academic Press; 2006:841-851 [Google Scholar]
- 15. Bolton MC, Dudhia J, Bayliss MT. Age-related changes in the synthesis of link protein and aggrecan in human articular cartilage: implications for aggregate stability. Biochem J. 1999;337(pt 1):77-82 [PMC free article] [PubMed] [Google Scholar]
- 16. Bolton MC, Dudhia J, Bayliss MT. Quantification of aggrecan and link-protein mRNA in human articular cartilage of different ages by competitive reverse transcriptase-PCR. Biochem J. 1996;319(pt 2):489-498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brama PA, van den Boom R, DeGroott J, Kiers GH, van Weeren PR. Collagenase-1 (MMP-1) activity in equine synovial fluid: influence of age, joint pathology, exercise and repeated arthrocentesis. Equine Vet J. 2004;36:34-40 [DOI] [PubMed] [Google Scholar]
- 18. Briston L, Dudhia J, Lees P. Age-related differences in prostaglandin E2 synthesis by equine cartilage explants and synoviocytes. J Vet Pharmacol Ther. 2010;33:268-276 [DOI] [PubMed] [Google Scholar]
- 19. Buckwalter JA. Should bone, soft-tissue, and joint injuries be treated with rest or activity [reprinted from J Orthop Res 1995;13:155-156]. J Orthop Sport Phys. 1995;22:181-182 [DOI] [PubMed] [Google Scholar]
- 20. Buckwalter JA, Hunzinker E, Rosenberg L, et al. Articular Cartilage: Composition and Structure. Chicago, IL: American Academy of Orthopaedic Surgeons; 1988 [Google Scholar]
- 21. Buckwalter JA, Kuettner KE, Thonar EJ, et al. Age-related changes in articular cartilage proteoglycans: electron microscopic studies. J Orthop Res. 1985;3:251-257 [DOI] [PubMed] [Google Scholar]
- 22. Buckwalter JA, Mankin HJ. Articular cartilage: tissue design and chondrocyte-matrix interactions. J Bone Joint Surg Am. 1997;79:600-611 [PubMed] [Google Scholar]
- 23. Buckwalter JA, Mankin HJ. Articular cartilage: tissue design and chondrocyte-matrix interactions. Instr Course Lect. 1998;47:477-486 [PubMed] [Google Scholar]
- 24. Buckwalter JA, Martin J, Mankin HJ. Synovial joint degeneration and the syndrome of osteoarthritis. Instr Course Lect. 2000;49:481-489 [PubMed] [Google Scholar]
- 25. Buckwalter JA, Pita JC, Muller FJ, Nessler J. Structural differences between 2 populations of articular-cartilage proteoglycan aggregates. J Orthop Res. 1994;12:144-148 [DOI] [PubMed] [Google Scholar]
- 26. Buckwalter JA, Roughley PJ, Rosenberg LC. Age-related changes in cartilage proteoglycans: quantitative electron-microscopic studies. Microsc Res Techniq. 1994;28:398-408 [DOI] [PubMed] [Google Scholar]
- 27. Buckwalter JA, Simon SRE. Orthopaedic Basic Science: Biology and Biomechanics of the Musculoskeletal System. 2nd ed. Chicago, IL: American Academy Orthopedic Surgeons; 2000 [Google Scholar]
- 28. Chubinskaya S, Kumar B, Merrihew C, Heretis K, Rueger DC, Kuettner KE. Age-related changes in cartilage endogenous osteogenic protein-1 (OP-1). Biochim Biophys Acta. 2002;1588:126-134 [DOI] [PubMed] [Google Scholar]
- 29. Creamer P, Hochberg MC. Osteoarthritis. Lancet. 1997;350:503-508 [DOI] [PubMed] [Google Scholar]
- 30. DeGroot J, Verzijl N, Wenting-Van Wijk MJ, et al. Age-related decrease in susceptibility of human articular cartilage to matrix metalloproteinase-mediated degradation: the role of advanced glycation end products. Arthritis Rheum. 2001;44:2562-2571 [DOI] [PubMed] [Google Scholar]
- 31. DeHaven KE, Lintner DM. Athletic injuries: comparison by age, sport, and gender. Am J Sports Med. 1986;14:218-224 [DOI] [PubMed] [Google Scholar]
- 32. Dore S, Pelletier JP, DiBattista JA, Tardif G, Brazeau P, Martel-Pelletier J. Human osteoarthritic chondrocytes possess an increased number of insulin-like growth factor 1 binding sites but are unresponsive to its stimulation: possible role of IGF-1-binding proteins. Arthritis Rheum. 1994;37:253-263 [DOI] [PubMed] [Google Scholar]
- 33. Duan W, Wei L, Zhang J, et al. Alteration of viscoelastic properties is associated with a change in cytoskeleton components of ageing chondrocytes from rabbit knee articular cartilage. Mol Cell Biomech. 2011;8:253-274 [PubMed] [Google Scholar]
- 34. Eden S, Cedar H. Role of DNA methylation in the regulation of transcription. Curr Opin Genet Dev. 1994;4:255-259 [DOI] [PubMed] [Google Scholar]
- 35. Evans CH. Nitric oxide: what role does it play in inflammation and tissue destruction? Agents Actions Suppl. 1995;47:107-116 [DOI] [PubMed] [Google Scholar]
- 36. Fan Z, Bau B, Yang H, Aigner T. IL-1beta induction of IL-6 and LIF in normal articular human chondrocytes involves the ERK, p38 and NFkappaB signaling pathways. Cytokine. 2004;28:17-24 [DOI] [PubMed] [Google Scholar]
- 37. Felson DT. The epidemiology of knee osteoarthritis: results from the Framingham Osteoarthritis Study. Semin Arthritis Rheum. 1990;20:42-50 [DOI] [PubMed] [Google Scholar]
- 38. Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39:237-246 [PubMed] [Google Scholar]
- 39. Flanagan SR, Ragnarsson KT, Ross MK, Wong DK. Rehabilitation of the geriatric orthopaedic patient. Clin Orthop Relat Res. 1995;316:80-92 [PubMed] [Google Scholar]
- 40. Flatt T. A new definition of aging? Front Genet. 2012;3:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Flechtenmacher J, Huch K, Thonar EJ, et al. Recombinant human osteogenic protein 1 is a potent stimulator of the synthesis of cartilage proteoglycans and collagens by human articular chondrocytes. Arthritis Rheum. 1996;39:1896-1904 [DOI] [PubMed] [Google Scholar]
- 42. Forsyth CB, Cole A, Murphy G, Bienias JL, Im HJ, Loeser RF., Jr. Increased matrix metalloproteinase-13 production with aging by human articular chondrocytes in response to catabolic stimuli. J Gerontol A Biol Sci Med Sci. 2005;60:1118-1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Goldring MB. The role of the chondrocyte in osteoarthritis. Arthritis Rheum. 2000;43:1916-1926 [DOI] [PubMed] [Google Scholar]
- 44. Goldring MB. The role of cytokines as inflammatory mediators in osteoarthritis: lessons from animal models. Connect Tissue Res. 1999;40:1-11 [DOI] [PubMed] [Google Scholar]
- 45. Goldring SR, Goldring MB. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res. 2004;427:S27-S36 [DOI] [PubMed] [Google Scholar]
- 46. Green DM, Noble PC, Ahuero JS, Birdsall HH. Cellular events leading to chondrocyte death after cartilage impact injury. Arthritis Rheum. 2006;54:1509-1517 [DOI] [PubMed] [Google Scholar]
- 47. Grushko G, Schneiderman R, Maroudas A. Some biochemical and biophysical parameters for the study of the pathogenesis of osteoarthritis: a comparison between the processes of ageing and degeneration in human hip cartilage. Connect Tissue Res. 1989;19:149-176 [DOI] [PubMed] [Google Scholar]
- 48. Guarente L. Diverse and dynamic functions of the Sir silencing complex. Nat Genet. 1999;23:281-285 [DOI] [PubMed] [Google Scholar]
- 49. Guerne PA, Blanco F, Kaelin A, Desgeorges A, Lotz M. Growth factor responsiveness of human articular chondrocytes in aging and development. Arthritis Rheum. 1995;38:960-968 [DOI] [PubMed] [Google Scholar]
- 50. Hardingham TE, Muir H. The specific interaction of hyaluronic acid with cartillage proteoglycans. Biochim Biophys Acta. 1972;279:401-405 [DOI] [PubMed] [Google Scholar]
- 51. Hascall VC, Heinegard D. Aggregation of cartilage proteoglycans: I. The role of hyaluronic acid. J Biol Chem. 1974;249:4232-4241 [PubMed] [Google Scholar]
- 52. Hascall VC, Heinegard D. Aggregation of cartilage proteoglycans: II. Oligosaccharide competitors of the proteoglycan-hyaluronic acid interaction. J Biol Chem. 1974;249:4242-4249 [PubMed] [Google Scholar]
- 53. Heinegard D, Hascall VC. Aggregation of cartilage proteoglycans: III. Characteristics of the proteins isolated from trypsin digests of aggregates. J Biol Chem. 1974;249:4250-4256 [PubMed] [Google Scholar]
- 54. Heinegard D, Hascall VC. Characterization of chondroitin sulfate isolated from trypsin-chymotrypsin digests of cartilage proteoglycans. Arch Biochem Biophys. 1974;165:427-441 [DOI] [PubMed] [Google Scholar]
- 55. Henrotin YE, Bruckner P, Pujol JP. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthritis Cartilage. 2003;11:747-755 [DOI] [PubMed] [Google Scholar]
- 56. Hickery MS, Bayliss MT, Dudhia J, Lewthwaite JC, Edwards JC, Pitsillides AA. Age-related changes in the response of human articular cartilage to IL-1alpha and transforming growth factor-beta (TGF-beta): chondrocytes exhibit a diminished sensitivity to TGF-beta. J Biol Chem. 2003;278:53063-53071 [DOI] [PubMed] [Google Scholar]
- 57. Holmes MW, Bayliss MT, Muir H. Hyaluronic acid in human articular cartilage: age-related changes in content and size. Biochem J. 1988;250:435-441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hudelmaier M, Glaser C, Hohe J, et al. Age-related changes in the morphology and deformational behavior of knee joint cartilage. Arthritis Rheum. 2001;44:2556-2561 [DOI] [PubMed] [Google Scholar]
- 59. Inerot S, Heinegard D, Audell L, Olsson SE. Articular-cartilage proteoglycans in aging and osteoarthritis. Biochem J. 1978;169:143-156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Isogai S, Murakami G, Wada T, Ishii S. Which morphologies of synovial folds result from degeneration and/or aging of the radiohumeral joint: an anatomic study with cadavers and embryos. J Shoulder Elbow Surg. 2001;10:169-181 [DOI] [PubMed] [Google Scholar]
- 61. Jackson RW, Rouse DW. The results of partial arthoscopic meniscectomy in patients over 40 years of age. J Bone Joint Br. 1982;64:481-485 [DOI] [PubMed] [Google Scholar]
- 62. Jay GD, Elsaid KA, Zack J, et al. Lubricating ability of aspirated synovial fluid from emergency department patients with knee joint synovitis. J Rheumatol. 2004;31:557-564 [PubMed] [Google Scholar]
- 63. Jerosch J. Effects of glucosamine and chondroitin sulfate on cartilage metabolism in OA: outlook on other nutrient partners especially omega-3 fatty acids [published online August 2, 2011]. Int J Rheumatol. 2011;2011:969012. 10.1155/2011/969012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jilani M, Ghadially FN. An ultrastructural study of age-associated changes in the rabbit synovial membrane. J Anat. 1986;146:201-215 [PMC free article] [PubMed] [Google Scholar]
- 65. Kallinen M, Markku A. Aging, physical activity and sports injuries: an overview of common sports injuries in the elderly. Sports Med. 1995;20:41-52 [DOI] [PubMed] [Google Scholar]
- 66. Kaplan FS, Hayes WC, Keaveny TM, Boskey A, Einhorn TA, Iannotti JP. Form and function of bone. In: Simon SR. ed. Orthopaedic Basic Science. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1994;127-184 [Google Scholar]
- 67. Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33-42 [DOI] [PubMed] [Google Scholar]
- 68. Karvonen RL, Negendank WG, Teitge RA, Reed AH, Miller PR, Fernandez-Madrid F. Factors affecting articular cartilage thickness in osteoarthritis and aging. J Rheumatol. 1994;21:1310-1318 [PubMed] [Google Scholar]
- 69. Kim YJ, Sah RL, Grodzinsky AJ, Plaas AH, Sandy JD. Mechanical regulation of cartilage biosynthetic behavior: physical stimuli. Arch Biochem Biophys. 1994;311:1-12 [DOI] [PubMed] [Google Scholar]
- 70. Kiviranta I, Tammi M, Jurvelin J, Saamanen AM, Helminen HJ. Moderate running exercise augments glycosaminoglycans and thickness of articular cartilage in the knee joint of young beagle dogs. J Orthop Res. 1988;6:188-195 [DOI] [PubMed] [Google Scholar]
- 71. Koepp H, Eger W, Muehleman C, et al. Prevalence of articular cartilage degeneration in the ankle and knee joints of human organ donors. J Orthop Sci. 1999;4:407-412 [DOI] [PubMed] [Google Scholar]
- 72. Koepp HE, Sampath KT, Kuettner KE, Homandberg GA. Osteogenic protein-1 (OP-1) blocks cartilage damage caused by fibronectin fragments and promotes repair by enhancing proteoglycan synthesis. Inflamm Res. 1999;48:199-204 [DOI] [PubMed] [Google Scholar]
- 73. Lee HY, Han L, Roughley PJ, Grodzinsky AJ, Ortiz C. Age-related nanostructural and nanomechanical changes of individual human cartilage aggrecan monomers and their glycosaminoglycan side chains. J Struct Biol. 2013;181:264-273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lee SW, Song YS, Lee SY, et al. Downregulation of protein kinase CK2 activity facilitates tumor necrosis factor-alpha-mediated chondrocyte death through apoptosis and autophagy. PLoS One. 2011;6:e19163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Li YP, Wei XC, Zhou JM, Wei L. The age-related changes in cartilage and osteoarthritis [published online July 22, 2013]. Biomed Res Int. 10.1155/2013/916530 [DOI] [PMC free article] [PubMed]
- 76. Loeser RF., Jr Aging and the etiopathogenesis and treatment of osteoarthritis. Rheum Dis Clin North Am. 2000;26:547-567 [DOI] [PubMed] [Google Scholar]
- 77. Loeser RF. Molecular mechanisms of cartilage destruction: mechanics, inflammatory mediators, and aging collide. Arthritis Rheum. 2006;54:1357-1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Loeser RF, Im HJ, Richardson B, Lu Q, Chubinskaya S. Methylation of the OP-1 promoter: potential role in the age-related decline in OP-1 expression in cartilage. Osteoarthritis Cartilage. 2009;17:513-517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Loeser RF, Shakoor N. Aging or osteoarthritis: which is the problem? Rheum Dis Clin North Am. 2003;29:653-673 [DOI] [PubMed] [Google Scholar]
- 80. Loeser RF, Shanker G, Carlson CS, Gardin JF, Shelton BJ, Sonntag WE. Reduction in the chondrocyte response to insulin-like growth factor 1 in aging and osteoarthritis: studies in a non-human primate model of naturally occurring disease. Arthritis Rheum. 2000;43:2110-2120 [DOI] [PubMed] [Google Scholar]
- 81. Lotz M. Cytokines in cartilage injury and repair. Clin Orthop Relat Res. 2001;391:S108-S115 [DOI] [PubMed] [Google Scholar]
- 82. Malemud CJ. Protein kinases in chondrocyte signaling and osteoarthritis. Clin Orthop Relat Res. 2004;427:S145-S151 [DOI] [PubMed] [Google Scholar]
- 83. Mankin HJ, Mow CV, Buckwalter JA, Iannotti JP, Ratcliffe A. Articular cartilage structure, composition, and function. In: Buckwalter JA, Einhorn TA, Simon SR, eds. Orthopaedic Basic Science. 2nd ed Rosemont, IL: American Academy of Orthopaedic Surgeons; 2000:443-470 [Google Scholar]
- 84. Maroudas A, Muir H, Wingham J. The correlation of fixed negative charge with glycosaminoglycan content of human articular cartilage. Biochim Biophys Acta. 1969;177:492-500 [DOI] [PubMed] [Google Scholar]
- 85. Martin JA, Buckwalter JA. Aging, articular cartilage chondrocyte senescence and osteoarthritis. Biogerontology. 2002;3:257-264 [DOI] [PubMed] [Google Scholar]
- 86. Martin JA, Buckwalter JA. The role of chondrocyte senescence in the pathogenesis of osteoarthritis and in limiting cartilage repair. J Bone Joint Surg Am. 2003;85(suppl 2):106-110 [DOI] [PubMed] [Google Scholar]
- 87. Martin JA, Buckwalter JA. Roles of articular cartilage aging and chondrocyte senescence in the pathogenesis of osteoarthritis. Iowa Orthop J. 2001;21:1-7 [PMC free article] [PubMed] [Google Scholar]
- 88. Martin JA, Buckwalter JA. Telomere erosion and senescence in human articular cartilage chondrocytes. J Gerontol A Biol Sci Med Sci. 2001;56:B172-B179 [DOI] [PubMed] [Google Scholar]
- 89. Martin JA, Ellerbroek SM, Buckwalter JA. Age-related decline in chondrocyte response to insulin-like growth factor-I: the role of growth factor binding proteins. J Orthop Res. 1997;15:491-498 [DOI] [PubMed] [Google Scholar]
- 90. Martin JA, Mitchell CJ, Klingelhutz AJ, Buckwalter JA. Effects of telomerase and viral oncogene expression on the in vitro growth of human chondrocytes. J Gerontol A Biol Sci Med Sci. 2002;57:B48-B53 [DOI] [PubMed] [Google Scholar]
- 91. Matyas JR, Huang D, Chung M, Adams ME. Regional quantification of cartilage type II collagen and aggrecan messenger RNA in joints with early experimental osteoarthritis. Arthritis Rheum. 2002;46:1536-1543 [DOI] [PubMed] [Google Scholar]
- 92. Mayne R, von der Mark K. Collagens and cartilage. In: Hall BK, ed. Cartilage: Structure, Function and Biochemistry. New York, NY: Academic Press; 1983:181-214 [Google Scholar]
- 93. Medawar PB. The definition and measurement of senescence. In: Wolstenholme GEW, Cameron MP, Etherington J, eds. Ciba Foundation Colloquia on Ageins: General Aspects. London: J&A Churchill; 1955:4-15 [Google Scholar]
- 94. Menard D, Stanish WD. The aging athlete. Am J Sports Med. 1989;17:187-196 [DOI] [PubMed] [Google Scholar]
- 95. Messai H, Duchossoy Y, Khatib AM, Panasyuk A, Mitrovic DR. Articular chondrocytes from aging rats respond poorly to insulin-like growth factor-1: an altered signaling pathway. Mech Ageing Dev. 2000;115:21-37 [DOI] [PubMed] [Google Scholar]
- 96. Moriyama H, Kanemura N, Brouns I, et al. Effects of aging and exercise training on the histological and mechanical properties of articular structures in knee joints of male rat. Biogerontology. 2012;13:369-381 [DOI] [PubMed] [Google Scholar]
- 97. Mort JS, Caterson B, Poole AR, Roughley PJ. The origin of human cartilage proteoglycan link-protein heterogeneity and fragmentation during aging. Biochem J. 1985;232:805-812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Mort JS, Poole AR, Roughley PJ. Age-related changes in the structure of proteoglycan link proteins present in normal human articular cartilage. Biochem J. 1983;214:269-272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Mow VC, Hung CT. Biomechanics of articular cartilage. In: Nordin M, Frankel VH, eds. Basic Biomechanics of the Musculoskeletal System. Philadelphia, PA: Lippincott Williams & Wilkins; 2001:60-97 [Google Scholar]
- 100. Nakayama Y, Narita T, Mori A, Uesaka S, Miyazaki K, Ito H. The effects of age and sex on chondroitin sulfates in normal synovial fluid. Arthritis Rheum. 2002;46:2105-2108 [DOI] [PubMed] [Google Scholar]
- 101. Newton CD, Nunamaker DM. Synovioum and cartilage in health and disease [chap 5]. In: Newton CD, ed. Textbook of Small Animal Orthopaedics. Philadelphia, PA: Lippincott Williams & Wilkins; 1985 [Google Scholar]
- 102. Nguyen Q, Mort JS, Roughley PJ. Preferential mRNA expression of prostromelysin relative to procollagenase and in situ localization in human articular cartilage. J Clin Invest. 1992;89:1189-1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Otterness IG, Eskra JD, Bliven ML, Shay AK, Pelletier JP, Milici AJ. Exercise protects against articular cartilage degeneration in the hamster. Arthritis Rheum. 1998;41:2068-2076 [DOI] [PubMed] [Google Scholar]
- 104. Pasquali-Ronchetti I, Frizziero L, Guerra D, et al. Aging of the human synovium: an in vivo and ex vivo morphological study. Semin Arthritis Rheum. 1992;21:400-414 [DOI] [PubMed] [Google Scholar]
- 105. Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001;44:1237-1247 [DOI] [PubMed] [Google Scholar]
- 106. Phillips PD, Kaji K, Cristofalo VJ. Progressive loss of the proliferative response of senescing WI-38 cells to platelet-derived growth factor, epidermal growth factor, insulin, transferrin, and dexamethasone. J Gerontol. 1984;39:11-17 [DOI] [PubMed] [Google Scholar]
- 107. Poschl E, Fidler A, Schmidt B, Kallipolitou A, Schmid E, Aigner T. DNA methylation is not likely to be responsible for aggrecan down regulation in aged or osteoarthritic cartilage. Ann Rheum Dis. 2005;64:477-480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Redini F, Mauviel A, Pronost S, Loyau G, Pujol JP. Transforming growth factor beta exerts opposite effects from interleukin-1 beta on cultured rabbit articular chondrocytes through reduction of interleukin-1 receptor expression. Arthritis Rheum. 1993;36:44-50 [DOI] [PubMed] [Google Scholar]
- 109. Richardson B. Impact of aging on DNA methylation. Ageing Res Rev. 2003;2:245-261 [DOI] [PubMed] [Google Scholar]
- 110. Roach HI, Aigner T. DNA methylation in osteoarthritic chondrocytes: a new molecular target. Osteoarthritis Cartilage. 2007;15:128-137 [DOI] [PubMed] [Google Scholar]
- 111. Roach HI, Yamada N, Cheung KSC, et al. Association between the abnormal expression of matrix-degrading enzymes by human osteoarthritic chondrocytes and demethylation of specific CpG sites in the promoter regions. Arthritis Rheum. 2005;52:3110-3124 [DOI] [PubMed] [Google Scholar]
- 112. Rose MR. Evolutionary Biology of Aging. Oxford, England: Oxford University Press; 1991 [Google Scholar]
- 113. Roughley PJ. Age-associated changes in cartilage matrix: implications for tissue repair. Clin Orthop Relat Res. 2001;391:S153-S160 [DOI] [PubMed] [Google Scholar]
- 114. Roughley PJ. The structure and function of cartilage proteoglycans. Eur Cell Mater. 2006;12:92-101 [DOI] [PubMed] [Google Scholar]
- 115. Roughley PJ, Lee ER. Cartilage proteoglycans: structure and potential functions. Microsc Res Tech. 1994;28:385-397 [DOI] [PubMed] [Google Scholar]
- 116. Roughley PJ, White RJ. Age-related changes in the structure of the proteoglycan subunits from human articular cartilage. J Biol Chem. 1980;255:217-224 [PubMed] [Google Scholar]
- 117. Sandelin J. Acute sports injuries requiring hospital care. Br J Sports Med. 1986;20:99-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Scharstuhl A, van Beuningen HM, Vitters EL, van der Kraan PM, van den Berg WB. Loss of transforming growth factor counteraction on interleukin 1 mediated effects in cartilage of old mice. Ann Rheum Dis. 2002;61:1095-1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Schmidt TA, Sah RL. Effect of synovial fluid on boundary lubrication of articular cartilage. Osteoarthritis Cartilage. 2007;15:35-47 [DOI] [PubMed] [Google Scholar]
- 120. Schwartz MA, Ciccotti MG. Cartilage biology: structure and function [chap 1]. In: Mirzayan R, ed. Cartilage Injury in the Athlete. New York, NY: Thieme; 2004 [Google Scholar]
- 121. Sharif M, Osborne DJ, Meadows K, et al. The relevance of chondroitin and keratan sulphate markers in normal and arthritic synovial fluid. Br J Rheumatol. 1996;35:951-957 [DOI] [PubMed] [Google Scholar]
- 122. Silver FH, Bradica G, Tria A. Elastic energy storage in human articular cartilage: estimation of the elastic modulus for type II collagen and changes associated with osteoarthritis. Matrix Biology. 2002;21:129-137 [DOI] [PubMed] [Google Scholar]
- 123. Smith MD. Immunohistochemistry of normal synovium. Ann Rheum Dis. 2004;63:1532-1533 [PMC free article] [PubMed] [Google Scholar]
- 124. Smith MD. The normal synovium. Open Rheumatol J. 2011;5:100-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Smith MD, Barg E, Weedon H, et al. Microarchitecture and protective mechanisms in synovial tissue from clinically and arthroscopically normal knee joints. Ann Rheum Dis. 2003;62:303-307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009;1:461-468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Stefanovic-Racic M, Mollers MO, Miller LA, Evans CH. Nitric oxide and proteoglycan turnover in rabbit articular cartilage. J Orthop Res. 1997;15:442-449 [DOI] [PubMed] [Google Scholar]
- 128. Taniguchi N, Carames B, Ronfani L, et al. Aging-related loss of the chromatin protein HMGB2 in articular cartilage is linked to reduced cellularity and osteoarthritis. Proc Natl Acad Sci U S A. 2009;106:1181-1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Thacker J, Zdzienicka MZ. The mammalian XRCC genes: their roles in DNA repair and genetic stability. DNA Repair. 2003;2:655-672 [DOI] [PubMed] [Google Scholar]
- 130. Thacker J, Zdzienicka MZ. The XRCC genes: expanding roles in DNA double-strand break repair. DNA Repair. 2004;3:1081-1090 [DOI] [PubMed] [Google Scholar]
- 131. Tyler JA. Insulin-like growth factor 1 can decrease degradation and promote synthesis of proteoglycan in cartilage exposed to cytokines. Biochem J. 1989;260:543-548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ulrich-Vinther M, Maloney MD, Schwarz EM, Rosier R, O’Keefe RJ. Articular cartilage biology. J Am Acad Orthop Surg. 2003;11:421-430 [DOI] [PubMed] [Google Scholar]
- 133. Verzijl N, Bank RA, TeKoppele JM, DeGroot J. AGEing and osteoarthritis: a different perspective. Curr Opin Rheumatol. 2003;15:616-622 [DOI] [PubMed] [Google Scholar]
- 134. Verzijl N, DeGroot J, Thorpe SR, et al. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem. 2000;275:39027-39031 [DOI] [PubMed] [Google Scholar]
- 135. Vilim V, Fosang AJ. Proteoglycans isolated from dissociative extracts of differently aged human articular cartilage: characterization of naturally occurring hyaluronan-binding fragments of aggrecan. Biochem J. 1994;304(pt 3):887-894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Vingard E, Alfredsson L, Goldie I, Hogstedt C. Sports and osteoarthrosis of the hip: an epidemiologic study. Am J Sports Med. 1993;21:195-200 [DOI] [PubMed] [Google Scholar]
- 137. Visser NA, Vankampen GP, Dekoning MH, Vanderkorst JK. The effects of loading on the synthesis of biglycan and decorin in intact mature articular cartilage in vitro. Connect Tissue Res. 1994;30:241-250 [DOI] [PubMed] [Google Scholar]
- 138. Visser NA, Vankampen GP, Dekoning MH, Vanderkorst JK. Mechanical loading affects the synthesis of decorin and biglycan in intact immature articular cartilage in vitro. Int J Tissue React. 1994;16:195-203 [PubMed] [Google Scholar]
- 139. Vo N, Niedernhofer LJ, Nasto LA, et al. An overview of underlying causes and animal models for the study of age-related degenerative disorders of the spine and synovial joints. J Orthop Res. 2013;31:831-837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Wang J, Rodova M, Lu Q, Woodbury B, Zhong XB, Anderson H. Nfat1 regulates adult articular chondrocyte function through its age-dependent expression mediated by epigenetic histone methylation. Bone. 2011;48:S142-S142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Wescoe KE, Schugar RC, Chu CR, Deasy BM. The role of the biochemical and biophysical environment in chondrogenic stem cell differentiation assays and cartilage tissue engineering. Cell Biochem Biophys. 2008;52:85-102 [DOI] [PubMed] [Google Scholar]
- 142. Woo SLY, Buckwalter JA. AAOS NIH ORS workshop: injury and repair of the musculoskeletal soft-tissues Savannah, Georgia, June 18-20, 1987 J Orthop Res. 1988;6:907-931 [DOI] [PubMed] [Google Scholar]
- 143. Xin H, Liu D, Songyang Z. The telosome/shelterin complex and its functions. Genome Biol. 2008;9:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Zhou HW, Lou SQ, Zhang K. Recovery of function in osteoarthritic chondrocytes induced by p16INK4a-specific siRNA in vitro. Rheumatology (Oxford). 2004;43:555-568 [DOI] [PubMed] [Google Scholar]